Avicenna Journal of Clinical Microbiology and Infection. 8(4):145-155.
doi: 10.34172/ajcmi.2021.27
Original Article
Identification of Potential Glucosyltransferase Inhibitors from Cinnamic Acid Derivatives Using Molecular Docking Analysis: A Bioinformatics Study
Amir Taherkhani 1  , Fateme Ghonji 2, Alireza Mazaheri 3, Mohammad Parsa Lohrasbi 4, Zeinab Mohamadi 3, Zahra Khamverdi 2, 5, *
, Fateme Ghonji 2, Alireza Mazaheri 3, Mohammad Parsa Lohrasbi 4, Zeinab Mohamadi 3, Zahra Khamverdi 2, 5, * 
Author information:
1Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2Faculty of Dentistry, Borujerd Branch, Islamic Azad University, Borujered, Iran
3Department of Operative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran
4Dental School, Silesia Medical University, Katowice, Poland
5Dental Research Center, Department of Operative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran
*
Corresponding author: Zahra Khamverdi, Faculty of Dentistry, Borujerd Branch, Islamic Azad University, Borujered, Iran; Dental Research Center, Department of Operative Dentistry, Dental School, Hamadan University of Medical Sciences, Hamadan, Iran. Fax: +98-8138381085, Phone: +98-9183122095, Email:
dr.zahra.khamverdi@gmail.com
Abstract
Background: Dental caries is one of the most common oral chronic diseases. Streptococcus mutans is the main pathogenic bacteria playing a role in degrading the mineral texture of the teeth. Glucosyltransferase (GTFase) of S. mutans is responsible for producing glucan, which is the main exopolysaccharide found in the cariogenic biofilms. Further, previous studies have reported that cinnamic acid diminished biofilm formation of S. mutans. Therefore, we hypothesized that cinnamic acid and its derivatives might act as GTFase inhibitors.
Methods: The binding affinity of a total of 12 plant-based compounds including cinnamic acid and its 11 derivatives to the GTFase active site were examined by utilizing the AutoDock tool. The possible interactions between top-ranked cinnamic acid derivatives and the residues within the GTFase catalytic site were also taken into consideration.
Results: Five of the cinnamic acid derivatives including rosmarinic acid (RA), cynarine, chlorogenic acid (CGA), caffeic acid 3-glucoside, and N-p-coumaroyltyramine demonstrated inhibitory effects on GTFase at nanomolar concentration. Stabilizing interactions such as π–π stack pairing and pi-charge interactions were detected between top-ranked GTFase inhibitors and residues within the enzyme active site.
Conclusions: The present study suggests that RA, cynarine, CGA, caffeic acid 3-glucoside, and N-p-coumaroyltyramine might have protective effects on dental caries, and therefore, may be considered as anti-tooth caries compounds.
Keywords: Cinnamic acid, Dental caries, Glucosyltransferase, Inhibitor, Molecular docking
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Dental caries (tooth decay) is the most frequent oral disorder worldwide that has destructive effects on the quality of life. According to the World Health Organization (WHO) report, it affects 60-90% of children, especially in developing countries, although it can affect people at any age. Several factors have been reported to be involved in the dental caries occurrence. While matrix metalloproteinases(MPPs) contribute to degrading the organic tissue within the teeth, tooth-adherent bacteria are involved in metabolizing sucrose, leading to acid production and demineralization of the mineral structure of teeth (1-6). Streptococcus mutans is the most prevalent bacteria extracted from human cariogenic dental cavities (7,8). It mediates the synthesis of exopolysaccharides, the main texture of cariogenic biofilms, resulting in more bacterial adhesion (9,10). Glucan is found to be the most common exopolysaccharides synthesized by glucosyltransferase (GTFase) of S. mutans. Therefore, GTFase inhibition has been considered as an effective strategy to diminish dental biofilm formation and to prevent dental caries occurrence (11-13).
Cinnamic acid is an aromatic carboxylic acid compound that can be synthesized by deamination of phenylalanine and is primarily found in Cinnamomum cassia, Panax ginseng, vegetables, grains, and honey (14,15). Figure 1 illustrates the chemical structure of cinnamic acid achieved by the ACD/ChemSketch version 12.01. Cinnamic acid derivatives are naturally produced by modifying their aromatic ring and the acrylic acid group (16). Several pharmaceutical features (i.e., antimicrobial, anticancer, and anti-inflammatory) have been reported for cinnamic acid and its derivatives (17,18). In addition to antibacterial activities of cinnamic acid derivatives, Mojtabavi et al (19) demonstrated that the combination of cinnamic acid and laccase resulted in approximately 90% reduction in S. mutans biofilm formation.
Figure 1.
Chemical Structure of cis-Cinnamic Acid.
.
Chemical Structure of cis-Cinnamic Acid.
In the present study, we hypothesized that cinnamic caid and its derivatives might act as GTFase inhibitors in S. mutans. The binding affinity of cinnamic acid and its 11 derivatives to the GTFase active site were estimated by molecular docking analysis. Five of the tested compounds were revealed to block the GTFase catalytic site at the nanomolar scale. Two-dimensional structures of the tested compounds in this study are presented in Table 1.
Table 1.
Two-dimensional Structures of the Tested Ligands in This Study for the Identification of Potential GTFase Inhibitors
|
Compound Name
|
Sources
|
Two-Dimensional Structure
|
Reference
|
| RA |
Rosemary, Perilla frutescens, and Salvia miltiorrhiza |
|
(56) |
| Cynarine |
Vernonia anthelmintica
|
|
(57) |
| CGA |
Apples, artichoke, betel, burdock, carrots, coffee beans, eggplants, Eucommia, and grapes |
|
(58) |
| Caffeic Acid 3-glucoside |
American cranberry |
|
(59) |
| N-p-Coumaroyltyramine |
Crinum biflorum Rottb |
|
(60) |
| CAPE |
Propolis and grains |
|
(61,62) |
| O-Coumaric Acid |
Barley, rye, corn, berries, grapes, apples, beans, peas, hazelnut, pecan, celery, tomato, garlic, flax, mustard, and tea |
|
(63-66) |
| CA |
Blueberries, kiwis, plums, cherries, and apples |
|
(67) |
| Ferulic acid |
Grains, spinach, parsley, grapes, rhubarb, and cereal seeds |
|
(68) |
| Sinapinic Acid |
Rhizome of Hydnophytum formicarum |
|
(69) |
| P-Coumaric acid |
Barley, rye, corn, berries, grapes, apples, beans, peas, hazelnut, pecan, celery, tomato, garlic, flax, mustard, and tea |
|
(63-66) |
| Cinnamic acid |
Cinnamomum cassia, Panax ginseng, grains, and honey |
|
(15) |
Note. GTFase: Glucosyltransferase; RA: Rosmarinic acid; CGA: Chlorogenic acid; CAPE: Caffeic acid phenethyl ester; CA: Caffeic acid.
Methods
Structural Preparation
The three-dimensional structure of GTFase was collected from the Protein Data Bank (PDB ID: 3AIE) with a criteria of X-ray resolution = 2.10 Å, which is available at https://www.rcsb.org. A total of eight subunits were included within the 3AIE file. Chain A, with a total of 844 residues, was chosen for computational dockings. It should be noted that the molecular energy of GTFase was optimized prior to molecular docking analysis by using the Swiss-PdbViewer version 4.1.0, which is available at http://www.expasy.org/spdbv (20).
The binding affinity of 12 compounds including cinnamic acid and its derivatives to the GTFase catalytic site was examined by using the AutoDock software (version 4.0), which is available at http://autodock.scripps.edu (21). The components included rosmarinic acid (RA), cynarine, chlorogenic acid (CGA), caffeic acid 3-glucoside, N-p-Coumaroyltyramine, caffeic acid phenethyl ester (CAPE), o-Coumaric acid, caffeic acid (CA), ferulic acid, sinapinic acid, p-coumaric acid, and cinnamic acid. In addition, Acarbose (PubChem ID: 41774), Maltose (PubChem ID: 6255), and WP1066 (PubChem ID: 11210478) were considered as standard inhibitors of GTFase (22,23). All ligand structures were firstly achieved as SDF files from the public repository for information on chemical substances and their biological activities (PubChem database), which is available at https://pubchem.ncbi.nlm.nih.gov (24-26). Thereafter, the SDF files were converted to PDB formats using the web-server of the Computer-Aided Drug Design (CADD) Group of the Chemical Biology Laboratory (CBL), NCI, and NIH located at the Frederick National Laboratory for Cancer Research (FNLCR), formerly NCI-Frederick (http://cactus.nci.nih.gov/chemical/structure). The energy minimization of small molecules was also executed before binding energy predictions using the HyperChem software (version 8.0.10) (27).
Molecular Docking and Post-docking Analyses
A windows-based computer (with the criteria of installed memory: 32 GB, processor: Intel Core i7, and system type: 64-bit) was used for in silico simulations. The AutoDock tool imposes limited flexibility on the protein. It uses an accurate free energy force field based on a Lamarckian genetic algorithm, leading to a rapid ligand conformation prediction within the binding site and estimating the Gibbs free binding energy from the following algorithm (28-30):
∆Gbinding = Intermolecular Energy + Total Internal Energy + Torsional Free Energy - Unbound System’s Energy
The active site of GTFase was considered as a receptor for the ligands. The grid box settings in the AutoDock tool (included spacing, 0.375 Å; X-dimension, 58; Y-dimension, 74; Z-dimension, 52; X-center, 190.161; Y-center, 46.104; and Z-center, 191.584. A total of 14 amino acids were identified to be located within the GTFase catalytic site from the Ito and colleagues’ study (22), including Tyr430, Leu433, Leu434, Arg475, Asp477, Asn481, Glu515, Trp517, Arg540, His587, Asp588, Asp909, Tyr916, and Gln960. It is worth mentioning that a total of 50 runs were set for each ligand.
For each ligand, the lowest ∆Gbinding within the largest cluster of results was considered for post-docking analyses including protein-ligand complex imaging and interaction mode study. The BIOVIA Discovery Studio Visualizer version 19.1.0.18287 (https://discover.3ds.com/discovery-studio-visualizer-download) was used for visualizing the two-dimensional images of interactions between top-ranked inhibitors and residues within the GTFase active site as well as demonstrating the three-dimensional docked pose of the top-ranked CA derivatives.
Results
Binding Affinity and Interaction Modes Between GTFase and Small Molecules
The Gibbs free energy changes of interactions between GTFase and the studied compounds were estimated using the AutoDock tool to identify potential GTFase inhibitors for combating dental caries. According to the results, a total of five cinnamic acid derivatives including RA, cynarine, CGA, caffeic acid 3-glucoside, and N-p-coumaroyltyramine were predicted to bind to the GTFase catalytic site at the nanomolar scale (nM); therefore, these cinnamic acid derivatives were considered as top-ranked GTFase inhibitors in the present study. It was also estimated that CAPE, o-Coumaric acid, and CA could inhibit the GTFase activity at the micromolar scale (uM). Moreover, ferulic acid, sinapinic acid, p-coumaric acid, and cinnamic acid revealed a dismal affinity to the GTFase active site, based on the inhibition constant values (Ki) calculated for these molecules that were predicted to be at the millimolar (mM) scale. In addition, acarbose demonstrated the highest binding affinity to the GTFase active site among control inhibitors followed by maltose and WP1066. Moreover, the ΔGbinding of GTFase with RA, cynarine, and CGA was predicted to be more negative than that of WP1066, suggesting that these three compounds can attach to the GTFase catalytic site more tightly than the WP1066 (Figure 2).
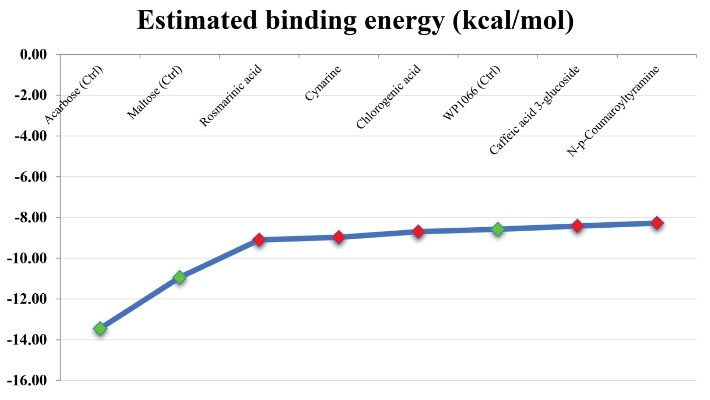
Figure 2.
Comparing the Binding Affinity Between the GTFase Catalytic Site and its Top-ranked Inhibitors From Cinnamic Acid Derivatives. Note. FTFase: Glucosyltransferase. Acarbose, Maltose, and WP1066 were considered as the standard GTFase inhibitors. The x-axis corresponds to the ligand name. The y-axis represents the score of ΔG binding in terms of kcal/mol.
.
Comparing the Binding Affinity Between the GTFase Catalytic Site and its Top-ranked Inhibitors From Cinnamic Acid Derivatives. Note. FTFase: Glucosyltransferase. Acarbose, Maltose, and WP1066 were considered as the standard GTFase inhibitors. The x-axis corresponds to the ligand name. The y-axis represents the score of ΔG binding in terms of kcal/mol.
The estimated ∆G binding and Ki values for all tested compounds in this study are presented in Table 2. The details of energies among top-ranked cinnamic acid derivatives and GTFase catalytic site are illustrated in Table 3. The interaction modes between top-ranked cinnamic derivatives and the residues within the GTFase active sites were also taken into consideration (Table 4). Accordingly, cynarine and N-p-coumaroyltyramine demonstrated the greatest number of hydrogen and hydrophobic interactions, respectively. It should be noticed that the H-bonds with the criteria of distance > 5 Å were not considered significant, and consequently, were removed from Table 4. Figure 3 illustrates the two-dimensional images of these interactions as well as the three-dimensional docked pose of top-ranked ligands. Figure 4 demonstrates all interactions between top-ranked cinnamic acid derivatives and their corresponding amino acids in a unique network achieved by Cytoscape version 3.8.0 software (https://cytoscape.org/download.html) (31).
Table 2.
Estimated Binding Energy and Ki Value of all Compounds Tested in This Study After Molecular Docking With GTFase
|
PubChem ID
|
Ligand Name
|
ΔG
binding
|
K
i
|
| 5281792 |
RA |
-9.10 |
212.34 nM |
| 6124212 |
Cynarine |
-8.97 |
265.18 nM |
| 1794427 |
CGA |
-8.70 |
419.70 nM |
| 5281759 |
Caffeic acid 3-glucoside |
-8.42 |
669.37 nM |
| 5372945 |
N-p-Coumaroyltyramine |
-8.27 |
864.04 nM |
| 5281787 |
CAPECAPE |
-7.92 |
1.56 uM |
| 637540 |
O-Coumaric acid |
-5.01 |
212.28 uM |
| 689043 |
CA |
-4.32 |
687.05 uM |
| 445858 |
Ferulic acid |
-4.01 |
1.16 mM |
| 637775 |
Sinapinic acid |
-3.99 |
1.18 mM |
| 637542 |
p-Coumaric acid |
-3.56 |
2.47 mM |
| 444539 |
Cinnamic acid |
-3.17 |
4.74 mM |
| 41774 |
Acarbose (Ctrl) |
-13.45 |
138.56 pM |
| 6255 |
Maltose (Ctrl) |
-10.94 |
9.53 nM |
| 11210478 |
WP1066 (Ctrl) |
-8.58 |
511.17 nM |
Note. GTFase: Glucosyltransferase; RA: Rosmarinic acid; CGA: Chlorogenic acid; CAPE: Caffeic acid phenethyl ester; CA: Caffeic acid.
Table 3.
Details of Energies Between Top-ranked Cinnamic Acid Derivatives, Control Inhibitors, and GTFase Catalytic Site Achieved From Molecular Docking Analysis
|
Ligand Name
|
Final Intermolecular Energy (kcal/mol)
|
Final Total Internal Energy (kcal/mol)
|
Torsional Free Energy (kcal/mol)
|
Unbound System’s Energy (kcal/mol)
|
Estimated Free Energy of Binding (kcal/mol)
|
| RA |
-8.1 |
-5.72 |
3.88 |
-0.84 |
-9.10 |
| Cynarine |
-10.97 |
-4.67 |
5.37 |
-1.3 |
-8.97 |
| CGA |
-6.53 |
-6.97 |
3.58 |
-1.22 |
-8.70 |
| N-p-coumaroyltyramine |
-8.32 |
-2.64 |
2.39 |
-0.3 |
-8.42 |
| Caffeic acid 3-glucoside |
-8.2 |
-4.75 |
3.58 |
-0.94 |
-8.27 |
| Acarbose (Ctrl) |
-11.33 |
-11.72 |
6.56 |
-3.04 |
-13.45 |
| Maltose (Ctrl) |
-8.95 |
-7.01 |
3.58 |
-1.44 |
-10.94 |
| WP1066 (Ctrl) |
-8.47 |
-2.6 |
1.79 |
-0.7 |
-8.58 |
Note. GTFase: Glucosyltransferase; RA: Rosmarinic acid; CGA: Chlorogenic acid.
Table 4.
Interaction Modes Between Top-ranked Cinnamic Acid Derivatives and Residues Inside the GTFase Active Site
|
Ligand Name
|
Hydrogen Bond (Distance Å)
|
Hydrophobic Interaction (Distance Å)
|
Electrostatic: Pi-charge (Distance Å)
|
Miscellaneous (Distance Å)
|
Halogen (Distance Å)
|
| RA |
Asp477 (4.11, 4.37, 4.39); |
NA |
Glu515 (7.65); Asp909 (6.40) |
Tyr916 (3.60) |
NA |
| Cynarine |
Asp909 (4.23); Asn481 (3.99); Glu509 (3.83); Ser589 (3.21); Asp593 (3.88) |
Tyr916 (6.97); Trp517 (4.91) |
NA |
NA |
NA |
| CGA |
Asn481 (3.98, 4.73) |
Trp517 (6.01) |
Asp909 (7.51) |
NA |
NA |
| N-p-Coumaroyltyramine |
Asn481 (4.86); Ala478 (3.53); Gln592 (4.73, 4.78) |
Phe907 (7.06); Tyr916 (5.55); His587 (7.17) |
NA |
NA |
NA |
| Caffeic acid 3-glucoside |
Asp477 (3.53, 4.31); Glu515 (4.83) |
Trp517 (6.06) |
Asp588 (5.86) |
NA |
NA |
| Acarbose (Ctrl) |
Gly429 (3.60); Asp477 (4.35); Asp909 (4.61); Glu515 (4.98); Asn481 (4.81); Trp517 (4.69); Gly428 (3.51); Ser518 (3.89) |
NA |
NA |
NA |
NA |
| Maltose (Ctrl) |
Asn481 (4.04, 4.98); Gln592 (4.62, 4.91) |
NA |
NA |
NA |
NA |
| WP1066 (Ctrl) |
Asp588 (4.29) |
Trp517 (5.99); His587 (5.90); Leu433 (7.12); Ala478 (6.31) |
Glu515 (7.37); Asp909 (6.20) |
NA |
His587 (5.72) |
Note. GTFase: Glucosyltransferase; RA: Rosmarinic acid; CGA: Chlorogenic acid. Acarbose, Maltose, and WP1066 were considered control inhibitors of the enzyme.
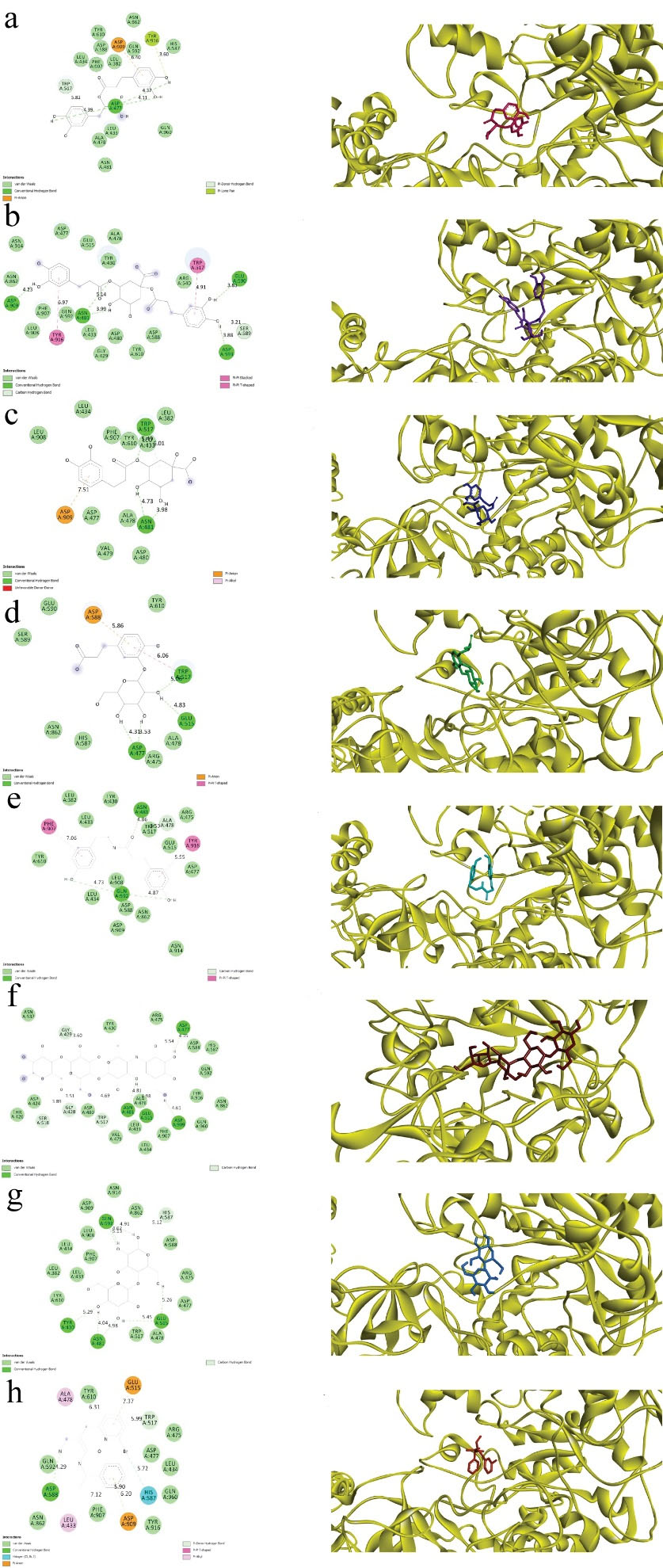
Figure 3.
Left: Two-dimensional Images of Interaction Modes Between (a) Rosmarinic Acid, (b) Cynarine, (c) Chlorogenic acid, (d) Caffeic acid 3-glucoside, (e) N-p-Coumaroyltyramine, (f) Acarbose, (g) Maltose, (h) WP1066, and Residues Within the GTFase Catalytic Site. Right: Three-dimensional Docked Pose of the Corresponding Ligands. Note. GTFase: Glucosyltransferase.
.
Left: Two-dimensional Images of Interaction Modes Between (a) Rosmarinic Acid, (b) Cynarine, (c) Chlorogenic acid, (d) Caffeic acid 3-glucoside, (e) N-p-Coumaroyltyramine, (f) Acarbose, (g) Maltose, (h) WP1066, and Residues Within the GTFase Catalytic Site. Right: Three-dimensional Docked Pose of the Corresponding Ligands. Note. GTFase: Glucosyltransferase.
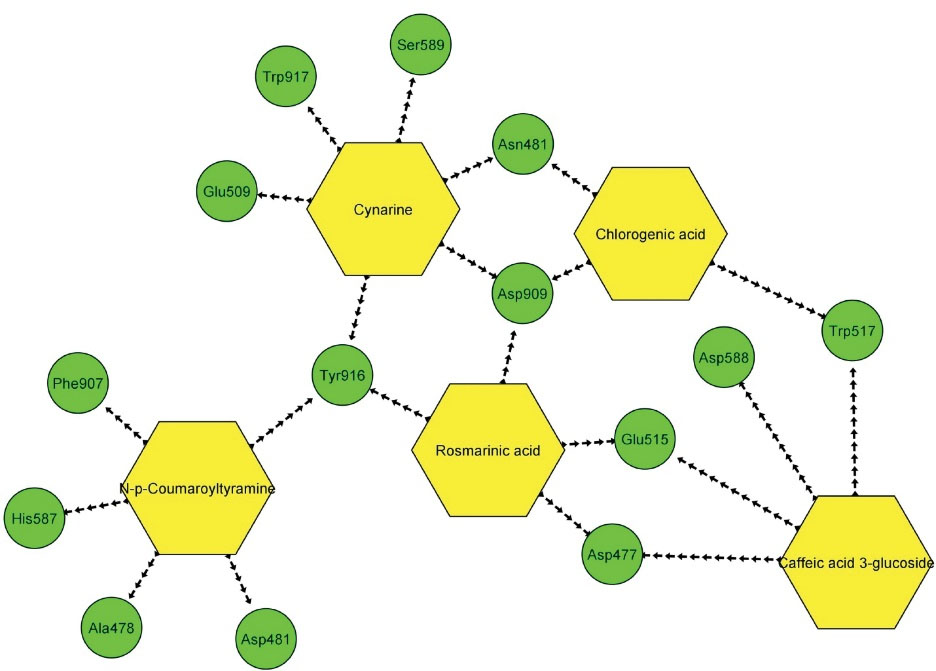
Figure 4.
Possible Connections Between Top-ranked Cinnamic Acid Derivatives and Amino Acids Incorporated Within the GTFase Catalytic Site. Note. GTFase: Glucosyltransferase.
.
Possible Connections Between Top-ranked Cinnamic Acid Derivatives and Amino Acids Incorporated Within the GTFase Catalytic Site. Note. GTFase: Glucosyltransferase.
Discussion
Tooth decay is one of the most common chronic diseases worldwide (32). It is a multifactorial disorder in which matrix metalloproteinases and S. mutans are most responsible for degrading the organic and mineral texture of the teeth, respectively (33,34). GTFase of S. mutans plays an essential role in biofilm formation, leading to more bacterial cohesion, acid production, and dental caries (11-13,35). To discover potential GTFase inhibitors, the binding affinity of several plant-based compounds including cinnamic acid and its 11 derivatives with GTFase catalytic sites were estimated using a molecular docking approach. The obtained results predicted that RA, cynarine, CGA, caffeic acid 3-glucoside, and N-p-Coumaroyltyramine could potentially inhibit the GTFase active site at the nanomolar scale. In addition, it was found that three of these compounds (i.e., RA, cynarine, and CGA) were more tightly bonded to the enzyme compared with WP1066 as one of the standard inhibitors of the enzyme.
CA is a water-soluble metabolite that can be synthesized in herbs with several beneficial properties such as antioxidant, antiviral, antibacterial, antitumorigenic, as well as liver and cardiovascular protective effects (36). Sorgi et al (37) conducted a study to examine CA’s antibacterial and anti-inflammatory properties in macrophage response against S. mutans. The authors demonstrated that S. mutans displayed an antibacterial effect at the half-maximal inhibitory concentration (IC50) = 2.938 mM without illustrating cytotoxicity. Moreover, CA led to downregulation of nitrite, tumor necrosis factor alpha, and prostaglandin E2 through the nuclear factor kappa B dependent pathway, demonstrating its anti-inflammatory effects within the macrophages. Furthermore, Nakamura et al (38) reported that CA solution significantly increased the antibacterial effect of 385 nM LED irradiation against cariogenic S. mutans biofilms. According to the present results, caffeic acid 3-glucoside was estimated to bond to the GTFase active site at the nanomolar scale (Ki = 669.37 nM) with a considerable ∆G binding of −8.42 kcal/mol, while the estimated binding energy between CA and GTFase catalytic site was −4.32 kcal/mol, suggesting that binding of a sugar moiety to CA has enhanced the binding affinity of the compound to GTFase active site. Caffeic acid 3-glucoside demonstrated three hydrogens one hydrophobic and one electrostatic interaction with the Asp477, Glu515, and Asp588 inside the GTFase active site. A pi-charge was detected between caffeic acid 3-glucoside and Asp588 (5.86 Å). It is worth mentioning that the π-π stack pairing, pi-charge, and salt bridges are the most stabilizing connections among ligands and proteins (33).
RA is a well-known antioxidant compound that exhibits antipathogenic activity in plants. It is an ester of CA and (R)-(+)-3-(3, 4-dihydroxy phenyl) lactic acid originating from L-phenylalanine and L-tyrosine, respectively. Many other beneficial properties have also been reported for RA including antinociceptive and neuroprotective effects. RA is found in a wide range of medicinal plant species including Rosmarinus officinalis L. (Lamiaceae), Apiaceae, Araliaceae, Cucurbitaceae, Rubiaceae, Plantaginaceae, and Polygonaceae (39-44).
Zdarilová et al (45) carried out a study to examine the effects of Prunella vulgaris L. extract (PVE) and RA, the main compound of PVE, on lipopolysaccharide -induced inflammation and oxidative impairment in human gingival fibroblasts. They reported that PVE and RA led to reduced reactive oxygen species production, resulting in down-regulation of interleukin 1b, interleukin 6, tumor necrosis factor-a, and inducible nitric oxide synthase. The authors demonstrated that PVE and RA could significantly reduce lipopolysaccharide-induced damages in gingival fibroblasts due to their anti-inflammatory properties. Therefore, they may be used for therapeutic purposes in periodontal diseases. Further, previous studies have reported a link between inflammatory periodontal diseases and dental plaque (46).
According to the present results, RA demonstrated the highest binding affinity to GTFase active site among 12 cinnamic acid derivatives. It was predicted that RA could attach to the GTFase catalytic site at the nanomolar concentration (Ki = 212.34 nM) with a salient ∆G binding of −9.10 kcal/mol. It revealed three hydrogen, two electrostatic, and one miscellaneous interactions with the Asp477, Glu515, Tyr916, and Asp909 within the GTFase active site. Further, the interactions among Glu515, Asp909, and SA were of pi-charge type.
CGA is an ester of CA and quinic acid with antioxidant activity (47,48) which is mainly found in coffee, apples, berries, pears, and aubergines (49).Previous studies have shown that coffee has exhibited anti GTFase activity in S. mutans, leading to dental caries prevention (11,50). Moreover, Lin et al (51) triggered a study to examine the effect of CGA on tooth decay in rats. The authors investigated the antibacterial properties of CGA on S. mutans ATCC 10449 and S. sobrinus OMZ65.The obtained results revealed that the MIC and MBC of S. mutans were 2.5 and 7.5 mg/mL, respectively. Therefore, the authors suggested that CGA may be considered as a potential anti tooth caries compound by inhibiting the growth of S. mutans. Hu et al (52) found that CGA elevated the osteogenic differentiation of human dental pulp stem cells through the Wnt signaling pathway. The authors suggested that CGA may be useful for alveolar bone damage repairmen in patients with periodontal disease. According to the results of the present study, CGA formed two hydrogen, one hydrophobic, and one pi-charged interactions with Asn481, Trp517, and Asp909 within the GTFase active site. It was also estimated that CGA can attach to the GTFase catalytic domain at the nanomolar concentration (Ki = 419.70 nM) with a ∆G binding of -8.70 kcal/mol.
N-p-coumaroyltyramine is a phenolic compound primarily found in Tribulus terrestris that has demonstrated several pharmaceutical properties such as anti-cariogenic effect against S. mutans. According to previous studies, T. terrestris have significantly reduced the growth, adhesion, acid production, as well as synthesis of glucan within the S. mutans (53,54). According to the results of the present study, N-p-Coumaroyltyramine could block the GTFase activity at the nanomolar scale (Ki = 864.04 nM) with a ∆G binding = -8.27 kcal/mol, suggesting the potential anti-tooth decay property of the compound. N-p-Coumaroyltyramine demonstrated four hydrogen and three hydrophobic interactions with Ala478, Asn481, His587, Gln592, Phe907, and Tyr916 inside the GTFase catalytic site.
Cynarine is a polar component mainly found in the roots of Echinacea angustifolia (55). It revealed a considerable binding affinity to the GTFase catalytic site (∆G binding = −8.97 kcal/mol) and was found to inhibit the enzyme activity at the nanomolar scale (Ki = 265.18 nM). Cynarine displayed five hydrogen and two hydrophobic interactions with the Asn481, Glu509, Trp517, Ser589, Asp593, Asp909, and Tyr916 within the GTFase active site. It should be noted that the electrostatic between Trp517 and cynarine is of pi-pi stack pairing type.
Previous studies have demonstrated that acarbose and maltose are potent GTFase inhibitors (22). Moreover, Tsurumaki et al (23) reported that WP1066 (PubChem ID: 11210478), a well-known JAK/STAT3 signaling pathway inhibitor, revealed inhibitory effects on ceramide GTFase. Therefore, these three compounds were considered as control inhibitors of GTFase in this study. Acarbose showed a salient binding affinity to the GTFase active site with ∆G binding and Ki values of -13.45 kcal/mol and 138.56 picomolar (pM), respectively. It demonstrated eight hydrogen bonds with the Gly428, Gly429, Asp477, Glu515, Trp517, Ser518, Asn481, and Asp909 residues within the GTFase catalytic site. In addition, maltose revealed a high binding affinity to the GTFase active site. The ∆G binding and Ki values for this compound were calculated to be −10.94 kcal/mol and 9.53 nM, respectively. It illustrated four hydrogen interactions with the Asn481 and Gln592 residues within the GTFase active site. Further, WP1066 formed one hydrogen, four hydrophobic, and two pi-charge interactions with the Leu433, Ala478, Glu515, Trp517, His587, Asp588, and Asp509 within the GTFase active site. The ∆G binding and Ki values of WP1066 regarding the enzyme were calculated to be −8.58 kcal/mol and 511.17 nM, respectively.
Conclusions
The present study suggests that RA, cynarine, CGA, caffeic acid 3-glucoside, and N-p-coumaroyltyramine potentially have inhibitory effects on GTFase of S. mutans at nanomolar concentration. Therefore, these compounds may be helpful for preventing dental caries; however, these findings should be confirmed by wet-lab techniques.
Acknowledgments
The authors would like to thank the Faculty of Dentistry, Borujerd Branch, Islamic Azad University, Borujered, Iran, for their support.
Authors’ Contributions
AT and ZK designed the study. FG and AT performed Docking operations. AT processed all images were. AT, ZK, and FG analyzed and discussed the results. AT wrote the manuscript, and finally, all authors read and approved the final version of the manuscript.
Availability of Data and Materials
The datasets used and/or analyzed during the current studyare available from the corresponding author on reasonablerequest.
Conflict of Interests
The authors declare no conflict of interests.
Ethical Approval
Not applicable.
Funding
This research received no specific grant from any fundingagency in the public, commercial, or not-for-profit sectors.
References
- Kale S, Kakodkar P, Shetiya S, Abdulkader R. Prevalence of dental caries among children aged 5-15 years from 9 countries in the Eastern Mediterranean Region: a meta-analysis. East Mediterr Health J 2020; 26(6):726-35. doi: 10.6719/emhj.20.050 [Crossref] [ Google Scholar]
- Sharma A, Bansal P, Grover A, Sharma S, Sharma A. Oral health status and treatment needs among primary school going children in Nagrota Bagwan block of Kangra, Himachal Pradesh. J Indian Soc Periodontol 2014; 18(6):762-6. doi: 10.4103/0972-124x.147421 [Crossref] [ Google Scholar]
- Al Agili DE. A systematic review of population-based dental caries studies among children in Saudi Arabia. Saudi Dent J 2013; 25(1):3-11. doi: 10.1016/j.sdentj.2012.10.002 [Crossref] [ Google Scholar]
- Prados-Privado M, García Villalón J, Martínez-Martínez CH, Ivorra C, Prados-Frutos JC. Dental caries diagnosis and detection using neural networks: a systematic review. J Clin Med 2020; 9(11):3579. doi: 10.3390/jcm9113579 [Crossref] [ Google Scholar]
- Rathee M, Sapra A. Dental caries. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- Taherkhani A, Orangi A, Moradkhani S, Khamverdi Z. Molecular docking analysis of flavonoid compounds with matrix metalloproteinase-8 for the identification of potential effective inhibitors. Lett Drug Des Discov 2021; 18(1):16-45. doi: 10.2174/1570180817999200831094703 [Crossref] [ Google Scholar]
- Fejerskov O, Nyvad B, Kidd E. Dental Caries: The Disease and its Clinical Management. John Wiley & Sons; 2015.
- Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ 2001; 65(10):1028-37. [ Google Scholar]
- Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol 2015; 5:10. doi: 10.3389/fcimb.2015.00010 [Crossref] [ Google Scholar]
- Flemming HC, Wingender J. Relevance of microbial extracellular polymeric substances (EPSs)--Part I: structural and ecological aspects. Water Sci Technol 2001; 43(6):1-8. [ Google Scholar]
- Daglia M, Tarsi R, Papetti A, Grisoli P, Dacarro C, Pruzzo C. Antiadhesive effect of green and roasted coffee on Streptococcus mutans’ adhesive properties on saliva-coated hydroxyapatite beads. J Agric Food Chem 2002; 50(5):1225-9. doi: 10.1021/jf010958t [Crossref] [ Google Scholar]
- Ham Y, Kim TJ. Inhibitory effect of phenolic acids in Rubus coreanus on glucosyltransferase of Streptococcus mutans. Curr Microbiol 2020; 77(11):3695-703. doi: 10.1007/s00284-020-02179-w [Crossref] [ Google Scholar]
- Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol 2010; 192(12):3024-32. doi: 10.1128/jb.01649-09 [Crossref] [ Google Scholar]
- Chandra S, Roy A, Jana M, Pahan K. Cinnamic acid activates PPARα to stimulate lysosomal biogenesis and lower amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol Dis 2019; 124:379-95. doi: 10.1016/j.nbd.2018.12.007 [Crossref] [ Google Scholar]
- Ruwizhi N, Aderibigbe BA. Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci 2020; 21(16):5712. doi: 10.3390/ijms21165712 [Crossref] [ Google Scholar]
- Zhang WX, Wang H, Cui HR, Guo WB, Zhou F, Cai DS. Design, synthesis and biological evaluation of cinnamic acid derivatives with synergetic neuroprotection and angiogenesis effect. Eur J Med Chem 2019; 183:111695. doi: 10.1016/j.ejmech.2019.111695 [Crossref] [ Google Scholar]
- de Almeida Lima GD, Rodrigues MP, de Oliveira Mendes TA, Moreira GA, Siqueira RP, da Silva AM. Synthesis and antimetastatic activity evaluation of cinnamic acid derivatives containing 1,2,3-triazolic portions. Toxicol In Vitro 2018; 53:1-9. doi: 10.1016/j.tiv.2018.07.015 [Crossref] [ Google Scholar]
- Gießel JM, Loesche A, Hoenke S, Csuk R. In search of new cinnamic acid derived flavours and fragrances. Results Chem 2019; 1:100010. doi: 10.1016/j.rechem.2019.100010 [Crossref] [ Google Scholar]
- Mojtabavi S, Khoshayand MR, Fazeli MR, Faramarzi MA, Samadi N. Development of an enzyme-enhancer system to improve laccase biological activities. Int J Biol Macromol 2021; 173:99-108. doi: 10.1016/j.ijbiomac.2021.01.068 [Crossref] [ Google Scholar]
- Johansson MU, Zoete V, Michielin O, Guex N. Defining and searching for structural motifs using DeepView/Swiss-PdbViewer. BMC Bioinformatics 2012; 13:173. doi: 10.1186/1471-2105-13-173 [Crossref] [ Google Scholar]
- Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J Mol Recognit 1996; 9(1):1-5. doi: 10.1002/(sici)1099-1352(199601)9:1<1::aid-jmr241>3.0.co;2-6. [Crossref] [ Google Scholar]
- Ito K, Ito S, Shimamura T, Weyand S, Kawarasaki Y, Misaka T. Crystal structure of glucansucrase from the dental caries pathogen Streptococcus mutans. J Mol Biol 2011; 408(2):177-86. doi: 10.1016/j.jmb.2011.02.028 [Crossref] [ Google Scholar]
- Tsurumaki H, Katano H, Sato K, Imai R, Niino S, Hirabayashi Y. WP1066, a small molecule inhibitor of the JAK/STAT3 pathway, inhibits ceramide glucosyltransferase activity. Biochem Biophys Res Commun 2017; 491(2):265-70. doi: 10.1016/j.bbrc.2017.07.115 [Crossref] [ Google Scholar]
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A. PubChem substance and compound databases. Nucleic Acids Res 2016; 44(D1):D1202-13. doi: 10.1093/nar/gkv951 [Crossref] [ Google Scholar]
- Wang Y, Suzek T, Zhang J, Wang J, He S, Cheng T. PubChem BioAssay: 2014 update. Nucleic Acids Res 2014; 42(Database issue):D1075-82. doi: 10.1093/nar/gkt978 [Crossref] [ Google Scholar]
- NCBI Resource Coordinators. Database resources of the national center for biotechnology information. Nucleic Acids Res 2015; 43(Database issue):D6-17. doi: 10.1093/nar/gku1130 [Crossref] [ Google Scholar]
- Froimowitz M. HyperChem: a software package for computational chemistry and molecular modeling. Biotechniques 1993; 14(6):1010-3. [ Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009; 30(16):2785-91. doi: 10.1002/jcc.21256 [Crossref] [ Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 1998; 19(14):1639-62. doi: 10.1002/(sici)1096-987x(19981115)19:14<1639::aid-jcc10>3.0.co;2-b. [Crossref] [ Google Scholar]
- Liu Z, Zhao J, Li W, Shen L, Huang S, Tang J. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci Rep 2016; 6:19095. doi: 10.1038/srep19095 [Crossref] [ Google Scholar]
- Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc 2007; 2(10):2366-82. doi: 10.1038/nprot.2007.324 [Crossref] [ Google Scholar]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007; 369(9555):51-9. doi: 10.1016/s0140-6736(07)60031-2 [Crossref] [ Google Scholar]
- Taherkhani A, Moradkhani S, Orangi A, Jalalvand A, Khamverdi Z. Molecular docking study of flavonoid compounds for possible matrix metalloproteinase-13 inhibition. J Basic Clin Physiol Pharmacol 2020. doi: 10.1515/jbcpp-2020-0036 [Crossref]
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F. Dental caries. Nat Rev Dis Primers 2017; 3:17030. doi: 10.1038/nrdp.2017.30 [Crossref] [ Google Scholar]
- Devulapalle KS, Mooser G. Glucosyltransferase inactivation reduces dental caries. J Dent Res 2001; 80(2):466-9. doi: 10.1177/00220345010800021301 [Crossref] [ Google Scholar]
- Wang RS, Wang S, Liang JW, Li T, Zhou L, Zhan ZL. (Cloning and functional analysis of caffeic acid and rosmarinic acid glycosyltransferases from Arnebia euchroma). Zhongguo Zhong Yao Za Zhi 2021; 46(1):86-93. doi: 10.19540/j.cnki.cjcmm.20200827.101.(Chinese) [Crossref] [ Google Scholar]
- Sorgi CA, de Campos Chaves Lamarque G, Verri MP, Nelson-Filho P, Faccioli LH, Paula-Silva FWG. Multifaceted effect of caffeic acid against Streptococcus mutans infection: microbicidal and immunomodulatory agent in macrophages. Arch Microbiol 2021; 203(6):2979-87. doi: 10.1007/s00203-021-02290-x [Crossref] [ Google Scholar]
- Nakamura K, Shirato M, Kanno T, Lingström P, Örtengren U, Niwano Y. Photo-irradiated caffeic acid exhibits antimicrobial activity against Streptococcus mutans biofilms via hydroxyl radical formation. Sci Rep 2017; 7(1):6353. doi: 10.1038/s41598-017-07007-z [Crossref] [ Google Scholar]
- Petersen M. Rosmarinic acid: new aspects. Phytochem Rev 2013; 12(1):207-27. doi: 10.1007/s11101-013-9282-8 [Crossref] [ Google Scholar]
- Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hücherig S. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009; 70(15-16):1663-79. doi: 10.1016/j.phytochem.2009.05.010 [Crossref] [ Google Scholar]
- Khojasteh A, Mirjalili MH, Alcalde MA, Cusido RM, Eibl R, Palazon J. Powerful plant antioxidants: a new biosustainable approach to the production of rosmarinic acid. Antioxidants (Basel) 2020; 9(12):1273. doi: 10.3390/antiox9121273 [Crossref] [ Google Scholar]
- Božin B, Gavrilović M, Kladar N, Rat M, Anačkov G, Gavarić N. Highly invasive alien plant Reynoutria japonica Houtt represents a novel source for pharmaceutical industry–evidence from phenolic profile and biological activity. J Serb Chem Soc 2017; 82(7-8):803-13. [ Google Scholar]
- Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons; 2002.
- Ghasemzadeh Rahbardar M, Hosseinzadeh H. Effects of rosmarinic acid on nervous system disorders: an updated review. Naunyn Schmiedebergs Arch Pharmacol 2020; 393(10):1779-95. doi: 10.1007/s00210-020-01935-w [Crossref] [ Google Scholar]
- Zdarilová A, Svobodová A, Simánek V, Ulrichová J. Prunella vulgaris extract and rosmarinic acid suppress lipopolysaccharide-induced alteration in human gingival fibroblasts. Toxicol In Vitro 2009; 23(3):386-92. doi: 10.1016/j.tiv.2008.12.021 [Crossref] [ Google Scholar]
- Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000 2006; 42:180-218. doi: 10.1111/j.1600-0757.2006.00192.x [Crossref] [ Google Scholar]
- Olthof MR, Hollman PC, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 2001; 131(1):66-71. doi: 10.1093/jn/131.1.66 [Crossref] [ Google Scholar]
- Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm 2011; 403(1-2):136-8. doi: 10.1016/j.ijpharm.2010.09.035 [Crossref] [ Google Scholar]
- Clifford MN. Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. J Sci Food Agric 1999; 79(3):362-72. doi: 10.1002/(sici)1097-0010(19990301)79:3<362::aid-jsfa256>3.0.co;2-d. [Crossref] [ Google Scholar]
- Daglia M, Racchi M, Papetti A, Lanni C, Govoni S, Gazzani G. In vitro and ex vivo antihydroxyl radical activity of green and roasted coffee. J Agric Food Chem 2004; 52(6):1700-4. doi: 10.1021/jf030298n [Crossref] [ Google Scholar]
- Lin JC, Zhao W, Lu JX. The inhibitory effects of chlorogenic acid on dental caries in rats. Journal of Guangdong Pharmaceutical University. 2012.
- Hu X, Wang L, He Y, Wei M, Yan H, Zhu H. Chlorogenic acid promotes osteogenic differentiation of human dental pulp stem cells through Wnt signaling. Stem Cells Dev 2021; 30(12):641-50. doi: 10.1089/scd.2020.0193 [Crossref] [ Google Scholar]
- Oh HK, Park SJ, Moon HD, Jun SH, Choi NY, You YO. Tribulus terrestris inhibits caries-inducing properties of Streptococcus mutans. J Med Plants Res 2011; 5(25):6061-6. doi: 10.5897/jmpr11.1008 [Crossref] [ Google Scholar]
- Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev 2014; 8(15):45-51. doi: 10.4103/0973-7847.125530 [Crossref] [ Google Scholar]
- Wölkart K, Gangemi D, Turner R, Bauer R. Enzymatic degradation of echinacoside and cynarine in Echinacea angustifolia Root Preparations. Pharm Biol 2004; 42(6):443-8. doi: 10.1080/13880200490894815 [Crossref] [ Google Scholar]
- Li M, Cui MM, Kenechukwu NA, Gu YW, Chen YL, Zhong SJ. Rosmarinic acid ameliorates hypoxia/ischemia induced cognitive deficits and promotes remyelination. Neural Regen Res 2020; 15(5):894-902. doi: 10.4103/1673-5374.268927 [Crossref] [ Google Scholar]
- Mamat N, Lu XY, Kabas M, Aisa HA. Potential anti-vitiligo properties of cynarine extracted from Vernonia anthelmintica (L) Willd. Int J Mol Med 2018; 42(5):2665-75. doi: 10.3892/ijmm.2018.3861 [Crossref] [ Google Scholar]
- Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017; 22(3):358. doi: 10.3390/molecules22030358 [Crossref] [ Google Scholar]
- Duke J. Dr. Duke’s Phytochemical and Ethnobotanical Databases. United States Department of Agriculture. https://phytochem.nal.usda.gov/phytochem/search.
- Masi M, Koirala M, Delicato A, Di Lecce R, Merindol N, Ka S. Isolation and biological characterization of homoisoflavanoids and the alkylamide N-p-coumaroyltyramine from Crinum biflorum Rottb, an amaryllidaceae species collected in Senegal. Biomolecules 2021; 11(9):1298. doi: 10.3390/biom11091298 [Crossref] [ Google Scholar]
- da Cunha FM, Duma D, Assreuy J, Buzzi FC, Niero R, Campos MM. Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radic Res 2004; 38(11):1241-53. doi: 10.1080/10715760400016139 [Crossref] [ Google Scholar]
- Dai G, Jiang Z, Sun B, Liu C, Meng Q, Ding K. Caffeic acid phenethyl ester prevents colitis-associated cancer by inhibiting NLRP3 inflammasome. Front Oncol 2020; 10:721. doi: 10.3389/fonc.2020.00721 [Crossref] [ Google Scholar]
- Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev 2010; 9(1):147-70. doi: 10.1007/s11101-009-9142-8 [Crossref] [ Google Scholar]
- Budryn G, Nebesny E. Fenolokwasy-ich wlasciwosci, wystepowanie w surowcach roslinnych, wchlanianie i przemiany metaboliczne. Bromatol Chem Toksykol 2006; 39(2):103-10. [ Google Scholar]
- Salameh D, Brandam C, Medawar W, Lteif R, Strehaiano P. Highlight on the problems generated by p-coumaric acid analysis in wine fermentations. Food Chem 2008; 107(4):1661-7. doi: 10.1016/j.foodchem.2007.09.052 [Crossref] [ Google Scholar]
- Kowczyk-Sadowy M, Świsłocka R, Lewandowska H, Piekut J, Lewandowski W. Spectroscopic (FT-IR, FT-Raman, 1H- and 13C-NMR), theoretical and microbiological study of trans o-coumaric acid and alkali metal o-coumarates. Molecules 2015; 20(2):3146-69. doi: 10.3390/molecules20023146 [Crossref] [ Google Scholar]
- Silva H, Lopes NMF. Cardiovascular effects of caffeic acid and its derivatives: a comprehensive review. Front Physiol 2020; 11:595516. doi: 10.3389/fphys.2020.595516 [Crossref] [ Google Scholar]
- Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 2018; 31(6):332-6. doi: 10.1159/000491755 [Crossref] [ Google Scholar]
- Senawong T, Misuna S, Khaopha S, Nuchadomrong S, Sawatsitang P, Phaosiri C. Histone deacetylase (HDAC) inhibitory and antiproliferative activities of phenolic-rich extracts derived from the rhizome of Hydnophytum formicarum Jack: sinapinic acid acts as HDAC inhibitor. BMC Complement Altern Med 2013; 13:232. doi: 10.1186/1472-6882-13-232 [Crossref] [ Google Scholar]