Avicenna Journal of Clinical Microbiology and Infection. 8(3):108-112.
doi: 10.34172/ajcmi.2021.20
Original Article
Epidemiological Distribution and Potential Risk Factors of Orientia tsutsugamushi Infection in Eastern Uttar Pradesh, India
Alka Shukla 1, Mayank Gangwar 1, Akanksha Srivastava 1, Sonam Rastogi 1, Deepak Kumar 1, Digvijay Singh 1, Rajesh Kumar 1, Pradyot Prakash 1, Gopal Nath 1, * 
Author information:
1Viral Research and Diagnostic Laboratory, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
*
Corresponding author: Gopal Nath, Viral Research and Diagnostic Laboratory, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Fax: +91-542-2367568, Tel: +91-542-2309506, +91-542-2316420, M: +91-9335058394, Email:
gopalnath@gmail.com
Abstract
Background: Scrub typhus (ST) is a rickettsial infection caused by Orientia tsutsugamushi, which presents with flu like symptoms. This disease has been reported from all over India but with slight variations in its pattern. For decreasing the prevalence, preventing new incidences, and predicting the course of the ST, therefore, it is crucial to gain knowledge and perception of local risk components associated with the disease. The present study aimed to investigate the epidemiological distribution and potential risk factors of O. tsutsugamushi Infection in Eastern Uttar Pradesh (EUP), India.
Methods: The serums of 211 samples were collected from the suspected cases along with the detailed information about the participants such as age, location, and place recorded in case history form (CRF). IgM estimation was performed using enzyme-linked immunosorbent assay (ELISA) assay.
Results: A total of 58 samples (27.4%) out of 211 ones were found to be positive for IgM antibodies against O. tsutsugamushi bacterium. Furthermore, the results were correlated with epidemiological data such as gender, rural or urban background, pets, and occupation. The results showed that 76.7% of the study participants were from rural areas or had bushes around their houses, 88.3% of them had pets/cattle or frequent encounter with rodents at their houses, and 30.3% of them had no toilet facilities at home.
Conclusions: It was concluded that the proximity to pets/cattle, having rodents in closer vicinity, residing in places surrounded by vegetation/farm/bushy areas, and following occupations involving field work increased the chances of getting bitten by mites/chiggers. Overall, Orientia tsutsugamushi prevalence increased in EUP, with respect to clinical features, disease presentation, and laboratory diagnosis can help our community to reduce the mortality caused by this infectious disease.
Keywords: Scrub typhus, Orientia tsutsugamushi, Zoonotic infection, Eastern Uttar Pradesh, Rickettsial infection
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Scrub typhus (ST) is a rickettsial zoonotic infectious disease, which is also referred to as Tsutsugamushi disease, Tsutsugamushi fever or bush typhus (1). It is caused by a gram negative obligate intracellular bacterium known as Orientia tsutsugamushi. This arthropod-borne bacterium which mainly survives in larvae (chiggers) of trombiculid mites is transmitted to human when the infected chiggers bite him (2,3). ST is often considered as occupational disease of rural population since the chances for exposure to infected chiggers are higher in rural areas (4).
This disease usually exhibits a spectrum of the signs and symptoms including pyrexia (may or may not with chills), headache, myalgia body-ache, nausea, vomiting, abdominal pain, non-pruritic macular or maculo-papular rash (not always), eschar, and a pathognomonic sign of ST which is a dark scab like lesion at the site of bite (sometimes goes unnoticed) (5). However, other symptoms such as enlarged lymph nodes and alteration in mental status ranging from confusion and encephalitis to coma have been also reported (1,3,5,6).
Epidemiology of Scrub Typhus
Globally, ST is endemic in certain geographical area known as “tsutsugamushi triangle”. This area covers around 8 million km2of land and extends from eastern Russia in the north to Australia in the south, and from Japan in the east to Pakistan in the west (7,8). People have recently showed interest in travelling and exploring different parts of the world, which has facilitated the transmission of such infections to non-endemic regions as well (1,9).
In India
As for India, this infection was first noted near Kumaon hills in 1938 (10). In 1945, few serological positive cases of ST were reported in Uttar Pradesh (11). In 2012, National Centre for Disease Control (NCDC) declared an outbreak of this bacterial infection for several states of Indian (12). Then, a large number of cases were reported from different parts of the country presenting with a wide range of signs and symptoms including even death (12-27).
This study aimed to investigate the distribution of the ST infection and identify the risk factors associated with it in Eastern Uttar Pradesh (EUP). The potential risk elements considered in this study were occupation, gender, age, seasonal variation, geographical location, surrounding of residence, and pets.
Methods
This study was conducted in the Viral Research and Diagnostic Laboratory (VRDL), Department of Microbiology, Institute of Medical Sciences, BHU, Varanasi, over a period of 6 months from September 2019 to February 2020. All suspected patients were required to submit the detailed case history form (CRF) filled by their physicians and provide their blood samples. The ST testing and this study were both approved by the Institutional Ethical Committee. The patients having fever of unknown origin (more than 6 day) and showing associated symptoms such as respiratory distress, acute renal failure, acute liver failure, and/or rash were included in this study; and their blood samples were also obtained for ST screening. It is noteworthy that these patients were from outpatient department (OPD) or indoor patients admitted to Medicine and Pediatric OPD.
Specimen Collection and Laboratory Testing
Blood samples were kept at room temperature for 30-60 minutes for separation of serum and centrifuged at 3000 rpm for 10 minutes to obtain serum (28); then they were stored in duplicate in VRDL lab for further analysis.
Serologic Analysis
Enzyme-linked immunosorbent assay (ELISA) was used to detect the presence of antibodies (IgM) against O. tsutsugamushi in the suspected serums, which was done using InBios Scrub Typhus DetectTM IgM ELISA kit. ELISA was performed following the instructions provided in the manufacturer’s manual and kit. Optical density (OD) value >0.5 was considered as cut off value since the same value had been used in previous studies (13,29,30).
Variables
CRFs of all positive cases were retrieved from VRDL data storage section. The data were analyzed for details like the age, gender, address of the patient, onset date of illness, duration of illness, duration of fever, systemic examination findings, signs and symptoms, as well as the contact numbers. Patients were contacted by VRDL staff to gather information regarding keeping pets at their houses (if any), having toilet facilities in their house, travel history, nature of the areas surrounding patients home, and occupational details.
Statistical Analysis
The data obtained from the CRFs and the number of cases with specific sign and symptoms were analyzed and presented as mean ± standard error of mean (SEM) using Sigma-Plot statistical software (version 11.0). Furthermore, the comparison was made in terms of frequency, percentage, and means values using Student t test. The P ≤ 0.05 was considered as statistically significant.
Results
Serological Assay Findings
Out of 211 serum samples which were subjected to ELISA for ST, 58 samples (27.4%) were found to be positive for IgM antibodies against O. tsutsugamushi bacterium. These patients were contacted to collect further information.
Epidemiological Data
As for 58 (30 males and 28 females), we failed to contact 15 patients; thus we were left with little details about them. Table 1 shows the information we gathered after telephone communication conducted with respective patients. Out of 43 patients who answered the phone calls, 76.7% were from rural area or had bushes around their houses, 88.3% had pets or cattle or frequent encounter with rodents at their houses, and 30.3% had no toilet facilities at their home. Occupation-wise, most of them were young students, and majority of the remaining participants followed occupation of keeping farms or cattle.
Table 1.
Detailed Description of Positive ST Cases
|
Total No. of Positive Cases=58
|
|
Information obtained from=43(100%)
|
|
Pets/rodents/cattle at home
|
Yes |
38 (88.3%) |
| No |
5 (11.7%) |
|
Bushes around house/village area
|
Yes |
33 (76.7%) |
| No |
10 (23.3%) |
|
Occupation
|
|
Most of them were students or associated with farm or cattle business (90%) |
|
Toilet facility at home
|
Yes |
30 (69.7%) |
| No |
13 (30.3%) |
Prominent Symptoms Reported
Most of the patients presented wide range of symptoms, ranging from fever to death. Ambit of most common symptoms included: headache, body-ache, chills, skin rashes, abdominal pain, vomiting, jaundice, and altered sensorium. Figure 1 shows age-wise distribution of the positive cases. Figure 2 depicts month-wise distribution of the cases for 6 studied months when November was found to be the month with the maximum reported cases. Figure 3 reflects geographical distribution.
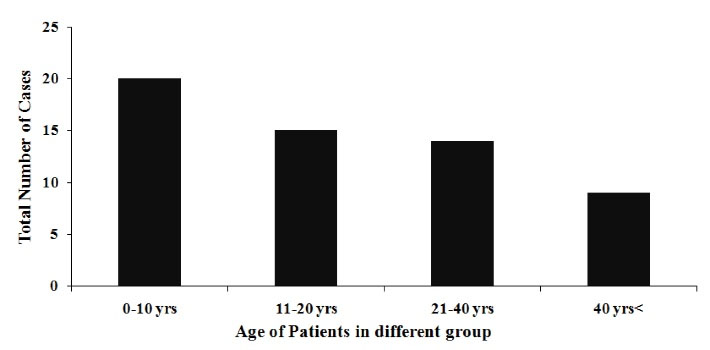
Figure 1.
Age-wise Distribution of Scrub Typhus Cases.
.
Age-wise Distribution of Scrub Typhus Cases.
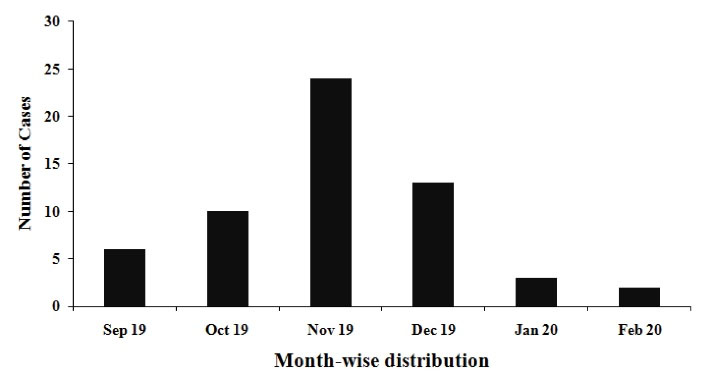
Figure 2.
Month-wise Distribution of Scrub Typhus Cases.
.
Month-wise Distribution of Scrub Typhus Cases.
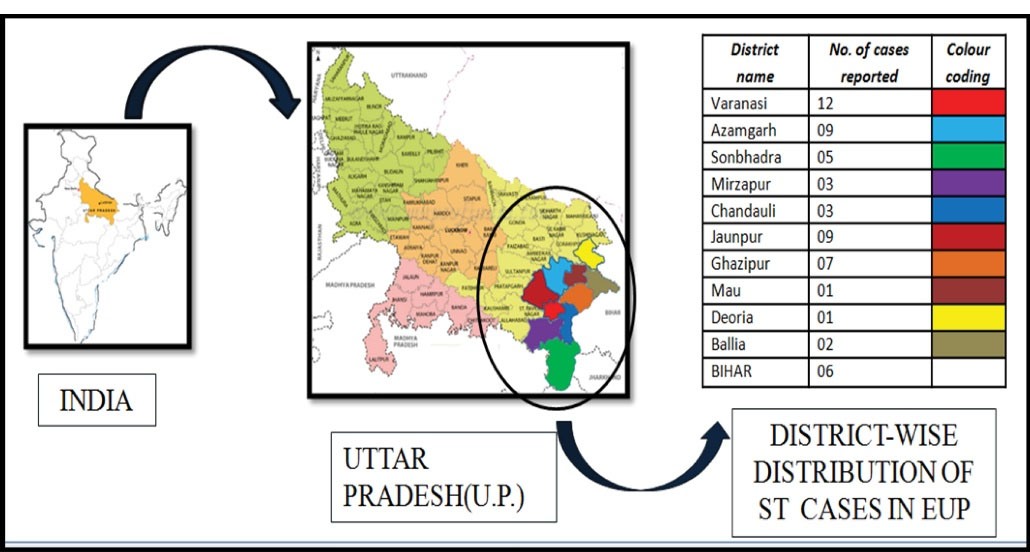
Figure 3.
Geographical Area-wise Distribution of Scrub Typhus.
.
Geographical Area-wise Distribution of Scrub Typhus.
Discussion
ST is a less-discussed and under-diagnosed bacterial infection in the world. It often mimics other infections, which makes it difficult for physician to diagnose as it does not have any specific pathognomonic set of clinical symptoms (1,31,32). If it goes undiagnosed, it can cause severe complications, including death in motion. An “Eschar” at the site of chigger-bite could be indicative of the disease, but its manifestation has been reported with a wide range of variations from 1% to 97% in various geographical regions, which does not make it a reliable manifestation of the disease (1,6).
ST surfaced in India in early 90s but failed to bloom out much due to advances in the application of insecticides, improved lifestyle, and empiric management of PUO (1). In the last decade, however, the attention was drawn towards this disease since an enormous number of ST positive cases was reported in India from 2010 to 2016 with around 20% of total acute encephalitic syndrome developed in the country (33,34). Epidemiological studies have concluded that ST occurs throughout India with prevalence rate ranging from 4.27% to 47.48%. The states with a history of presenting a good number of cases are Tamil Nadu, Karnataka, Odisha, West Bengal, Manipur, Tripura, Assam, and Uttar Pradesh (1,33,35,36).Prevalence rate unveiled by the present study was ~27%.
In our study, we obtained detailed data from 58 ST positive residents of EUP and adjacent Bihar. Most of them were from rural area and were involved with open field/farm work or cattle-keeping occupations, and belonged to younger age groups (Table 1 and Figure 1). These findings could have been attributed to the fact that the chiggers had easy access to human in rural areas since the houses were surrounded by scrub vegetation, bushes or farms, and the younger age group tended to involve in more outdoor activities enhancing their odds of getting in contact with chiggers. In a review by Xu et al, marked socioeconomic status and rural residential area were identified as two important risk factors associated with ST in India (1). Stephen et al reported that in parts of South India, field workers, patients who did not cover their bodies at home, and those who resided in bushy neighborhood were at greater risk of acquiring ST than others (20). The results regarding age distribution in the present study, however, were not consistent with the results from other studies conducted in countries like Japan where 62% of ST positive cases were reported to be under 51-75 age group (37). Since the female and male ratio was 1:1.1, no gender predilection was observed, which was in agreement with previous studies (13,38).However, increased inclination of ST towards females was reported by studies carried out in few countries like South Korea (1,39).
Approximately, 30% of ST positive patients did not have toilet facilities. Squatting to defecate or urinating in agricultural field/bushes increases the chances of getting in contact with mites by many folds (23,40,41). Thangaraj et al found open-field defecation as the most common element among ST patients (30). Data on ST outbreak in Manipur also revealed that population reliving themselves in bushy areas/jungles proved to be a “high risk” group for ST (23).
Our study results also demonstrated that EUP was an endemic region for this vector-borne infection since the infection could have been detected in full period of 6 months investigated in this study. The highest percentage of the cases was observed in November (41.3%). This finding was consistence with the result from a study by Bhargava et al.13 Maximum number of the cases during cooler season was recorded in south Indian states compared to other states of India (20,41).
In this study, keeping pets/rodents/cattle at home was identified as the highest risk factor for getting infected with OT. About 88.3% of the patients were in frequent contact with pets/rodents or kept cattle at their homes. Rodents, pets, and cattle are often infested with OT vectors and thus help it to get in contact with humans. Other risk factors highlighted in previous studies included water-body close to residence, preparation of meals outside the residence, children travelling to school by vehicles, drying clothes on grasses or bushes, taking bath in water-bodies, as well as storing firewood inside the house and carrying fodder/grass stacks on the head (30,42). Geographical distribution data from this study determined that EUP was in endemic proportion as far as the ST was concerned (Figure 2). Majority of the cases were from Varanasi, which could have been due to proximity of the university hospital to these people.
Taking into account the findings from this study, it was recommended that preventive measures be followed. Therefore, using insect-repellents as well as wearing long clothes, close-shoes, and hat/head cover were suggested for the population working in field/farms/vegetable gardens or being in contact with animal husbandry in order to decrease the infection of ST. In addition, using insecticides in disease-prone area, maintaining clean surrounding habitat, and improving sanitary standards along with avoiding open field defecation were also recommended in order to reduce the odds of ST infection.
Conclusions
Despite the changing epidemiology of ST and some limitations of this study, the following conclusions were drawn from this study:
-
Proximity to pets/cattle, having rodents in closer vicinity, residing in places surrounded by vegetation/farm/bushy area, and following occupations involving field work increased the chances of getting bitten by mites/chiggers.
-
An early diagnosis and management of the disease could have facilitated preventing its complications.
-
Overall, in EUP, an increasing awareness regarding clinical features, disease presentation, and laboratory diagnosis could have helped our community to reduce the mortality caused by this infectious disease.
Acknowledgement
Authors gratefully acknowledged the support provided by Department of Health Research under the Ministry of Health & Family Welfare (Government of India), New Delhi, India, as well as Indian Council of Medical Research (ICMR) in the form of establishment of State Level Viral Research and Diagnostic Laboratory (VRDL) network under scheme 5066.
Conflict of Interests
The authors have no conflict of interests to declare.
References
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 2017; 11(11):e0006062. doi: 10.1371/journal.pntd.0006062 [Crossref] [ Google Scholar]
- Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 2013; 89(2):301-7. doi: 10.4269/ajtmh.13-0064 [Crossref] [ Google Scholar]
-
https://www.cdc.gov/typhus/scrub/index.html.
- World Health Organization. Frequently Asked Questions: Scrub Typhus. Available from: http://www.searo.who.int/entity/emerging_diseases/CDS_faq_Scrub_Typhus.pdf.
- Shukla A, Gangwar M, Rastogi S, Nath G. Viral encephalitis: a hard nut to crack. Ann Natl Acad Med Sci (India) 2019; 55(2):98-109. doi: 10.1055/s-0039-1697767 [Crossref] [ Google Scholar]
- Shikino K, Ohira Y, Ikusaka M. Scrub typhus (Tsutsugamushi disease) presenting as fever with an eschar. J Gen Intern Med 2016; 31(5):582. doi: 10.1007/s11606-015-3371-x [Crossref] [ Google Scholar]
- Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect 2001; 3(1):11-21. doi: 10.1016/s1286-4579(00)01352-6 [Crossref] [ Google Scholar]
- Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N. Isolation of a novel Orientia species (O chuto sp nov) from a patient infected in Dubai. J Clin Microbiol 2010; 48(12):4404-9. doi: 10.1128/jcm.01526-10 [Crossref] [ Google Scholar]
- Kuo CC, Huang JL, Shu PY, Lee PL, Kelt DA, Wang HC. Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl 2012; 22(6):1803-16. doi: 10.1890/12-0031.1 [Crossref] [ Google Scholar]
- Blewitt B. Fevers of the typhus group in the Bhimtal area of Kumaon hills. J Royal Armed Corps 1938; 70:241-5. [ Google Scholar]
- Padbidri VS, Gupta NP. Rickettsiosis in India: a review. J Indian Med Assoc 1978; 71(4):104-7. [ Google Scholar]
- Mittal V, Bhattacharya D, Chhabra M. Multi-state outbreak of scrub typhus. NCDC Newsl 2013; 2:3-4. [ Google Scholar]
- Bhargava A, Kaushik R, Kaushik RM, Sharma A, Ahmad S, Dhar M. Scrub typhus in Uttarakhand & adjoining Uttar Pradesh: seasonality, clinical presentations & predictors of mortality. Indian J Med Res 2016; 144(6):901-9. doi: 10.4103/ijmr.IJMR_1764_15 [Crossref] [ Google Scholar]
- Batra HV. Spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res 2007; 126(2):101-3. [ Google Scholar]
- Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res 2007; 126(2):128-30. [ Google Scholar]
- Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M. Outbreak of scrub typhus in Southern India during the cooler months. Ann N Y Acad Sci 2003; 990:359-64. doi: 10.1111/j.1749-6632.2003.tb07391.x [Crossref] [ Google Scholar]
- Isaac R, Varghese GM, Mathai E, J M, Joseph I. Scrub typhus: prevalence and diagnostic issues in rural Southern India. Clin Infect Dis 2004; 39(9):1395-6. doi: 10.1086/424748 [Crossref] [ Google Scholar]
- Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 2010; 58:24-8. [ Google Scholar]
- Stephen S, Kandhakumari G, Vinithra SM, Pradeep J, Venkatesh C, Namachivayam V. Outbreak of pediatric scrub typhus in South India: a preliminary report. J Pediatr Infect Dis 2013; 8(3):125-9. doi: 10.3233/jpi-130392 [Crossref] [ Google Scholar]
- Stephen S, Kandhakumari G, Pradeep J, Vinithra SM, Siva PK, Hanifah M. Scrub typhus in South India: a re-emerging infectious disease. Jpn J Infect Dis 2013; 66(6):552-4. doi: 10.7883/yoken.66.552 [Crossref] [ Google Scholar]
- Boorugu H, Dinaker M, Roy ND, Jude JA. Reporting a case of scrub typhus from Andhra Pradesh. J Assoc Physicians India 2010; 58:519-20. [ Google Scholar]
- Sundhindra BK, Vijayakumar S, Kutty KA, Tholpadi SR, Rajan RS, Mathai E. Rickettsial spotted fever in Kerala. Natl Med J India 2004; 17(1):51-2. [ Google Scholar]
- Singh SI, Devi KP, Tilotama R, Ningombam S, Gopalkrishna Y, Singh TB. An outbreak of scrub typhus in Bishnupur district of Manipur, India, 2007. Trop Doct 2010; 40(3):169-70. doi: 10.1258/td.2010.090468 [Crossref] [ Google Scholar]
- Ahmad S, Srivastava S, Verma SK, Puri P, Shirazi N. Scrub typhus in Uttarakhand, India: a common rickettsial disease in an uncommon geographical region. Trop Doct 2010; 40(3):188-90. doi: 10.1258/td.2010.090447 [Crossref] [ Google Scholar]
- Chaudhry D, Garg A, Singh I, Tandon C, Saini R. Rickettsial diseases in Haryana: not an uncommon entity. J Assoc Physicians India 2009; 57:334-7. [ Google Scholar]
- Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the himalayan region of India. Jpn J Infect Dis 2005; 58(4):208-10. [ Google Scholar]
- Digra SK, Saini GS, Singh V, Sharma SD, Kaul R. Scrub typhus in children: Jammu experience. JK Sci 2010; 12(2):95-7. [ Google Scholar]
- Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 2009; 8(1):113-7. doi: 10.1021/pr800545q [Crossref] [ Google Scholar]
- Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis 2014; 23:39-43. doi: 10.1016/j.ijid.2014.02.009 [Crossref] [ Google Scholar]
- Thangaraj JWV, Vasanthapuram R, Machado L, Arunkumar G, Sodha SV, Zaman K. Risk factors for acquiring scrub typhus among children in Deoria and Gorakhpur districts, Uttar Pradesh, India, 2017. Emerg Infect Dis 2018; 24(12):2364-7. doi: 10.3201/eid2412.180695 [Crossref] [ Google Scholar]
- Watt G. Scrub typhus. In: Warrell DA, Cox TM, Firth JD, eds. Oxford Textbook of Medicine. 5th ed. Oxford: Oxford University Press; 2010.
- Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, Frances SP. Influence of Orientia tsutsugamushi infection on the developmental biology of Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae). J Med Entomol 2012; 49(6):1270-5. doi: 10.1603/me12100 [Crossref] [ Google Scholar]
- National Vector borne Disease Control Program. Ministry of Health and Family Welfare, Government of India. Statewise number of AES/JE cases and deaths from 2010–2017 [cited 2017 May 26]. http://www.nvbdcp.gov.in/Doc/je-aes-cd-April17.pdf.
- Khan SA, Bora T, Laskar B, Khan AM, Dutta P. Scrub typhus leading to acute encephalitis syndrome, Assam, India. Emerg Infect Dis 2017; 23(1):148-50. doi: 10.3201/eid2301.161038 [Crossref] [ Google Scholar]
- Sethi S, Prasad A, Biswal M, Hallur VK, Mewara A, Gupta N. Outbreak of scrub typhus in North India: a re-emerging epidemic. Trop Doct 2014; 44(3):156-9. doi: 10.1177/0049475514523761 [Crossref] [ Google Scholar]
- Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, Abraham OC. Scrub typhus: an unrecognized threat in South India - clinical profile and predictors of mortality. Trop Doct 2010; 40(3):129-33. doi: 10.1258/td.2010.090452 [Crossref] [ Google Scholar]
- Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, Furuya Y. Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg 2002; 67(2):162-5. doi: 10.4269/ajtmh.2002.67.162 [Crossref] [ Google Scholar]
- Zhang WY, Wang LY, Ding F, Hu WB, Soares Magalhaes RJ, Sun HL. Scrub typhus in mainland China, 2006-2012: the need for targeted public health interventions. PLoS Negl Trop Dis 2013; 7(12):e2493. doi: 10.1371/journal.pntd.0002493 [Crossref] [ Google Scholar]
- Lee HW, Cho PY, Moon SU, Na BK, Kang YJ, Sohn Y. Current situation of scrub typhus in South Korea from 2001-2013. Parasit Vectors 2015; 8:238. doi: 10.1186/s13071-015-0858-6 [Crossref] [ Google Scholar]
- Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, Ryu SY. A community-based case-control study of behavioral factors associated with scrub typhus during the autumn epidemic season in South Korea. Am J Trop Med Hyg 2009; 80(3):442-6. [ Google Scholar]
- Varghese GM, Raj D, Francis MR, Sarkar R, Trowbridge P, Muliyil J. Epidemiology & risk factors of scrub typhus in South India. Indian J Med Res 2016; 144(1):76-81. doi: 10.4103/0971-5916.193292 [Crossref] [ Google Scholar]
- Rose W, Kang G, Verghese VP, Candassamy S, Samuel P, Prakash JJA. Risk factors for acquisition of scrub typhus in children admitted to a tertiary centre and its surrounding districts in South India: a case control study. BMC Infect Dis 2019; 19(1):665. doi: 10.1186/s12879-019-4299-2 [Crossref] [ Google Scholar]