Avicenna Journal of Clinical Microbiology and Infection. 11(2):74-81.
doi: 10.34172/ajcmi.3543
Original Article
Antibacterial Properties of Mint Nanoemulsion Against Uropathogenic Escherichia coli Carrying Beta-Lactamase Resistance Genes
Hala Maitham Saker Alali 1  , Ashraf Kariminik 2, 3, *
, Ashraf Kariminik 2, 3, *  , Maryam Ghane 4
, Maryam Ghane 4
Author information:
1Department of Microbiology, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran
3Food and Agricultural Safety Research Center, Kerman Branch, Islamic Azad University, Kerman, Iran
4Department of Biology, Islamshahr Branch, Islamic Azad University, Islamshahr, Iran
Abstract
Background: Uropathogenic Escherichia coli (UPEC) is a significant contributor to urinary tract infections (UTIs). The inappropriate prescription and utilization of antibiotics in treating UTIs have resulted in a rise in antibiotic resistance. The aim of this study was to investigate the antibacterial properties of mint nanoemulsion against beta-lactam-resistant strains of UPEC.
Methods: Three hundred fifty urine samples were collected in sterile containers and cultured on eosin-methylene blue agar and blood agar media under sterile conditions. Microbial sensitivity testing was conducted using the standard disk diffusion method. Molecular analysis was performed to identify the blaTEM, blaSHV, and blaCTX genes in the samples. The mint nanoemulsion was produced using the low-energy method of spontaneous emulsion formation and characterized using scanning electron microscopy (SEM), dynamic light scattering (DLS), and Fourier-transform infrared spectroscopy (FTIR). The antibacterial activity of the mint nanoemulsion against multidrug-resistant (MDR) isolates underwent assessment.
Results: One hundred UPEC strains were isolated and identified, showing differing levels of resistance and sensitivity to various antibiotics. Nitrofurantoin exhibited the highest resistance (22%), while amikacin had the lowest resistance (4%). The prevalence of the blaTEM, blaSHV, and blaCTX genes among the UPEC isolates was 93.33%, 100%, and 73.33%, respectively. The mint nanoemulsion demonstrated effective antibacterial activity against resistant isolates, with a minimum inhibitory concentration of 0.2 mg/µL and a minimum bactericidal concentration of 0.3 mg/µL.
Conclusion: The resistance of UPEC against conventional antibiotics commonly used to treat UTIs was consistently high and demonstrated an increasing trend over time. This escalating situation could potentially become more severe in the future if the rational consumption of antibiotics is not carefully managed. Nanoemulsions synthesized using mint revealed promising stability and robust antimicrobial properties against UPEC isolates. Consequently, it is evident that these synthesized nanoemulsions represent a viable candidate as effective antimicrobial agents in combating antibiotic resistance in UTI treatment.
Keywords: Urinary tract infection, Antibacterial properties, Beta-lactam, Mint nanoemulsion
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Maitham Saker Alali H, Kariminik A, Ghane M. Antibacterial properties of mint nanoemulsion against uropathogenic Escherichia coli carrying beta-lactamase resistance genes. Avicenna J Clin Microbiol Infect. 2024; 11(2):74-81. doi:10.34172/ ajcmi.3543
Introduction
The emergence of antimicrobial-resistant strains has made infectious diseases; hence, their treatment has been a significant concern and problem in human society. Infections caused by resistant organisms have become prevalent and are now recognized as important contributors to morbidity, mortality, disability, extended hospital stays, financial burdens, and increased healthcare challenges. Treating these infections is challenging, often resulting in patient fatalities and posing an elevated risk. Gram-negative bacilli are particularly noteworthy as they are responsible for nosocomial infections and are a leading cause of morbidity and mortality in patients (1). The Enterobacteriaceae family is a key group of bacteria that contributes to these infections. Two significant genera within this family are Escherichia coli and Klebsiella, both of which are opportunistic nosocomial pathogens (2). E. coli is known to be an opportunistic pathogen, causing a range of infections such as septicemia, bacteremia, infantile enteritis, meningitis, and urinary tract infections (UTIs), as well as soft tissue infections (3). This pathogen primarily affects hospitalized patients with compromised immune systems, as well as individuals with underlying conditions such as diabetes mellitus and chronic pulmonary disorders (4). β-Lactams are a widely prescribed class of antibiotics for the treatment of bacterial infections. They work by inhibiting the trans-peptidase enzyme, which disrupts the synthesis of the bacterial cell wall, leading to the death of the bacteria. However, bacteria have developed a defense mechanism by producing β-lactamase enzymes that can destroy the β-lactam ring found in β-lactam antibiotics (5). There is a group of β-lactamases known as extended-spectrum β-lactamases, which exhibit three different phenotypes, namely, extended-spectrum β-lactamases, metallo-β-lactamases, and AMPC β-lactamases. Antibiotic resistance is a significant global health issue, and its spread in developing countries is not well understood yet (5). Consequently, it is crucial to detect these enzymes in vitro to prevent clinical failures resulting from inappropriate antimicrobial treatments. By identifying and understanding the presence of antibiotic-resistance enzymes, healthcare teams can make more informed decisions regarding the selection and administration of appropriate antibiotics. This approach can help improve treatment outcomes and reduce the risk of further dissemination of antibiotic-resistant bacteria (6). Nanotechnology, as an interdisciplinary and pioneering technology, along with biotechnology, has proven its ability to address deficiencies in various scientific and industrial fields, particularly in agricultural sciences and related industries (7). In the last three decades, the introduction of various technologies in the field of agriculture has brought about significant changes, such as the Green Revolution and the transition from traditional agriculture to commercial agriculture (8). Living organisms have great potential for the production of nanoparticles (NPs) and nanodevices with a wide range of applications. NPs with the desired shape and size can be produced using a simple bacterium to complex eukaryotes in the reaction mixture. Although chemical and physical methods may successfully lead to the production of pure NPs, this process is economically expensive and potentially dangerous for the environment (9). The use of biological organisms such as microorganisms, plant extracts, or plant biomass can be an alternative to physical and chemical methods for the production of NPs in a way that does not harm the environment (10). Nanoemulsions are heterogeneous systems consisting of two immiscible liquids, with one liquid phase being dispersed as nanometric droplets into another continuous liquid phase and stabilized through an appropriate emulsifier (11). In particular, oil-in-water nanoemulsions, which are of prevalent interest for food delivery systems, are composed of oil droplets dispersed in an aqueous medium and stabilized by surfactants approved for human consumption and common food substances that are generally recognized as safe by the United States Food and Drug Administration (12,13). A wide range of emulsions can be assembled with biomaterials. Mint is one of hundreds of different types of essential oils and the most popular. Mint essential oils can be used as an oil-soluble part of an emulsion (14). Thus, this study aimed at determining the frequency of beta-lactamase resistance genes in uropathogenic Escherichia coli (UPEC) and assessing the antibacterial effects of mint nanoemulsion against MDR isolates.
Materials and Methods
Sampling and Bacterial Identification
A total of one hundred bacterial strains were isolated from 350 samples collected from patients with UTIs at a hospital in Tehran, Iran, between September and December 2023. The urine samples were collected midstream in 10 mL sterile containers, centrifuged at 5000 rpm for 5 minutes, and 0.1 mL of the lower pellet portion was inoculated into 5 mL of sterile transport medium. The samples were promptly transported to the laboratory for analysis within 1–2 hours of collection. Patient age and gender data were obtained from their medical records. A loopful of the bacterial culture was diluted (10-2) with 5 mL of sterile 0.1 N normal saline and streaked onto MacConkey and sheep blood agar medium (Merck, Germany). The plates were then incubated for 24 hours at 37 °C. Bacterial identification was performed using standard biochemical tests, including Gram-staining, motility, lactose fermentation, H2S production, urease production, triple sugar iron agar test, phenylalanine deaminase test, lysine decarboxylase test, methyl red test, Voges-Proskauer test, and indole test (15).
Antimicrobial Susceptibility Testing
The susceptibility of E. coli isolates to some antibiotics was determined using the Kirby-Bauer disk-diffusion assay according to the methods of the Clinical and Laboratory Standards Institute (CLSI). Briefly, antimicrobial disks were placed on the surfaces of Mueller–Hinton agar (Merck, Germany) plates inoculated with E. coli. The inoculated plates were incubated at 37 °C for 24 hours before the diameters of the zones of inhibition were measured and interpreted following CLSI guidelines. The antibiotic disks (Padtan Teb, Iran) contained nitrofurantoin (300 μg), amikacin (30 μg), ciprofloxacin (5 μg), ceftriaxone (30 μg), nalidixic acid (30 μg), ticarcillin (10 μg), gentamicin (10 μg), trimethoprim-sulfamethoxazole (25 μg), cephalothin (30 μg), co-amoxiclav (10 μg), and ampicillin (10 μg). To conduct an antibiogram test, each strain was cultured on MacConkey agar for 24 hours at a temperature of 37 °C. Subsequently, 2–3 colonies were transferred to a test tube containing Tryptic Soy Broth and incubated for 3 hours at 37 °C to achieve a turbidity equivalent to 0.5 McFarland standard. Once the turbidity of the bacterial suspension was adjusted to match the 0.5 McFarland standard, 150 μL of the test suspension was inoculated onto Mueller-Hinton agar plates. Antibiotic discs were then placed onto the plates, which were subsequently incubated at 37 °C for 18 hours. The test results were interpreted as either susceptible or resistant based on the criteria recommended by the CLSI and the manufacturer’s protocols (16,17). Isolates that exhibited resistance to at least one antimicrobial agent in three or more categories were classified as MDR.
Molecular Identification (Polymerase Chain Reaction) of Virulence Genes
The genomic DNA of all samples was extracted using a genomic DNA isolation kit (Karmania Pars Gene, Iran). The primers for the blaTEM, blaSHV, and blaCTX genes utilized in this research are detailed in Table 1. The amplification process consisted of 35 cycles, including 1 minute at 94 °C for denaturation, 1 minute at 57 °C for annealing, 1 minute at 72 °C for extension, and a final extension step at 72 °C for 5 minutes. Subsequently, the PCR products were separated by agarose gel electrophoresis (Sinaclon, Iran) and visualized using a UV transilluminator (Ultraviolet Transilluminator, UVT-20M, KIGEN).
Table 1.
Primer Sequences Used in This Research
|
Gene
|
Primer Sequences (5’ to 3’)
|
Product Size (bp)
|
Reference
|
|
blaTEM
|
ATAAAATTCTTGAAGACGAAA
GACAGTTACCAATGCTTAATC |
1080 |
(20) |
|
blaHSV
|
AGGATTGACTGCCTTTTTG
ATTTGCTGATTTCGCTCG |
392 |
(18) |
|
blaCTX
|
CGCTTTGCGATGTGCAG
ACCGCGATATCGTTGGT |
550 |
(19) |
Preparation of Nanoemulsion Mint
The nanoemulsion system was produced using the low-energy method of spontaneous emulsion formation, in which mint was distilled in water twice by adding the dropwise non-ionic hydrophilic surfactant (Tween 80) to deionized water (21). For this purpose, initially, 50 μL of the Tween 80 surfactant was exposed to direct reflux conditions for 30 minutes with the help of non-toxic surfactant sodium dodecyl sulfate in a certain ratio with water. The formed emulsion was continuously stirred at a speed of 400 rpm by a magnetic stirrer equipped with a temperature regulation sensor (to keep the temperature of the solution constant during the formation of the emulsion). After the oil phase was added to the system containing luteolin and water, the new system was given 30 minutes to stir and reach equilibrium. It was then irradiated with 300-watt microwaves for 5 minutes (with a 1-minute break for every 30 seconds of irradiation). The oil phase in this design was the non-polar part of Twin 80, and the aqueous phase was distilled water. To produce nanoemulsions of water in oil in water, a 40% internal phase percentage was used in the presence of ultrasonic devices with power of 40 and 60 W. All nanoemulsions should have a clear appearance, without bubbles and uniform texture (non-granular texture), and have good consistency and expandability, which were investigated by the observations.
Characterization of Biosynthesized Nanoparticles
Fourier-transform infrared spectroscopy (FTIR) was utilized to identify the functional groups and proteins crucial for NP stabilization. The NPs in powder form were combined with potassium bromide (KBr) to create a solid, transparent film using a hydraulic press. The asymmetry stretching mode of the CH2 groups was related to mint nanoemulsion reflectance near 1864 cm-1. A strength vibrational frequency of C–OH bands of mint nanoemulsion, which is observed at about 954 cm-1, shows the coordination of non-ionic hydrophilic surfactants between carbon chains in final nanoemulsion structures (22). Scanning electron microscopy (SEM) with a LEO-1455VP instrument (Sweden) was employed to examine NP morphology. The particle size distribution of the NPs was determined through dynamic light scattering (DLS) measurements, which assess particle size by monitoring fluctuations in laser light intensity scattered by particles as they diffuse through a solvent (23).
Antibacterial Activity
A single colony of E. coli was inoculated in 5 mL LB liquid culture medium at 37 °C for 15 hours at 180 rpm. In addition, 100 µL of bacterial inoculum was mixed with 900 µL of H2O as a control. In addition, bacterial inoculum of 100 µL was mixed with mint nanoemulsion with the final concentration of 0.03 mg/µL, 0.05 mg/µL, 0.07 mg/µL, 0.1 mg/µL, 0.2 mg/µL, and 0.3 mg/µL as the treatment group. The bacteria were counted, and CFU/mL was determined after incubation at 37°C for 24 hours (24).
Results
Distribution of Uropathogenic Escherichia coli and Antimicrobial Susceptibility
Figure 1 shows the distribution of patients with UTIs according to gender and age. The highest incidence of UTIs was observed in the age group of 3140 years, while the lowest prevalence was found in the age range of 6170 years. Furthermore, the majority of infections were reported among females. Figure 2 outlines the frequency of sensitivity and resistance of E. coli isolates to 11 antibiotics. The sensitivity rates of the isolated strains to nitrofurantoin, amikacin, ciprofloxacin, ceftriaxone, nalidixic acid, ticarcillin, gentamicin, cotrimoxazole, cephalothin, co-amoxiclav, and ampicillin were 46%, 67%, 44%, 20%, 73%, 64%, 86%, 52%, 48%, 32%, and 69%, respectively. The highest resistance was observed against nitrofurantoin at 22%, while the lowest resistance was detected against amikacin at 4%. The highest and lowest sensitivity rates were related to nalidixic acid (73%) and ceftriaxone (20%), respectively. In addition, the frequency of the blaTEM, BlaHSV, and BlaCTX genesamong the UPEC isolates was 93.33%, 100%, and 73.33%, respectively (Figure 3). The results indicated a high prevalence of resistance genes in the examined bacteria.
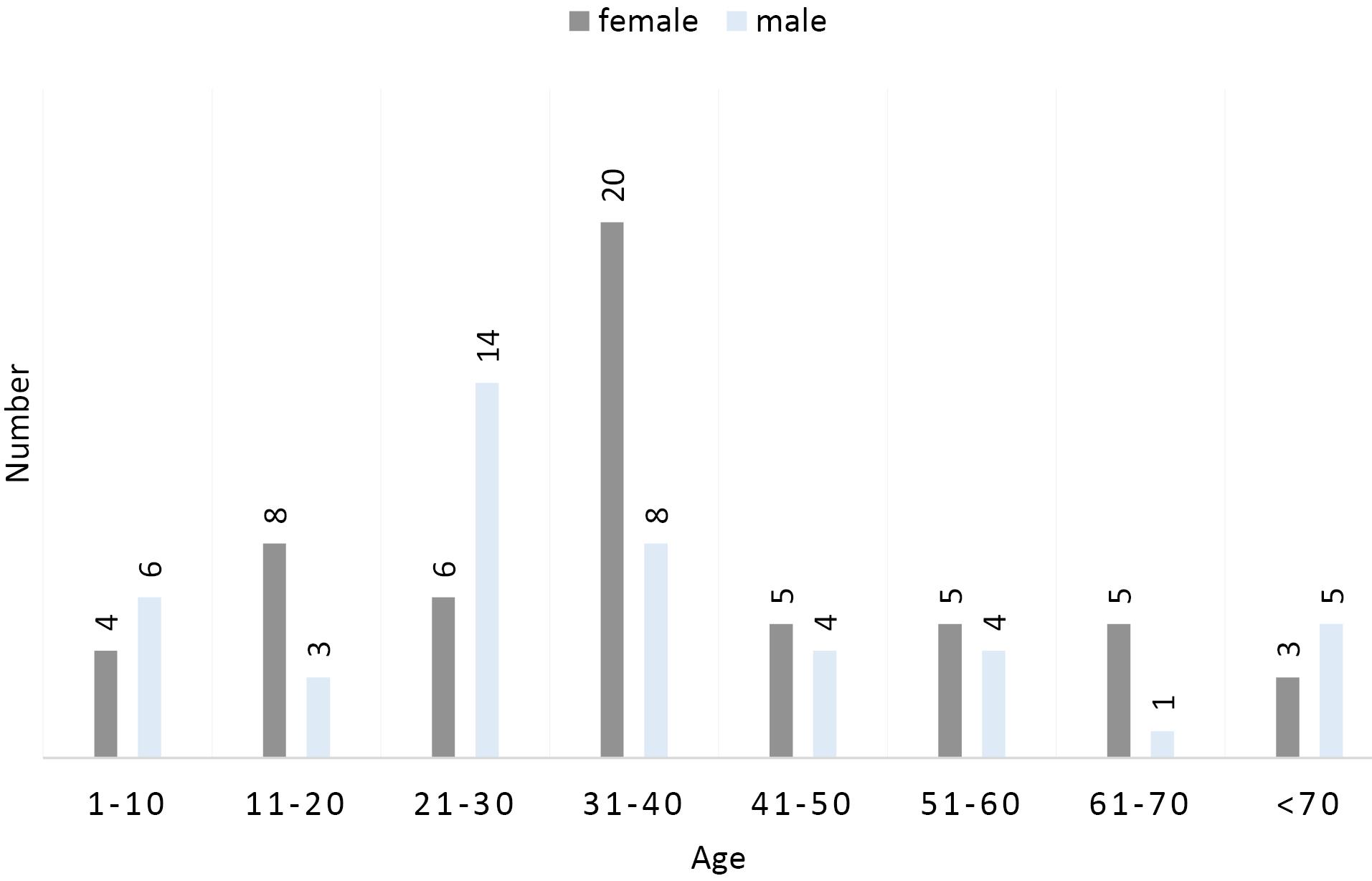
Figure 1.
Frequency Distribution of Uropathogenic Escherichia coli Isolates According to Age and Gender
.
Frequency Distribution of Uropathogenic Escherichia coli Isolates According to Age and Gender
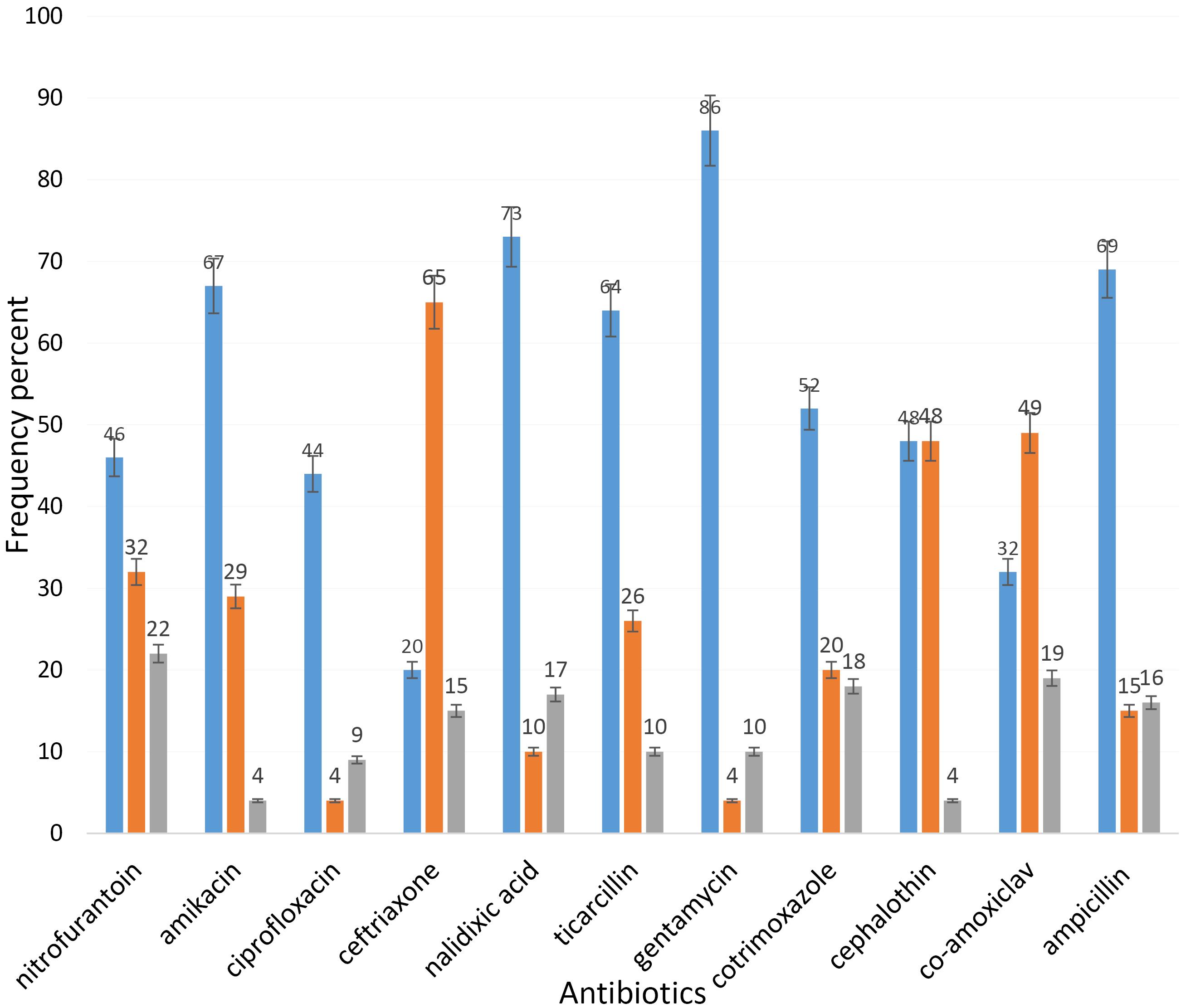
Figure 2.
Antibiotic Resistance Pattern of Uropathogenic Escherichia coli Isolates
.
Antibiotic Resistance Pattern of Uropathogenic Escherichia coli Isolates
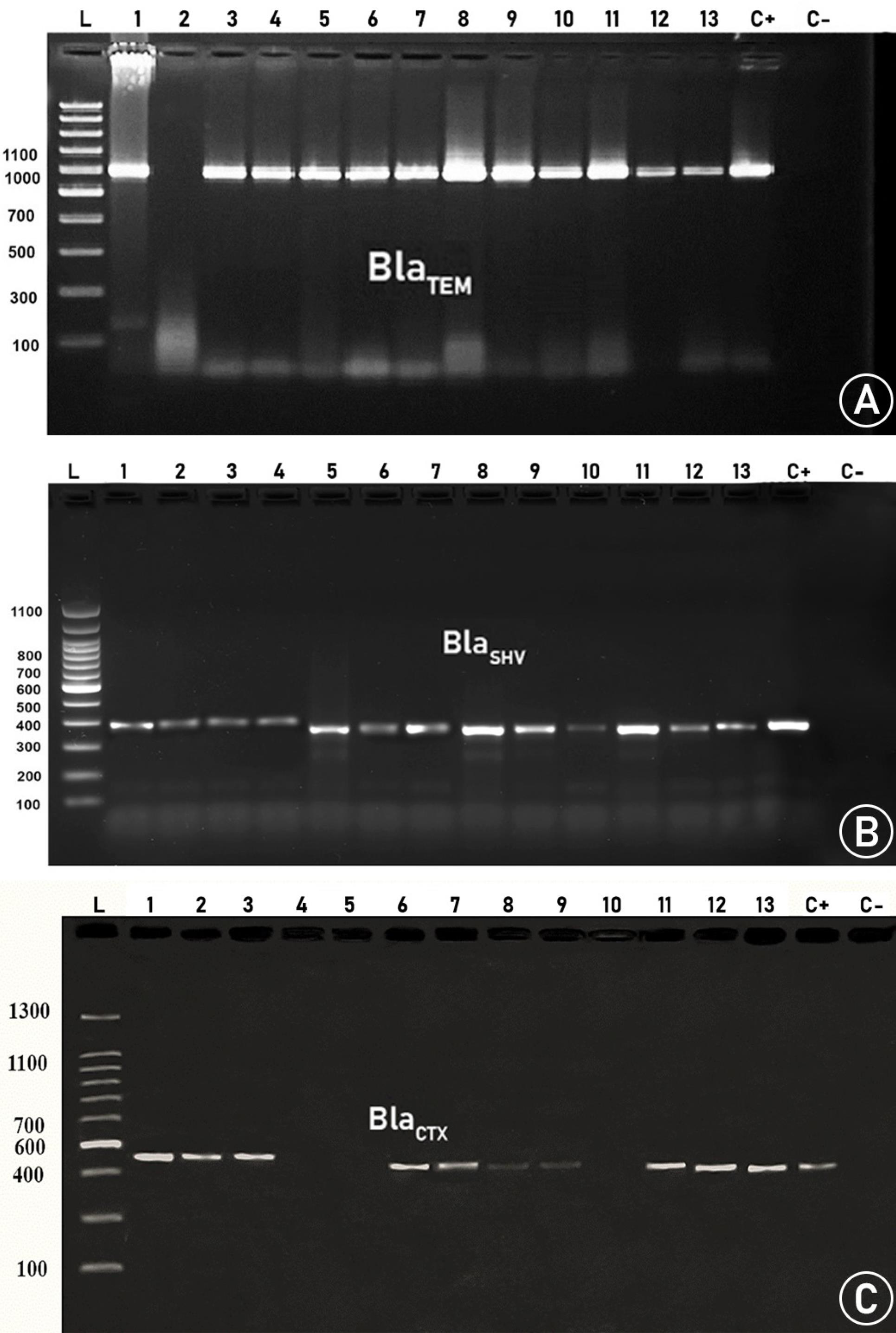
Figure 3.
Agarose Gel Electrophoresis of PCR Amplified β-Lactamases Genes. Note. PCR: Polymerase chain reaction. L: Ladder. (A) blaTEM gene appears at 1080 bp, (B) blaHSV gene appears at 392bp, and (C) blaCTX gene appears at 550 bp.
.
Agarose Gel Electrophoresis of PCR Amplified β-Lactamases Genes. Note. PCR: Polymerase chain reaction. L: Ladder. (A) blaTEM gene appears at 1080 bp, (B) blaHSV gene appears at 392bp, and (C) blaCTX gene appears at 550 bp.
Mint Nanoemulsion Characterization Results
According to the SEM image (Figure 4), the nanoemulsion was formed, and the size distribution was highly suitable; it was formed in the range below 500 nm and was mostly spherical with an average size of 70 nm. The SEM image also displayed the appropriate distribution of nanostructures. Figure 5 depicts the results of FTIR to describe functional groups in nanofibers to ensure the presence of NPs in the structure of the nanocapsule. The information obtained from DLS analysis of the mint nanoemulsion (Figure 6) demonstrates that Di 90%: 786.29 nm. It is predicted that this is the size of the mother nanoemulsions that contain smaller nanoemulsions. The particle size diagram shows the accumulation of particles in some places, which is due to the active surface of the NPs and their adhesion. It can be indicated that usually DLS analysis due to diffraction with analytical light has errors compared to microscopic imaging.

Figure 4.
Scanning Electron Microscopy Image of Mint Nanoemulsion
.
Scanning Electron Microscopy Image of Mint Nanoemulsion
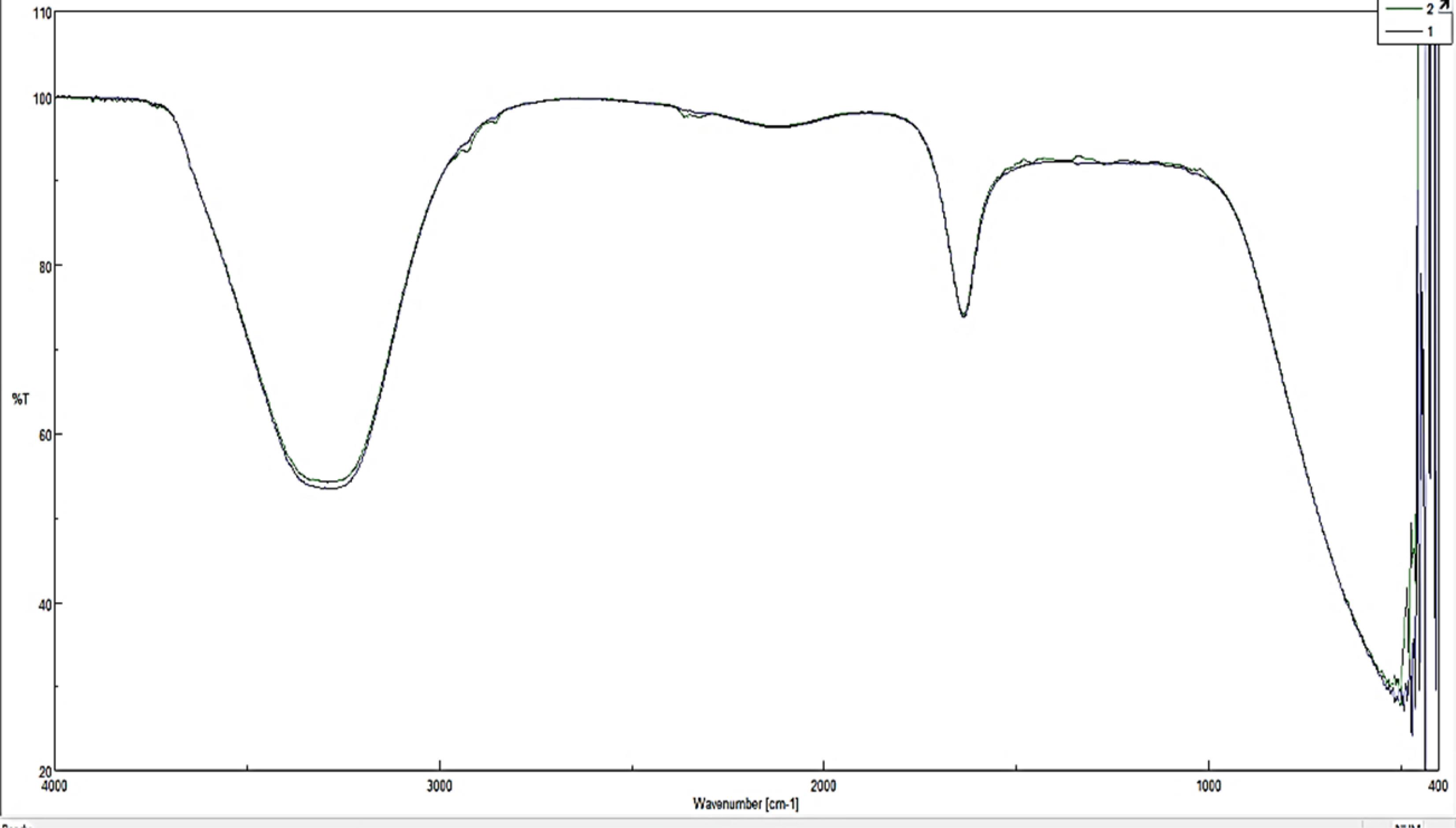
Figure 5.
Fourier-Transform Infrared Spectroscopy Spectrum Obtained From the Extract Containing Synthesized Mint Nanoemulsion
.
Fourier-Transform Infrared Spectroscopy Spectrum Obtained From the Extract Containing Synthesized Mint Nanoemulsion
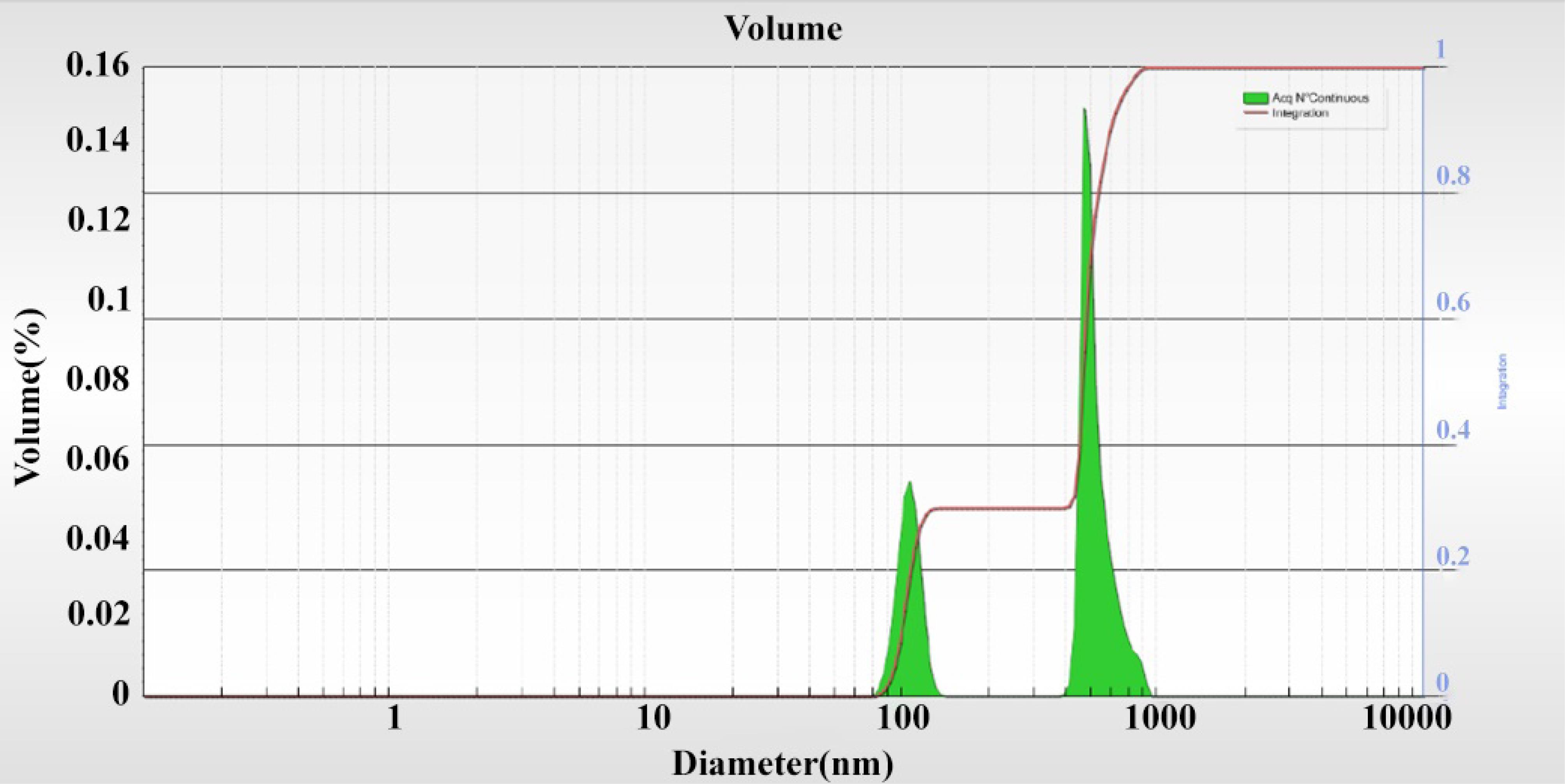
Figure 6.
Dynamic Light Scattering Diagram of the Mint Nanoemulsion
.
Dynamic Light Scattering Diagram of the Mint Nanoemulsion
Antibacterial Activity of Mint Nanoemulsion
The antibacterial activity of mint nanoemulsion was performed on 4 (42, 85, 95, and 96) UPEC isolates that had the highest resistance to the used antibiotics. The results of the antibacterial effects of these isolates are presented in Table 2.
Table 2.
Minimum Inhibitory Concentration of Resistant UPEC Bacteria Treated With Different Mint Nanoemulsion Concentrations
|
Nano Mint (mg/µL)
|
0 (Control)
|
0.03
|
0.05
|
0.07
|
0.1
|
0.2
|
0.3
|
| Isolate 42 |
0.560 |
0.540 |
0.462 |
0.405 |
0.245 |
0.199 |
0.181 |
| Percentage reduction of bacteria after 24 hours |
- |
3.57 |
17.50 |
27.68 |
56.25 |
64.46 |
67.68 |
| Isolate 85 |
0.370 |
0.262 |
0.253 |
0.321 |
0.202 |
0.181 |
0.174 |
| Percentage reduction of bacteria after 24 hours |
- |
29.18 |
31.62 |
13.25 |
45.41 |
51.09 |
52.98 |
| Isolate 95 |
0.480 |
0.387 |
0.301 |
0.286 |
0.255 |
0.094 |
0.054 |
| Percentage reduction of bacteria after 24 hours |
- |
19.38 |
37.29 |
40.42 |
46.87 |
80.42 |
88.75 |
| Isolate 96 |
0.430 |
0.401 |
0.398 |
0.356 |
0.333 |
0.103 |
0.094 |
| Percentage reduction of bacteria after 24 hours |
- |
6.74 |
7.44 |
7.40 |
22.41 |
76.05 |
78.13 |
Note. UPEC: Uropathogenic Escherichia coli.
Discussion
The traditional antibacterial method involves the use of antibiotics, but many clinically significant bacterial infections are increasingly becoming resistant to antibiotics (25). For thousands of years, natural products have been used to treat various bacterial infections all over the world (26). Historically, the major contribution to pharmacotherapy and medicine arrived from different medicinal plant products and their structural analogs (27). Nanoemulsions of plant origin have garnered attention for their potential as potent antibacterial agents, attributed to their small droplet size, which enhances the stability, surface area, and bioavailability of active compounds in plant extracts (28). In this study, the highest incidence of the UTI was observed in the age group of 31–40 years, while the lowest prevalence was related to the age range of 61–70 years. Furthermore, most infections were reported among females. The findings are consistent with those of other similar studies, indicating that women are more susceptible to UTIs than men, and this could be due to the shorter urethra in women compared to men. Moreover, women’s tendency to get UTIs can also depend on their behavioral patterns. Nitrofurantoin is one of a limited number of preferred antimicrobials, prescribed for the treatment of UTIs in adults, and as such should be closely monitored (29). In our investigation, the highest resistance was observed against nitrofurantoin, while the lowest resistance was found against amikacin. This is a crucial caution that must be taken into account. Similar to some studies (18,19), among the UPEC isolates, blaTEM, BlaHSV, and BlaCTX genesshowed a high prevalence. Mint nanoemulsion demonstrated significant antibacterial effects on four isolates with the highest antibiotic resistance. In line with the current research, the results indicated that the activity of cinnamaldehyde nanoemulsion at concentrations of 1,000 and 3,000 mg/L surpassed that of the control group (30). Furthermore, Almadiy et al, comparing the antibacterial effects of essential oils and nanoemulsions from Achillea species against various bacteria, reported a significant increase in the activity of essential oils and fractions, with inhibition zones reaching 34.5 mm and minimum inhibitory concentration and minimum bactericidal concentration values of 15.0 μg/mL observed with Achillea biebersteinii nanoemulsions against S. aureus. The study suggests utilizing these plant oils as antimicrobial biorationals, particularly at the nanoscale, following the necessary toxicological assessments (31). In a separate study, the antibacterial efficacy of the essential oil against E. coli was significantly enhanced upon conversion into a nanoemulsion. This enhancement was attributed to the improved accessibility of the essential oils to bacterial cells, leading to the amplification of their antibacterial activity against E. coli by enhancing their ability to disrupt cell membrane integrity (32). The observations align with our findings, demonstrating that nanoemulsions have the capability to eradicate resistant bacteria and exhibit greater efficacy compared to pure essential oils.
Conclusion
The effectiveness of mint nanoemulsion against MDR UPEC was confirmed, showing promise in combating infections that are resistant to traditional antibiotics. The use of plant nanoemulsions as antibacterial agents presents a novel and potentially more effective strategy in antimicrobial research, offering a promising approach to addressing bacterial infections while reducing the risk of resistance development. In summary, nanoemulsions serve as effective delivery systems for essential oils, enhancing antimicrobial application and efficacy in combating bacterial infections.
Acknowledgments
This study is the result of the Master’s thesis submitted to Islamic Azad University, Science and Research Branch, Tehran, Iran (Researcher ID: 162797873 and thesis code: 1237522607982396932162797873).
Authors’ Contribution
Conceptualization: Hala Maitham Saker Alali.
Data curation: Hala Maitham Saker Alali.
Investigation: Hala Maitham Saker Alali.
Supervision: Ashraf Kariminik.
Validation: Ashraf Kariminik.
Writing–original draft: Ashraf Kariminik.
Writing–review & editing: Maryam Ghane.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research was supported by the Science and Research Branch and Kerman Branch, Islamic Azad University, Iran.
References
- Huemer M, Mairpady Shambat S, Brugger SD, Zinkernagel AS. Antibiotic resistance and persistence-implications for human health and treatment perspectives. EMBO Rep 2020; 21(12):e51034. doi: 10.15252/embr.202051034 [Crossref] [ Google Scholar]
- Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed 2015; 5(7):509-14. doi: 10.1016/j.apjtb.2015.05.001 [Crossref] [ Google Scholar]
- Zhou Y, Zhou Z, Zheng L, Gong Z, Li Y, Jin Y. Urinary tract infections caused by uropathogenic Escherichia coli: mechanisms of infection and treatment options. Int J Mol Sci 2023; 24(13):10537. doi: 10.3390/ijms241310537 [Crossref] [ Google Scholar]
- Mann R, Mediati DG, Duggin IG, Harry EJ, Bottomley AL. Metabolic adaptations of uropathogenic E coli in the urinary tract. Front Cell Infect Microbiol 2017; 7:241. doi: 10.3389/fcimb.2017.00241 [Crossref] [ Google Scholar]
- Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob Agents Chemother 2018; 62(2):e01882-17. doi: 10.1128/aac.01882-17 [Crossref] [ Google Scholar]
- Ubah CS, Pokhrel LR, Williams JE, Akula SM, Richards SL, Kearney GD. Antibacterial efficacy, mode of action, and safety of a novel nano-antibiotic against antibiotic-resistant Escherichia coli strains. Sci Total Environ 2024; 925:171675. doi: 10.1016/j.scitotenv.2024.171675 [Crossref] [ Google Scholar]
- Parmanik A, Das S, Kar B, Bose A, Dwivedi GR, Pandey MM. Current treatment strategies against multidrug-resistant bacteria: a review. Curr Microbiol 2022; 79(12):388. doi: 10.1007/s00284-022-03061-7 [Crossref] [ Google Scholar]
- Abbas F, Maqbool Q, Nazar M, Jabeen N, Hussain SZ, Anwaar S. Green synthesised zinc oxide nanostructures through Periplocaaphylla extract shows tremendous antibacterial potential against multidrug resistant pathogens. IET Nanobiotechnol 2017; 11(8):935-41. doi: 10.1049/iet-nbt.2016.0238 [Crossref] [ Google Scholar]
- Habeeb Rahuman HB, Dhandapani R, Narayanan S, Palanivel V, Paramasivam R, Subbarayalu R. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol 2022; 16(4):115-44. doi: 10.1049/nbt2.12078 [Crossref] [ Google Scholar]
- Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2017; 2(1):18. doi: 10.1007/s41204-017-0029-4 [Crossref] [ Google Scholar]
- Sheth T, Seshadri S, Prileszky T, Helgeson ME. Multiple nanoemulsions. Nat Rev Mater 2020; 5(3):214-28. doi: 10.1038/s41578-019-0161-9 [Crossref] [ Google Scholar]
- Soni H, Sharma S. Current update on nanoemulsion: a review. Sch Int J Anat Physiol 2021; 4(1):6-13. doi: 10.36348/sijap.2021.v04i01.002 [Crossref] [ Google Scholar]
- Ghosh S, Nandi S, Basu T. Nano-antibacterials using medicinal plant components: an overview. Front Microbiol 2021; 12:768739. doi: 10.3389/fmicb.2021.768739 [Crossref] [ Google Scholar]
- Mohkami Z, Ranjbar A, Bidarnamani F. Essential oil compositions and antibacterial properties of mint (Mentha longifolia L) and rosemary (Rosmarinus officinalis). Annu Res Rev Biol 2014; 4(17):2675-83. doi: 10.9734/arrb/2014/7899 [Crossref] [ Google Scholar]
- Mandal D, Dash SK, Das B, Sengupta M, Kundu PK, Roy S. Isolation and characterization of multi-drug resistance proteus vulgaris from clinical samples of UTI infected patients from Midnapore, West Bengal. Int J Life Sci Pharm Res 2015; 5(2):32-45. [ Google Scholar]
- Fernández-Espigares L, Hernández-Chico I, Expósito-Ruiz M, Rosales-Castillo A, Navarro-Marí JM, Gutiérrez-Fernández J. Antibiotic resistance changes in gram-positive bacteria from urine cultures: development analysis in a health area of south-east Spain. Antibiotics (Basel) 2023; 12(7):1133. doi: 10.3390/antibiotics12071133 [Crossref] [ Google Scholar]
- Azar N, Khodor M, Choucair J, Hamze M, Pirenne H. Susceptibility of urinary Enterobacteriaceae to selected antimicrobials in an out-patient setting in Wallonia-Belgium: retrospective analysis over 5 years period (2018-2022). Ann Biol Clin (Paris) 2023; 81(1):52-60. doi: 10.1684/abc.2023.1780 [Crossref] [ Google Scholar]
- Aleisa AM, Ashgan MH, Alnasserallah AA, Mahmoud MH, Moussa IM. Molecular detection of β-lactamases and aminoglycoside resistance genes among Escherichia coli isolates recovered from medicinal plant. Afr J Microbiol Res 2013; 7(20):2305-10. [ Google Scholar]
- Polse RF, Yousif SY, Assafi MS. Prevalence and molecular characterization of extended spectrum beta-lactamases-producing uropathogenic Escherichia coli isolated in Zakho, Iraq. J Microbiol Infect Dis 2016; 6(4):163-7. doi: 10.5799/jmid.328863 [Crossref] [ Google Scholar]
- Zongo KJ, Dabire AM, Compaore LG, Sanou I, Sangare L, Simpore J. First detection of bla TEM, SHV and CTX-M among gram-negative bacilli exhibiting extended spectrum β-lactamase phenotype isolated at University Hospital Center, Yalgado Ouedraogo, Ouagadougou, Burkina Faso. Afr J Biotechnol 2015; 14(14):1174-80. doi: 10.5897/ajb2014.13908 [Crossref] [ Google Scholar]
- Komaiko J, McClements DJ. Low-energy formation of edible nanoemulsions by spontaneous emulsification: factors influencing particle size. J Food Eng 2015; 146:122-8. doi: 10.1016/j.jfoodeng.2014.09.003 [Crossref] [ Google Scholar]
- Moghaddasi F, Housaindokht MR, Darroudi M, Bozorgmehr MR, Sadeghi A. Synthesis of nano curcumin using black pepper oil by O/W Nanoemulsion Technique and investigation of their biological activities. LWT 2018; 92:92-100. doi: 10.1016/j.lwt.2018.02.023 [Crossref] [ Google Scholar]
- Kumari S, Kumaraswamy RV, Choudhary RC, Sharma SS, Pal A, Raliya R. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci Rep 2018; 8(1):6650. doi: 10.1038/s41598-018-24871-5 [Crossref] [ Google Scholar]
- Liu Q, Gao Y, Fu X, Chen W, Yang J, Chen Z. Preparation of peppermint oil nanoemulsions: Investigation of stability, antibacterial mechanism and apoptosis effects. Colloids Surf B Biointerfaces 2021; 201:111626. doi: 10.1016/j.colsurfb.2021.111626 [Crossref] [ Google Scholar]
- Etebu E, Arikekpar I. Antibiotics: classification and mechanisms of action with emphasis on molecular perspectives. Int J Appl Microbiol Biotechnol Res 2016; 4:90-101. [ Google Scholar]
- Qadri H, Shah AH, Ahmad SM, Alshehri B, Almilaibary A, Mir MA. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J Biol Sci 2022; 29(9):103376. doi: 10.1016/j.sjbs.2022.103376 [Crossref] [ Google Scholar]
- Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med 2011; 2011:680354. doi: 10.1093/ecam/nep067 [Crossref] [ Google Scholar]
- Behera A, Mittu B, Padhi S, Singh A. Antimicrobial efficacy of essential oil nanoemulsions. In: Nanotechnological Approaches in Food Microbiology. CRC Press; 2020. p. 293-309.
- Guy RL, Rudman J, Higgins H, Carter E, Henderson KL, Demirjian A. Nitrofurantoin resistance as an indicator for multidrug resistance: an assessment of Escherichia coli urinary tract specimens in England, 2015-19. JAC Antimicrob Resist 2023; 5(6):dlad122. doi: 10.1093/jacamr/dlad122 [Crossref] [ Google Scholar]
- Abdelrasoul MA, Eid AR, Badawy MEI. Preparation, characterizations and antibacterial activity of different nanoemulsions incorporating monoterpenes: in vitro and in vivo studies. Arch Phytopathol Plant Prot 2020; 53(7-8):310-34. doi: 10.1080/03235408.2020.1744977 [Crossref] [ Google Scholar]
- Almadiy AA, Nenaah GE, Al Assiuty BA, Moussa EA, Mira NM. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT Food Sci Technol 2016; 69:529-37. doi: 10.1016/j.lwt.2016.02.009 [Crossref] [ Google Scholar]
- Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E coli. Food Chem 2016; 194:410-5. doi: 10.1016/j.foodchem.2015.07.139 [Crossref] [ Google Scholar]