Avicenna Journal of Clinical Microbiology and Infection. 11(1):17-21.
doi: 10.34172/ajcmi.3504
Original Article
Molecular Detection of Cylindrospermopsin-Producing Cyanobacteria in the Coasts of Hormozgan Province, Iran
Elmira Eivazpour Nami 1  , Mohammad Hassan Shah Hoseini 2, 3, *
, Mohammad Hassan Shah Hoseini 2, 3, *  , Alireza Dehnad 1, 4
, Alireza Dehnad 1, 4
Author information:
1Rob’ Rashid Higher Education Institution, Tabriz, Iran
2Iran Gene Fanavar (IGF) Higher Education Institution, Tehran, Iran
3Microbiology group, Islamic Azad University of Shahr-e-Qods, Tehran, Iran
4Department of Biotechnology of Agriculture and Natural Resources Research and Education Center of East Azerbaijan, Agricultural Research and Education Organization, Tabriz, Iran
Abstract
Background: In recent years, water and environmental pollution by cyanobacteria have been increasingly reported as a serious hazard to human health in the world. Cylindrospermopsin is one of the most important algal toxins produced by cyanobacteria that can cause damage to the liver, kidneys, thymus, and heart. The aim of this study was to detect cylindrospermopsin-producing cyanobacteria accurately and fast in the water resources of the Hormozgan province coast by molecular method.
Methods: We collected 20 water samples (2 L) from 20 sampling stations on the coasts of Hormozgan province in October 2017. The genomic DNA was extracted from water samples. Finally, molecular detection was conducted by the polymerase chain reaction (PCR) method.
Results: The optimized PCR successfully detected 20 cyanobacteria strains from all 20 samples. In addition, the presence of cylindrospermopsin-producing cyanobacteria was confirmed in 2 (10%) stations.
Conclusion: Generally, our study confirmed the risk of the presence of toxigenic cyanobacteria on the coasts of Hormozgan province and demonstrated that PCR assay is an accurate and fast method for early detection of cylindrospermopsin-producing cyanobacteria in water resources.
Keywords: Cyanobacteria, Cylindrospermopsin, Molecular detection, Province coasts
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Eivazpour Nami E, Shah Hoseini MH, Dehnad A. Molecular detection of cylindrospermopsin-producing cyanobacteria in the coasts of Hormozgan Province, Iran. Avicenna J Clin Microbiol Infect. 2024; 11(1):17-21. doi:10.34172/ajcmi.3504
Introduction
Cyanobacteria are photosynthetic prokaryotic blue-green algae that are present in warm, fresh, and eutrophic waters. The presence of cyanobacteria in local waters causes problems on a global scale by producing dangerous toxins called cyanotoxins (1). In addition, many of these species of cyanobacteria cause significant problems in rivers, freshwater lakes, oceans, and stored and drinking water (2). Therefore, in order to protect water consumers from exposure to cyanobacteria toxins, it is necessary to quantitatively and qualitatively investigate water sources for the presence of these dangerous toxins and to prevent possible risks caused by their consumption.
Two toxin types, cylindrospermopsin and microcystins, are the main causes of cyanobacterial poisoning (3). Both of these toxins can be produced by various cyanobacterial strains in the world (4); consequently, monitoring of cyanotoxins in water bodies has been increasing in recent years. Cylindrospermopsin was first detected in 1979 when 148 patients were hospitalized at the Australian Hospital in Palm Island with liver inflammation symptoms. The disease was associated with the presence of Cylindrospermopsin raciborskii in the source of drinking water (5). Cylindrospermopsin is an alkaloid toxin produced by a variety of cyanobacteria and uracil and hydroxyl-carbon-7 group play an important role in its toxicity (6). Cylindrospermopsin is also biologically active and inhibits several metabolic pathways, including the synthesis of glutathione and cytochrome P450 (7,8).
Since phenotypical and morphological investigations of cyanobacteria cannot be proper and accurate ways to detect toxigenic strains, methods with higher efficiency should be used. With the development of molecular genetics methods in recent years, molecular methods can be alternatively used to detect toxigenic cyanobacteria. Particularly, conventional polymerase chain reaction (PCR) methods for detection of toxigenic cyanobacterial strains have been confirmed as fast and inexpensive methods (9,10).
Persian Gulf region and Hormozgan province coasts in the Middle East have been considered the most important channels of communication between continents, rich resources of oil and gas, and strategic locations. On the other hand, this area is important for the diversity of plants and animals (11). In addition, due to climatic conditions and the presence of effective factors, the coasts of Hormozgan province are susceptible to phenomena such as the accumulation of cylindrospermopsin-producing cyanobacteria (12).
Therefore, the aim of the present study was to investigate the efficiency of molecular PCR assay in detection of cyanobacteria and cylindrospermopsin-producing cyanobacteria in the water resources of Hormozgan province.
Materials and Methods
Sample Collection
In the present study, 20 water samples were collected from 20 stations on the coast of Hormozgan province in October 2017. For this purpose, we collected 2-L water samples in dark glass bottles from 100 m away from the coast. The sampling was performed in triplicate, and the obtained samples were transferred to the laboratory immediately in cold conditions within 3 hours. The coordinates of the sampling stations are presented in Figure 1. The water samples were centrifuged at 8000 rpm for 12 minutes, and the residual sediment was resuspended with 500 µL of deionized water.
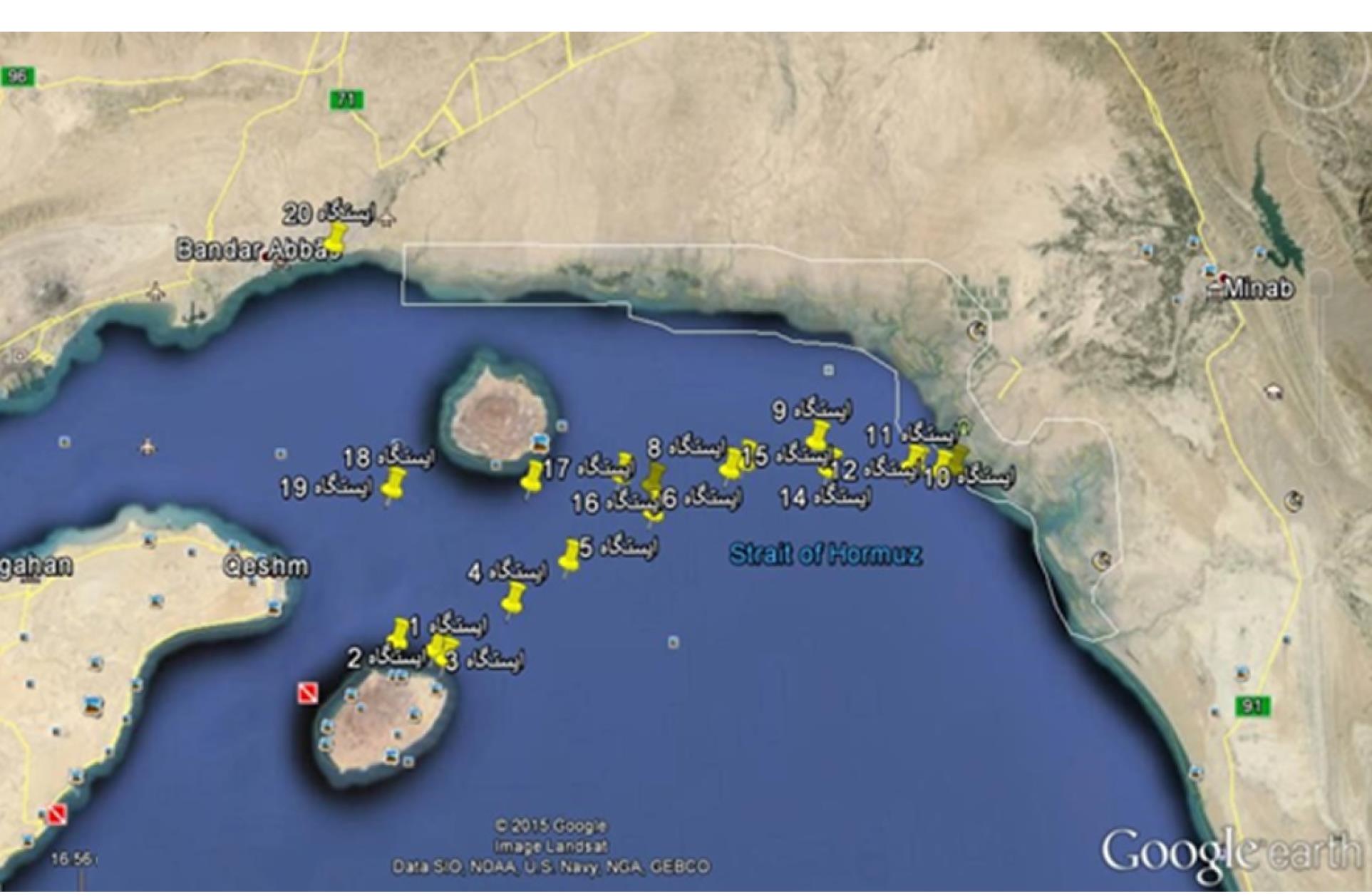
Figure 1.
The Coordinates of the Sampling Stations on the Coasts of Hormozgan Province
.
The Coordinates of the Sampling Stations on the Coasts of Hormozgan Province
Genomic DNA Extraction
The genomic DNA was extracted from the samples by a DNG-Plus extraction kit according to the manufacturer’s instructions. The quantity and quality of extracted DNA samples were evaluated using a NanoDrop spectrophotometer and electrophoresis on 1% agarose gel, respectively. The obtained DNA samples were stored at -20°C until molecular detection.
PCR Amplification
Molecular detection of cyanobacteria and Cylindrospermopsin was performed by PCR method. For this purpose, PCR amplification was optimized by different concentrations of genomic DNA of Microcystis aeruginosa PCC 7806 strain. Universal primers were used for the detection of cyanobacteria, and specific primers were used for the detection of Cylindrospermopsin (Table 1). PCR amplification was performed in a total volume of 25 μL containing 0.5 μL of each primer (25 pmol), 1 μg of template DNA, 1.5 mmol/L of Mgcl2, 0.1 mmol of dNTP, 1.5 units of Taq DNA polymerase, and 12.5 μL of PCR buffer. The following thermal condition was used for the reaction: initial denaturation (1 cycle at 94 °C for 4 minutes), denaturation (40 cycles at 94 °C for 40 seconds), annealing (40 cycles at 50 °C for 30 seconds), extension (40 cycles at 72 °C for 25 seconds), and final extension (1 cycle at 72 °C for 5 minutes). The obtained PCR products were electrophoresed on 1.5% agarose gel, and the bands were visualized by a gel documentation system.
Table 1.
Primers Used to Detect Cyanobacteria and Cylindrospermopsin
|
Target Gene
|
Sequences Of Primers
|
Product Size
|
References
|
CYA359F
CYA781R |
5΄-GGGGAATYYYCCGCAATGGG-3΄
5΄-GACTACWGGGGTATCTAATCCCWTT-3΄ |
487 bp |
13 |
CynsulfF
CylnamR |
5΄-ACTTCTCTCCTTTCCCTATC-3΄
5΄-GAGTGAAAATGCGTAGAACTTG-3΄ |
578 bp |
14 |
Results
Specificity of the PCR Test
In order to investigate the specificity of PCR test for the detection of cyanobacteria, DNA samples of Staphylococcus aureus, Hepatitis B virus, Fusarium solani, and Saccharomyces cerevisiae were used, and an optimized PCR test was evaluated with positive and negative control DNA samples. We also used DNA samples of Microcystis aeruginosa, Nostoc, Anabaena, and Fischerella to optimize detection of cylindrospermopsin. The obtained results indicated that PCR assay was able to detect cyanobacteria and cylindrospermopsin-producing cyanobacteria, whereas DNA samples of the other microorganisms were not detectable with the used primers. In addition, the limit of detection (LOD) of PCR was 100 copies per reaction for both cyanobacteria and cylindrospermopsin.
Frequency of Cyanobacteria
The frequency of cyanobacteria was investigated by universal primers in 20 water samples from different stations of Hormozgan province coasts. We detected cyanobacteria in all water samples (Figure 2). Moreover, the presence of cylindrospermopsin-producing cyanobacteria was observed in the samples of 2 stations (Figure 3).
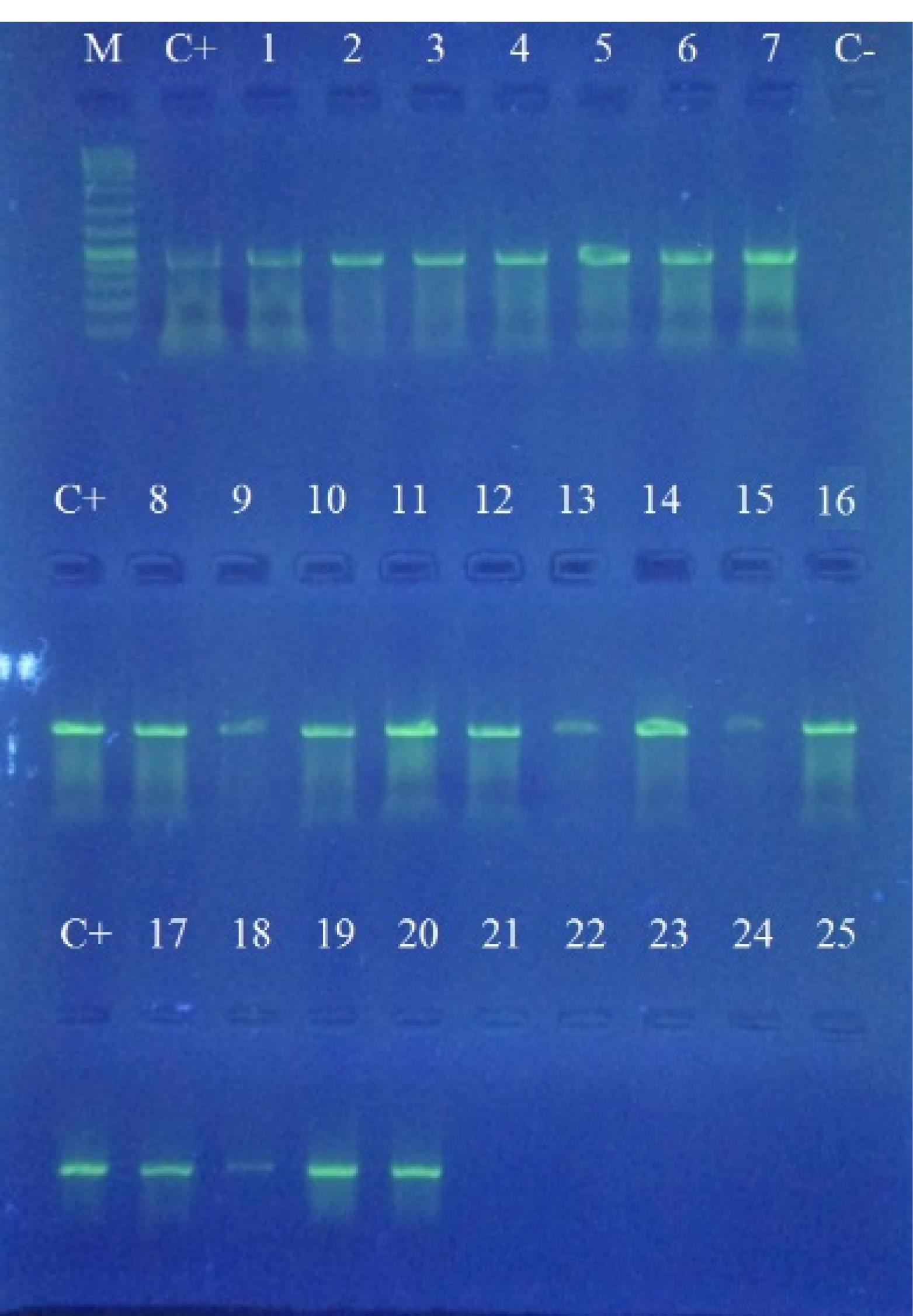
Figure 2.
PCR Assay for Detection of Cyanobacteria with a Product Size of 487 bp. (M) 1 kb DNA Ladder; (C + ) Positive Control of Cylindrospermopsin Raciborskii AWT205 Sample; (C-) Negative Control; (1-25) Positive Cyanobacteria Samples
.
PCR Assay for Detection of Cyanobacteria with a Product Size of 487 bp. (M) 1 kb DNA Ladder; (C + ) Positive Control of Cylindrospermopsin Raciborskii AWT205 Sample; (C-) Negative Control; (1-25) Positive Cyanobacteria Samples

Figure 3.
PCR Assay for Detection of cylindrospermopsin with a Product Size of 578 bp. (M) 1 kb DNA Ladder; (C + ) Positive Control of Cylindrospermopsin Raciborskii AWT205 Sample; (C-) (1-25) Cyanobacteria Samples; (1-4 and 7-25) Negative Sample; (5 and 6) Positive Sample
.
PCR Assay for Detection of cylindrospermopsin with a Product Size of 578 bp. (M) 1 kb DNA Ladder; (C + ) Positive Control of Cylindrospermopsin Raciborskii AWT205 Sample; (C-) (1-25) Cyanobacteria Samples; (1-4 and 7-25) Negative Sample; (5 and 6) Positive Sample
Discussion
The emergence of cyanobacteria is observed in tropical and subtropical areas, and usually in freshwater, brackish water, seawater, and eutrophic waters. On the other hand, some cyanobacteria species produce dangerous toxins causing a variety of diseases and even death (15). Considering that the Persian Gulf region and Hormozgan coasts are susceptible to such phenomenon, due to climatic conditions and the availability of effective factors, one of the concerns is the accumulation of cylindrospermopsin-producing cyanobacteria in the water resources of this region. Cylindrospermopsin can accumulate in tissues of aquatic animals and other organisms and play an important role in the prevalence of human poisoning and livestock losses (16). Since the morphological and microscopic methods cannot be suitable and accurate methods for the detection of toxigenic strains, other efficient methods should be developed. In this case, it is possible to develop high-performance analytical methods such as enzyme-linked immunosorbent assay (ELISA), protein phosphatase inhibition assay, high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and solid phase adsorption toxin tracking (17,18).
In a previous study by Rasmussen et al, the real-time PCR method was used to detect the specific gene of Cylindrospermopsin raciborskii. They reported that real-time PCR is a rapid and sensitive test; however, because of the costly nature of this method, which requires the purchase of expensive instruments, it is commonly used in reference laboratories (19). In another study, Manali et al identified the presence of specific genes of cyanobacteria that encode cylindrospermopsin and microcystins, using multiplex PCR. They suggested that multiplex PCR provides low sensitivity, due to the competitive state of genes in this method (20). In another similar study, Gaget et al identified the presence of cylindrospermopsin and microcystins in Australian waters by ELISA, chromatography, real-time PCR, PCR, and LC-MS methods. They reported that PCR-based methods provide high sensitivity in the detection of cylindrospermopsin and microcystins (21). Liyanage et al detected cylindrospermopsin by amplification of 16S rRNA gene and reported that 16S rRNA gene is a suitable target for the detection of cylindrospermopsin (22). In addition, Barón-Sola et al identified the presence of cylindrospermopsin-producing cyanobacteria using the real-time PCR method by amplification of 6 target genes and reported that nTCA gene is a suitable target for detection of cylindrospermopsin (23). In this regard, we also found that PCR is a precise, fast, and highly sensitive method for the detection of cylindrospermopsin-producing cyanobacteria in water sources.
In this study, we detected 20 cyanobacterial isolates from the coasts of Hormozgan province by molecular method, 2 of which were cylindrospermopsin-producing cyanobacteria. A high level of cylindrospermopsin production can be observed in various regions of the world, but there is limited information on aquatic toxicity (24). This toxic cyanobacterium was a minor component of the mixed algal assemblage on coasts. However, cylindrospermopsin-producing Kamptonema may dominate the community composition in some areas (24). Previous studies have indicated that abiotic factors influence the accumulation and release of cylindrospermopsin (25). Lower concentrations of phosphorus and nitrogen have been related to a higher accumulation of cylindrospermopsins in cyanobacterial cells (26,27). Lower concentrations of nutrients in the coasts of Hormozgan could trigger an increase in the detection of toxins such as cylindrospermopsin, and there is a constant need to monitor such changes in this environment. According to the World Health Organization, safe levels of cylindrospermopsin for lifetime drinking water, short-term drinking water, and recreational exposure are 0.7 μg/L, 3 μg/L, and 6 μg/L. However, environments are not well surveyed for the production of cyanobacterial toxins, and the microbial diversity of cyanobacteria is poorly understood.
In general, our study confirmed the presence of cylindrospermopsin-producing cyanobacteria in 10% of the stations in Hormozgan province by PCR. Accordingly, the PCR technique is proposed as a precise, rapid, inexpensive, and sensitive method for the detection of cylindrospermopsin-producing cyanobacteria in water sources.
Acknowledgments
Hereby, the contributions of the Iran Gene Fanavar Institute and Mrs. Mahsa Malek Mohammadi in providing the scientific and laboratory facilities for this project are appreciated. The authors also appreciate Prof. Nilan who provided the strains required in this study and all those who collaborated in sample collection.
Authors’ Contribution
Conceptualization: Mohammad Hassan Shah Hoseini.
Data collection: Elmira Eivazpour Nami.
Formal analysis: Alireza Dehnad.
Funding acquisition: Elmira Eivazpour Nami.
Investigation: Elmira Eivazpour Nami.
Methodology: Mohammad Hassan Shah Hoseini.
Project administration: Mohammad Hassan Shah Hoseini.
Resources: Elmira Eivazpour Nami.
Software: Alireza Dehnad.
Supervision: Mohammad Hassan Shah Hoseini.
Validation: Elmira Eivazpour Nami, Mohammad Hassan Shah Hoseini, Alireza Dehnad.
Writing–original draft: Elmira Eivazpour Nami.
Competing Interests
The authors declare that there is no conflict of interests.
Ethical Approval
Not applicable.
References
- de Figueiredo DR, Azeiteiro UM, Esteves SM, Gonçalves FJ, Pereira MJ. Microcystin-producing blooms--a serious global public health issue. Ecotoxicol Environ Saf 2004; 59(2):151-63. doi: 10.1016/j.ecoenv.2004.04.006 [Crossref] [ Google Scholar]
- Cheung MY, Liang S, Lee J. Toxin-producing cyanobacteria in freshwater: a review of the problems, impact on drinking water safety, and efforts for protecting public health. J Microbiol 2013; 51(1):1-10. doi: 10.1007/s12275-013-2549-3 [Crossref] [ Google Scholar]
- Miner G. Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. J Am Water Works Assoc 2006; 98(6):138-9. [ Google Scholar]
- Cheung MY, Liang S, Lee J. Toxin-producing cyanobacteria in freshwater: a review of the problems, impact on drinking water safety, and efforts for protecting public health. J Microbiol 2013; 51(1):1-10. doi: 10.1007/s12275-013-2549-3 [Crossref] [ Google Scholar]
- Messineo V, Melchiorre S, Di Corcia A, Gallo P, Bruno M. Seasonal succession of Cylindrospermopsisraciborskii and Aphanizomenonovalisporum blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, Central Italy. Environ Toxicol 2010; 25(1):18-27. doi: 10.1002/tox.20469 [Crossref] [ Google Scholar]
- Mathe C, M-Hamvas M, Garda T, Beyer D, Vasas G. Cellular effects of cylindrospermopsin (cyanobacterial alkaloid toxin) and its potential medical consequences. Curr Med Chem 2017; 24(1):91-109. doi: 10.2174/0929867323666161028153814 [Crossref] [ Google Scholar]
- Adamski M, Chrapusta E, Bober B, Kamiński A, Białczyk J. Cylindrospermopsin: cyanobacterial secondary metabolite Biological aspects and potential risk for human health and life. Oceanol Hydrobiol Stud 2014; 43(4):442-9. doi: 10.2478/s13545-014-0148-5 [Crossref] [ Google Scholar]
- Adamski M, Kaminski A. Impact of cylindrospermopsin and its decomposition products on antioxidant properties of glutathione. Algal Res 2021; 56:102305. doi: 10.1016/j.algal.2021.102305 [Crossref] [ Google Scholar]
- Sidelev SI. A novel multiplex PCR-based technique for detection of toxigenic cyanobacteria. Microbiology 2019; 88(3):375-7. doi: 10.1134/s0026261719030123 [Crossref] [ Google Scholar]
- Moreira C, Ramos V, Azevedo J, Vasconcelos V. Methods to detect cyanobacteria and their toxins in the environment. Appl Microbiol Biotechnol 2014; 98(19):8073-82. doi: 10.1007/s00253-014-5951-9 [Crossref] [ Google Scholar]
- Bayani N. Ecology and environmental challenges of the Persian Gulf. Iran Stud 2016; 49(6):1047-63. doi: 10.1080/00210862.2016.1241569 [Crossref] [ Google Scholar]
- Zaheri A, Bahador N, Yousefzadi M, Arman M. Molecular identification and toxicity effects of cyanobacteria species isolated from the Khoor-e-Khooran mangrove forest, Persian Gulf. Iran J Fish Sci 2021; 20(2):572-89. doi: 10.22092/ijfs.2021.123905 [Crossref] [ Google Scholar]
- Massahi S, Emtyazjoo M, Shahhossieni H. Diagnosis of microcystin-producing cyanobacteria of Anzali Lagoon using an optimized PCR method. Pejouhandeh 2014;19(2):99-106. [Persian].
- Nikoei N, Bahador N. Study on destruction of extracted toxins from isolated cyanobacteria using titanium dioxide. Int J Mol Clin Microbiol 2017; 7(2):841-8. [ Google Scholar]
- Paerl HW, Otten TG. Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 2013; 65(4):995-1010. doi: 10.1007/s00248-012-0159-y [Crossref] [ Google Scholar]
- Mohamed ZA, Bakr A. Concentrations of cylindrospermopsin toxin in water and tilapia fish of tropical fishponds in Egypt, and assessing their potential risk to human health. Environ Sci Pollut Res Int 2018; 25(36):36287-97. doi: 10.1007/s11356-018-3581-y [Crossref] [ Google Scholar]
- Chinnappan R, Alzabn R, Fataftah AK, Alhoshani A, Zourob M. Probing high-affinity aptamer binding region and development of aptasensor platform for the detection of cylindrospermopsin. Anal Bioanal Chem 2020; 412(19):4691-701. doi: 10.1007/s00216-020-02723-4 [Crossref] [ Google Scholar]
- Guzmán-Guillén R, Prieto AI, González AG, Soria-Díaz ME, Cameán AM. Cylindrospermopsin determination in water by LC-MS/MS: optimization and validation of the method and application to real samples. Environ Toxicol Chem 2012; 31(10):2233-8. doi: 10.1002/etc.1954 [Crossref] [ Google Scholar]
- Rasmussen JP, Giglio S, Monis PT, Campbell RJ, Saint CP. Development and field testing of a real-time PCR assay for cylindrospermopsin-producing cyanobacteria. J Appl Microbiol 2008; 104(5):1503-15. doi: 10.1111/j.1365-2672.2007.03676.x [Crossref] [ Google Scholar]
- Manali KM, Arunraj R, Ramakrishnan GS, Ramya M. Development of sensitive and specific multiplex PCR method for the detection of microcystin producing cyanobacteria in spirulina food supplements. Food Sci Biotechnol 2019; 28(2):609-14. doi: 10.1007/s10068-018-0476-0 [Crossref] [ Google Scholar]
- Gaget V, Humpage AR, Huang Q, Monis P, Brookes JD. Benthic cyanobacteria: a source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Res 2017; 124:454-64. doi: 10.1016/j.watres.2017.07.073 [Crossref] [ Google Scholar]
- Liyanage HM, Magana Arachchi DN, Chandrasekaran NV. Genetic divergence among toxic and non-toxic cyanobacteria of the dry zone of Sri Lanka. Springerplus 2016; 5(1):2026. doi: 10.1186/s40064-016-3680-5 [Crossref] [ Google Scholar]
- Barón-Sola Á, del Campo FF, Sanz-Alférez S. Dynamics of cylindrospermopsin production and toxin gene expression in Aphanizomenonovalisporum. Adv Microbiol 2016; 6(5):381-90. doi: 10.4236/aim.2016.65037 [Crossref] [ Google Scholar]
- Scarlett KR, Kim S, Lovin LM, Chatterjee S, Scott JT, Brooks BW. Global scanning of cylindrospermopsin: critical review and analysis of aquatic occurrence, bioaccumulation, toxicity and health hazards. Sci Total Environ 2020; 738:139807. doi: 10.1016/j.scitotenv.2020.139807 [Crossref] [ Google Scholar]
- Bormans M, Lengronne M, Brient L, Duval C. Cylindrospermopsin accumulation and release by the benthic cyanobacterium Oscillatoria sp PCC 6506 under different light conditions and growth phases. Bull Environ Contam Toxicol 2014; 92(2):243-7. doi: 10.1007/s00128-013-1144-y [Crossref] [ Google Scholar]
- Bar-Yosef Y, Sukenik A, Hadas O, Viner-Mozzini Y, Kaplan A. Enslavement in the water body by toxic Aphanizomenonovalisporum, inducing alkaline phosphatase in phytoplanktons. Curr Biol 2010; 20(17):1557-61. doi: 10.1016/j.cub.2010.07.032 [Crossref] [ Google Scholar]
- Cirés S, Wörmer L, Timón J, Wiedner C, Quesada A. Cylindrospermopsin production and release by the potentially invasive cyanobacterium Aphanizomenonovalisporum under temperature and light gradients. Harmful Algae 2011; 10(6):668-75. doi: 10.1016/j.hal.2011.05.002 [Crossref] [ Google Scholar]