Avicenna Journal of Clinical Microbiology and Infection. 10(3):120-125.
doi: 10.34172/ajcmi.3496
Original Article
Antimicrobial Photodynamic Therapy Using Zinc Phthalocyanine Nanoemulsion Against Infected Wounds in Diabetic Rats
Sara Soleymani Sedeh 1  , Mahnoosh Fatemi 2, *
, Mahnoosh Fatemi 2, *  , Fereshte Ghandehari 1
, Fereshte Ghandehari 1 
Author information:
1Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
2Department of Biology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
Abstract
Background: Chronic diabetic infections commonly involve highly antibiotic-resistant pathogens, such as Staphylococcus aureus and streptococci. This study aimed to assess the impact of zinc (Zn) phthalocyanine incorporated into nanoemulsions on diabetic wound infections.
Methods: Thirty-six adult male rats were divided into control, diabetic wound, diabetic wound infected with S. aureus, and three diabetic wound infected with S. aureus groups, which were treated with laser, medication, and a combination of medication and laser therapy. After the treatment period, wound diameter was measured, and blood samples and wound tissue were collected to evaluate antioxidant factors.
Results: The results demonstrated a significant reduction in wound diameter in the group treated with both the drug and laser compared to the other groups. The activity of superoxide dismutase (SOD) and glutathione peroxidase (GPX), along with the concentration of glutathione (GSH) in the blood of all groups, exhibited a significant decrease in comparison to the control group. However, the activity of these factors in both blood and tissue showed a noteworthy increase in the rats treated with both the drug and laser, as opposed to diabetic rats infected with S. aureus.
Conclusion: Photodynamic therapy (PDT) for infectious wounds, employing nanoemulsions containing Zn phthalocyanine, appears to enhance the body’s antioxidant system by eradicating bacteria and, ultimately, expediting wound healing. This approach may be considered a potential candidate for treating antibiotic-resistant infections.
Keywords: Zinc phthalocyanine, Photodynamic therapy, Diabetic ulcers, Staphylococcus aureus, Nanoemulsion
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Soleymani Sedeh S, Fatemi M, Ghandehari F. Antimicrobial photodynamic therapy using zinc phthalocyanine nanoemulsion against infected wounds in diabetic rats. Avicenna J Clin Microbiol Infect. 2023; 10(3):120-125. doi:10.34172/ajcmi.3496
Introduction
Elevated blood glucose levels in diabetic patients have detrimental effects, including the reduction of growth factors and fibroblast proliferation, damage to the blood vessel endothelium, increased expression of inflammatory cytokines, and cellular matrix metalloproteinases. These factors ultimately result in apoptosis and tissue degradation (1). In diabetic patients, impaired tissue granulation, reduced collagen production, and compromised angiogenesis exacerbate infection complications and delay wound healing in the presence of infectious microbes (2). Most diabetic wound infections are polymicrobial, with Staphylococcus aureus being the most common causative organism. Pathogens such as S. aureus possess various antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and alkane hydroperoxide reductase, as well as small molecules such as coenzyme A, staphylococcal oxide, and nitrous oxide oxides, which protect them from oxidative damage (3).
Currently, one of the treatment methods gaining attention for such wounds is photodynamic therapy (PDT). PDT relies on inducing oxidative stress through light exposure and a photosensitive drug. In this approach, active oxygen mediators and free radicals are produced, weakening the antioxidant defenses of microorganisms and ultimately leading to their elimination (4). This method proves effective in inhibiting bacteria and reducing the need for oral antibiotics in patients. A significant advantage of PDT is the reduction in antibiotic use, which translates to cost savings in treatment (5). Phthalocyanines are among the most common and effective optical sensitizers used in PDT due to their high absorption in the red light region, efficient production of reactive oxygen species (ROS), and low toxicity (6). However, most phthalocyanines tend to aggregate in biological solutions due to their hydrophobic macrocyclic skeleton structure. Enhancing hydrophilicity and mitigating aggregation by introducing cationic groups to the phthalocyanine structure is an effective strategy (7). Recently, several potential systems, such as liposomes, nanoparticles, microemulsions, and nanoemulsions, have been employed for the controlled delivery of sensitizers and optimal distribution of the active substance into cells and tissues (8). Nanoemulsions, in particular, offer a solution for encapsulating hydrophobic drugs and enhancing their solubility in aqueous environments. Their small size and efficient drug targeting make them highly effective for transferring lipophilic compounds. Additionally, nanoemulsions offer advantages such as high stability, low toxicity, and the potential for gradual and sustained drug release (9). In the present study, the impact of PDT using a nanoemulsion containing zinc (Zn) phthalocyanine was assessed in the context of infectious wounds in diabetic rats.
Materials and Methods
Animal Grouping
Thirty-six adult male rats (150–250 g) were kept at 20–25 °C, a humidity of 50 ± 5%, and 12–12 hours light/dark. They were fed with sterile water and food. After a week of adaptation to environmental conditions, the rats were divided into six groups.
-
Control group: The animals were fed with sterile water and food from the beginning to the end of the experiment.
-
Diabetic wounded rat group: Diabetes was induced in rats, and then a wound with a diameter of 1.5 cm was created on their backs.
-
Diabeticrats withinfected wound group: Similar to the previous group, after those rats became diabetic and wounds were created on their back, the wound surface became infected with 100 μL of S. aureus (1.5 × 108 CFU/mL) (10).
-
Diabeticrats withinfected wounds treated with laser: Rats were prepared in the same way as the previous group. Then, the wound surface was exposed to laser irradiation with a wavelength of 670 nm and a laser dose of 203.85 J/cm2 for 40 minutes.
-
Diabeticrats withinfected wounds treated with drug: The rats were prepared in the same way as the previous group, and the surface of the wound was impregnated with phthalocyanine chloride. Then, these rats were placed in the dark for forty minutes.
-
Diabeticrats withinfected wounds treated with laser and drug: Similar to the previous two groups, the rats were prepared and treated with laser and drug.
Method of Inducing Diabetes in Rats
Using a glucometer, rat blood glucose was measured from the tail vein. Then, to induce diabetes in them, streptozotocin (55 mg/kg) was injected subcutaneously into rats of all groups, except for the control group. After four days, while observing the symptoms of diabetes in the form of weight loss, polydipsia, and polyuria, after 12 hours of fasting, blood glucose levels were measured again from the tail vein. The diabetes induction criterion was glucose levels higher than 200 mg/dL (11).
Preparation of Microbial Suspension
To prepare a microbial suspension from the fresh culture of S. aureus on the Müller-Hinton agar medium, several colonies were transferred to the tryptic soy broth medium and mixed well. Then, the light absorption of the suspension prepared with the spectrophotometer at 620 nm was adjusted between 0.8 and 0.13. This turbidity is equivalent to 1.5 × 108 CFU/mL, according to the McFarland standard (12).
Measurement of Antioxidant Factors in Serum and Tissue
The serum of the groups was isolated to measure the level of antioxidant factors in the blood. To measure the same factors in skin tissue, several pieces of tissue weighing 1 mg were randomly taken from the wound of each rat. After freezing and thawing each tissue twice, 1 mL of phosphate-buffered saline was added to each tissue. The samples were completely homogenized and centrifuged (15 minutes at 5000 rpm at 4 °C), and then the supernatants were collected.
To determine the activity of SOD, glutathione peroxidase (GPX), and glutathione (GSH) concentrations in the tissue and serum, the samples and blanks were prepared according to the instructions of ZellBio GmbH (Germany) kits and added to special wells. In addition, the turbidity of the samples was read at 420 nm (for SOD) and 412 nm (for GPX and GSH) by an enzyme-linked immunosorbent assay reader.
Statistical Analysis
The data were analyzed as means ± standard deviations by using SPSS software. The data related to wound diameter comparison at the beginning and end of the experiment were analyzed using a paired t test, and those associated with wound diameter comparison between experimental groups and antioxidant levels of blood and tissues were analyzed by one-way analysis of variance (ANOVA).
Results
Comparison of Blood and Tissue Antioxidant Factors Between Experimental Groups
According to Figure 1A, SOD activity significantly decreased in the blood of all groups compared to the control group (P≤ 0.001). After comparing the enzyme activity in blood among the experimental groups compared to the S. aureus-infected diabetic group, the activity of this enzyme was significantly higher only in the control group and the S. aureus-infected diabetic wounded group treated with laser and drug (P≤ 0.001). According to Figure 1B, in the wound tissue of the experimental groups in which diabetes was induced, no significant difference was found in the SOD activity in the groups whose infectious wounds were treated with either laser or drug alone compared to the group in which only the wound was created without infection and through staph. However, in the group in which the wound was infected, the level of this factor significantly decreased (P≤ 0.001), and vice versa. It significantly increased in the group in which the infected wound was treated with laser and Zn phthalocyanine (P≤ 0.001).
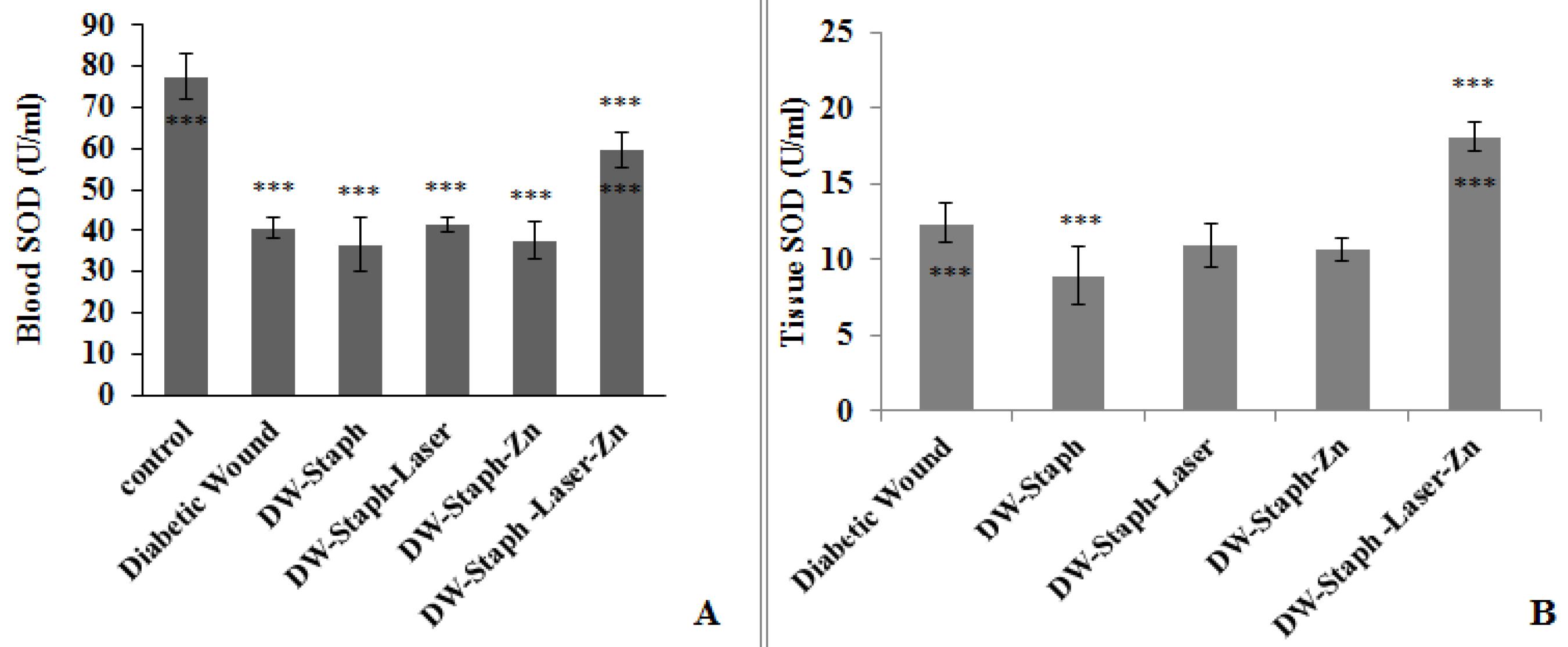
Figure 1.
Comparison of SOD Activity in the Blood (A) and Tissue (B) of Experimental Groups. Note. The data are expressed as the mean ± SD, and the significance level is considered ***P ≤ 0.001. The stars above the error bar indicate a comparison of this enzyme in the experimental groups relative to the first group, and the lower stars below the error bar represent a comparison of this enzyme in the experimental groups compared to the Staphylococcus aureus-infected diabetic group. SOD: Superoxide dismutase; SD: Standard deviation; DW: Diabetic wound; Staph: Staphylococcus aureus; Zn: Zinc phthalocyanine
.
Comparison of SOD Activity in the Blood (A) and Tissue (B) of Experimental Groups. Note. The data are expressed as the mean ± SD, and the significance level is considered ***P ≤ 0.001. The stars above the error bar indicate a comparison of this enzyme in the experimental groups relative to the first group, and the lower stars below the error bar represent a comparison of this enzyme in the experimental groups compared to the Staphylococcus aureus-infected diabetic group. SOD: Superoxide dismutase; SD: Standard deviation; DW: Diabetic wound; Staph: Staphylococcus aureus; Zn: Zinc phthalocyanine
According to Figure 2A, the GPX activity in the blood in all groups demonstrated a significant decrease. This decrease was at the level of P≤ 0.05 in rats whose wounds were treated with laser and drug, while in other groups, the decrease in the level of activity of this enzyme was more significant than in the control group.
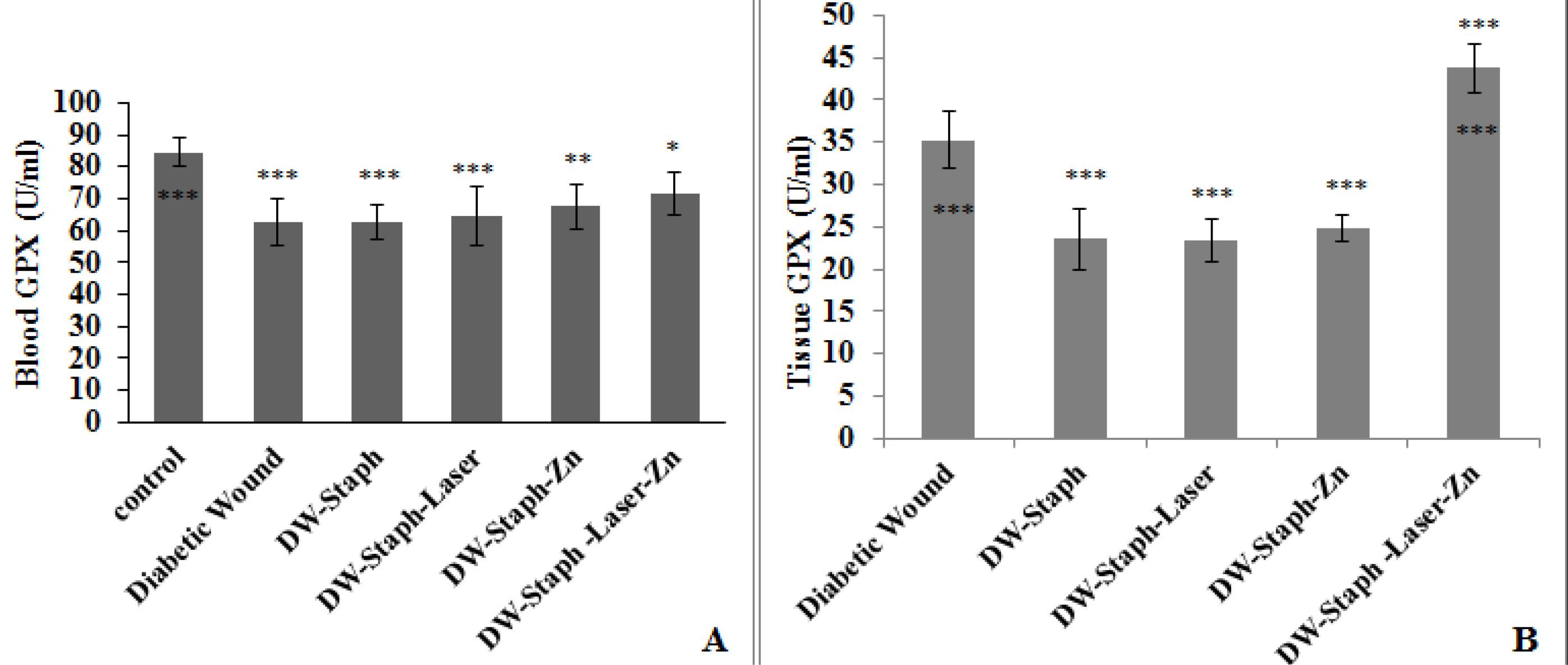
Figure 2.
Comparison of GPX Activity Levels in the Blood (A) and Tissue (B) of Experimental Groups. Note. SD: Standard deviation; GPX: Glutathione peroxidase. The data are expressed as the mean ± SD, and the significance level is considered *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. The stars above the error bar demonstrate a comparison of the levels of the GPX enzyme in the experimental groups relative to the column, and the lower stars below the error bar indicate a comparison of the levels of this enzyme in the experimental groups in comparison to the Staphylococcus aureus-infected diabetic group
.
Comparison of GPX Activity Levels in the Blood (A) and Tissue (B) of Experimental Groups. Note. SD: Standard deviation; GPX: Glutathione peroxidase. The data are expressed as the mean ± SD, and the significance level is considered *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. The stars above the error bar demonstrate a comparison of the levels of the GPX enzyme in the experimental groups relative to the column, and the lower stars below the error bar indicate a comparison of the levels of this enzyme in the experimental groups in comparison to the Staphylococcus aureus-infected diabetic group
Based on Figure 2B, the activity of this enzyme in the wound tissue of three groups of rats with infected wounds, infectious wounds treated with laser alone, and infectious wounds treated with drugs alone revealed a significant decrease compared to the group of rats in which only wounds had been created. However, a significant increase was found in the rats that were treated with drugs and the laser.
According to the data in Figure 3A, a comparison of the experimental groups with the control group represented that the level of GSH in the blood decreased, similar to the two previous factors. This decrease was significant in all groups (P≤ 0.001), but in the group of diabetic wounds infected with bacteria and treated with laser and drugs, it was significant (P≤ 0.05). According to Figure 3B, a comparison of tissue GSH levels in the experimental groups with the diabetic wounded group demonstrated that the level of this factor significantly decreased in all groups (P≤ 0.001), while it significantly increased only in the diabetic group infected with bacteria and treated with laser and drug (P≤ 0.001). Based on the comparison of the experimental groups with the diabetic wounded group infected with the bacterium, the level of tissue GSH concentration significantly increased in the group treated with laser and Zn phthalocyanine (P ≤ 0.00).
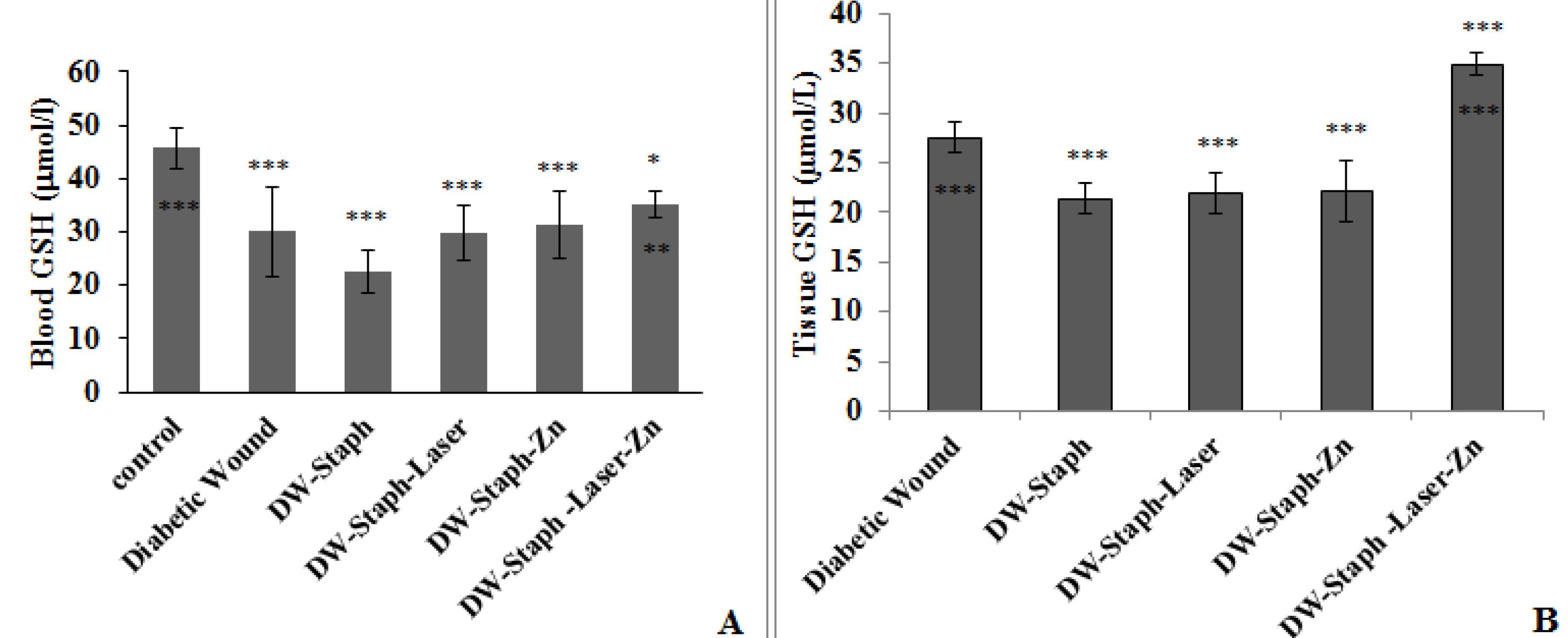
Figure 3.
Comparison of GSH Activity Levels in the Blood (A) and Tissue (B) of Experimental Groups. Note. SD: Standard deviation; GSH: Glutathione. The data are expressed as the mean ± SD, and the significance level is considered *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. The stars above the error bar represent a comparison of the levels of the GSH enzyme in the experimental groups relative to the first group, and the lower stars below the error bar demonstrate a comparison of the levels of this enzyme in the experimental groups compared to the Staphylococcus aureus-infected diabetic wound group
.
Comparison of GSH Activity Levels in the Blood (A) and Tissue (B) of Experimental Groups. Note. SD: Standard deviation; GSH: Glutathione. The data are expressed as the mean ± SD, and the significance level is considered *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. The stars above the error bar represent a comparison of the levels of the GSH enzyme in the experimental groups relative to the first group, and the lower stars below the error bar demonstrate a comparison of the levels of this enzyme in the experimental groups compared to the Staphylococcus aureus-infected diabetic wound group
Comparation of Diameter of Wounds in Experimental Groups
Wound diameters in all experimental groups were investigated at the end of the experiment compared to the beginning of the experiment. According to Figure 4A, in all treated and untreated groups, the reduction in wound diameter at the end of the experiment was quite evident (P≤ 0.001). The wound diameters of the experimental groups were compared at the end of the experiment. Based on Figure 4B, the diameter of the wounds did not change much in any of the experimental groups compared to the group in which only the wound had been created. However, the diameter of the wounds significantly decreased in the group that was treated with drugs and the laser (P≤ 0.01). The healing of the wound after the end of the treatment period was clearly evident in this group (Figure 5).
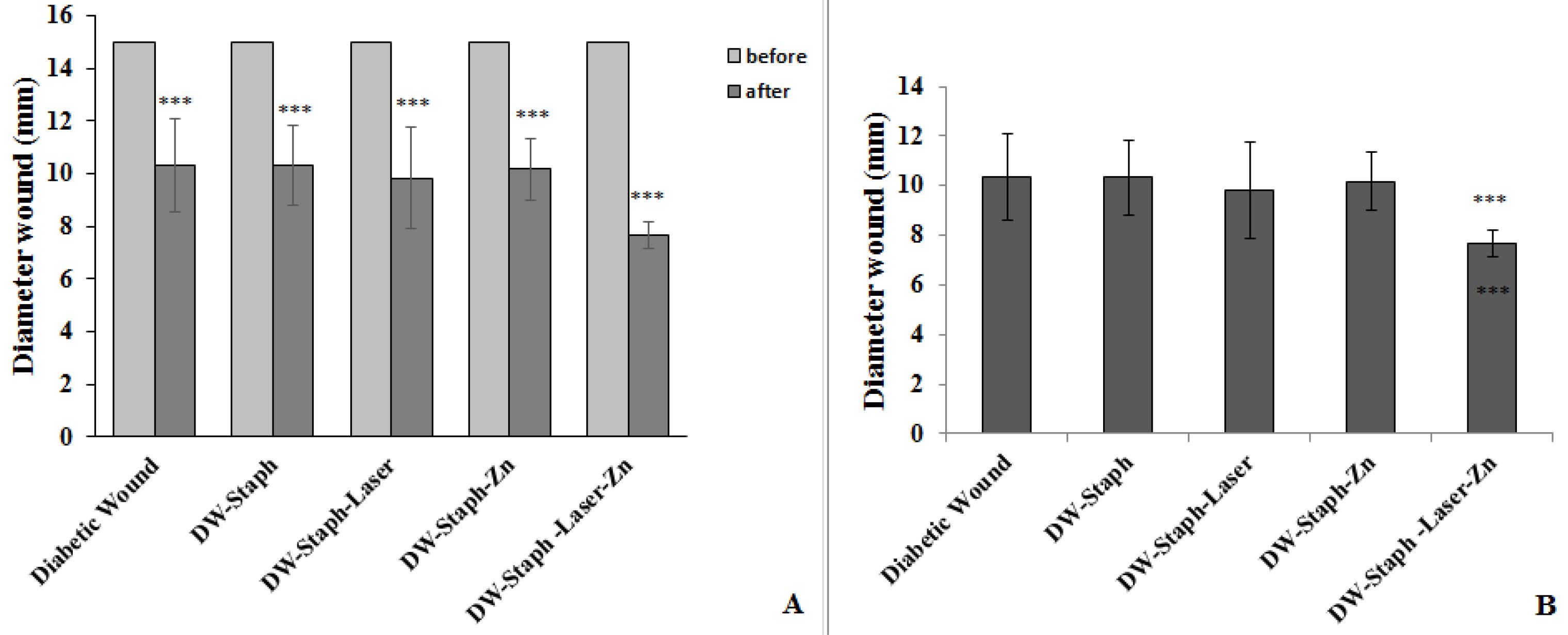
Figure 4.
(A) Comparison of Wound Diameters at the End of the Experiment and the Beginning of the Experiment in All Experimental Groups and (B) Comparison of Wound Diameters at the End of the Experiment in Experimental Groups in Comparison to Diabetic Wounded Group. Note. SD: Standard deviation. The data are expressed as the mean ± SD, and the significance level is considered *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001
.
(A) Comparison of Wound Diameters at the End of the Experiment and the Beginning of the Experiment in All Experimental Groups and (B) Comparison of Wound Diameters at the End of the Experiment in Experimental Groups in Comparison to Diabetic Wounded Group. Note. SD: Standard deviation. The data are expressed as the mean ± SD, and the significance level is considered *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001
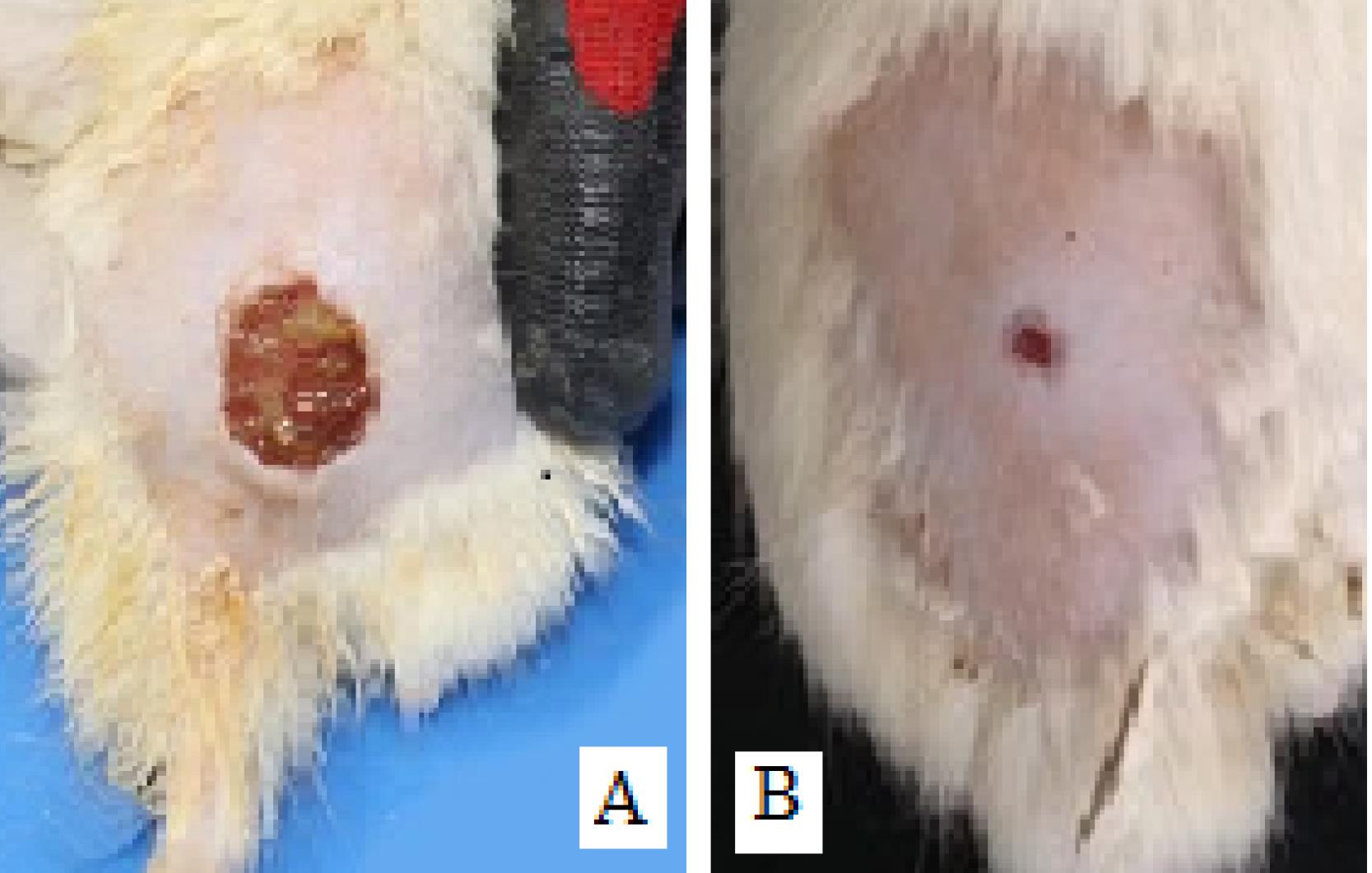
Figure 5.
Infectious Wounds in Diabetic Rats. (A) Wound surface in a group of untreated rats and (B) reduction of wound diameter and elimination of infection in rats treated with zinc phthalocyanine by photodynamic therapy
.
Infectious Wounds in Diabetic Rats. (A) Wound surface in a group of untreated rats and (B) reduction of wound diameter and elimination of infection in rats treated with zinc phthalocyanine by photodynamic therapy
Discussion
According to the most recent official statistics released in Iran, there are more than five million people with diabetes, and approximately 60 000 individuals develop diabetic wounds each year, with a particular focus on diabetic foot wounds. Tragically, this has led to 17 000 people losing their infected feet (13). In the context of the current study, diabetes was induced in rats, and the study focused on evaluating infectious wounds caused by S. aureus and the subsequent wound healing following PDT with the specified drug.
Based on observations of wound healing and the measurement of wound diameter in the experimental groups, it was noted that the wound diameter gradually reduced in all rats by the end of the experiment, irrespective of whether they were in the treatment or other groups. This suggests wound healing across the board. One contributing factor to this phenomenon might be the choice of animal subjects for the study. Rodents, particularly rats, are known to exhibit extremely greater resistance to infectious diseases compared to primates, including humans. This resilience may be attributed to their living environments, which frequently expose them to pathogens, prompting their immune systems to evolve a higher level of resistance to infectious diseases compared to other species (14). Consequently, wound diameters were compared among groups at the end of the experiment, and the reduction in wound diameter was statistically significant solely in the group of rats treated with both the drug and laser in comparison to the diabetic wounded group. In essence, while wound healing was observed in all groups, the treatment group that received both laser therapy and the drug exhibited an accelerated rate of healing.
In recent years, there has been a focus on the development of certain cationic Zn phthalocyanines (ZnPc) for use in antimicrobial and anticancer PDT. Zheng et al reported the in vitro and in vivo efficiency of a super cationic Zn (II) phthalocyanine as a promising photosensitizer against S. aureus (15). Kashef et al conducted a study on wounds infected with methicillin-resistant S. aureus and multidrug-resistant Escherichia coli isolates. Their findings suggested that PDT reduced wound diameter in cases of E. coli and S. aureus infections, offering an alternative treatment for localized bacterial infections in response to the challenge of antibiotic resistance (16).
In the present study, it appears that oxidative stress induced during PDT significantly weakened the antioxidant defense system of S. aureus. This reduction in pathogen antioxidant defenses likely led to a quicker healing process. The concern of whether this treatment method had an adverse effect on the rats’ antioxidant system was also addressed, in addition to evaluating the levels of antioxidant factors in the wound tissue and the host’s blood. The results indicated that the activity of SOD, GPX, and the concentration of GSH in both blood and tissue were significantly reduced in the diabetic group compared to the control group. This aligns with previous studies that have reported low levels of antioxidants, including GSH, GPX, and SOD, in diabetic rats (17). Furthermore, Sulaiman et al noted an inverse relationship between decreased GPX and GSH levels in diabetes patients and their blood sugar levels, implying that the levels of these antioxidant factors decrease with an increase in blood sugar levels (18).
The decrease in SOD, GPX, and GSH levels observed in diabetic rats with infected wounds in this study may be attributed to an increase in the production of ROS by the body’s immune cells to combat the pathogen (19). Additionally, the depletion of antioxidants due to polyuria in diabetic patients could be another factor contributing to the reduced levels of these factors (20). The most abundant non-enzymatic antioxidant in the body is GSH, which plays a vital role in maintaining the body’s redox state. It is converted to its oxidized form by GPX to neutralize peroxide ions and OH radicals when oxidative stress is induced. However, it is rapidly regenerated back to its active form with the help of nicotinamide adenine dinucleotide phosphate, thereby preventing an imbalance in the oxidant/antioxidant system. SOD, on the other hand, plays a direct role in reducing ROS metabolites by catalyzing superoxide radicals into H2O2, which is then further broken down into water and oxygen by CAT (21).
Following PDT of wounds with the combination of drugs and laser, the levels of these factors in the tissue increased in comparison to untreated wounds. The reduction in activity and infiltration of immune cells into the wound surface subsequently reduced ROS production. It is plausible that this treatment method, by eliminating pathogens from the wounds, contributed to this outcome. Furthermore, some studies have suggested that phthalocyanines have antioxidant properties (22). Thus, the combination of these factors may have led to an improvement in the levels of antioxidants. An additional justification for this claim is the observed reduction in wound diameter in the group of rats receiving this combined treatment. Further, the study results showed that, in addition to improving the levels of antioxidants in the treated tissues, the levels of these factors in the blood of these rats increased as well. This indicates that the treatment not only had no adverse effects on the blood system but indirectly reinforced the anti-inflammatory system by eliminating infections from the wounds. A group of researchers reported the deaths of S. aureus, E. coli, and E. faecalis following antimicrobial PDT with the assistance of hypericin optical sensitizers and emphasized that this method had no detrimental effects on primary human fibroblast cells (16). In another study, after PDT of Walker 256 tumors in rats using a 5-ALA light sensor plus chitosan, although the activity of SOD, GPX, and CAT enzymes in the tumors decreased significantly, the plasma levels of these factors increased, indicating that this treatment had a localized effect only on tumors (23).
Conclusion
The antioxidant system of pathogens, particularly antibiotic-resistant ones, serves as a robust defense against the free radicals generated by the body’s immune cells. Consequently, PDT appears to assist the body’s immune system by intensifying oxidative stress. Elevating the levels of ROS within bacteria effectively hinders their antioxidant defenses, ultimately leading to their demise. This mechanism potentially prevents the body from hyperactivating the immune system, thereby reducing the production of free radicals, notably H2O2, and preventing an excessive and overstimulated response from the natural antioxidants responsible for neutralizing these free radicals in tissues and blood.
Hence, the increase in antioxidant levels observed in the blood and tissue of rats treated with Zn phthalocyanine and laser can be attributed to the reduction in infection. The substantial decrease in wound diameter in rats subjected to drug and laser treatment provides further support for this assertion.
Acknowledgements
The authors are grateful to the Faculty of Basic Sciences, Islamic Azad University, Falavarjan Branch, for their cooperation and supply of experimental equipment.
Authors’ Contribution
Conceptualization: Mahnoosh Fatemi, Fereshte Ghandehari.
Data curation: Sara Soleymani Sedeh.
Formal analysis: Mahnoosh Fatemi.
Funding acquisition: Sara Soleymani Sedeh.
Investigation: Mahnoosh Fatemi,Sara Soleymani Sedeh, Fereshte Ghandehari.
Methodology: Mahnoosh Fatemi, Fereshte Ghandehari.
Project administration: Mahnoosh Fatemi, Fereshte Ghandehari.
Resources: Mahnoosh Fatemi.
Software: Sara Soleymani Sedeh.
Supervision: Mahnoosh Fatemi.
Validation: Mahnoosh Fatemi, Fereshte Ghandehari.
Visualization: Mahnoosh Fatemi, Fereshte Ghandehari.
Writing–original draft: Sara Soleymani Sedeh.
Writing–review & editing: Sara Soleymani Sedeh.
Competing Interests
The authors declare that there is no conflict of interests.
Ethical Approval
All stages of the project from the beginning to the end were performed according to the standards of working with laboratory animals, and the code of ethics for working with laboratory animals (IR.IAU.FALA.REC 1398/041) was obtained from the Ethics Committee of the Falavarjan branch.
Funding
No funding was obtained for this study.
References
- Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006; 23(6):594-608. doi: 10.1111/j.1464-5491.2006.01773.x [Crossref] [ Google Scholar]
- Chakraborty R, Borah P, Dutta PP, Sen S. Evolving spectrum of diabetic wound: mechanistic insights and therapeutic targets. World J Diabetes 2022; 13(9):696-716. doi: 10.4239/wjd.v13.i9.696 [Crossref] [ Google Scholar]
- Linzner N, Loi VV, Fritsch VN, Antelmann H. Thiol-based redox switches in the major pathogen Staphylococcus aureus. Biol Chem 2021; 402(3):333-61. doi: 10.1515/hsz-2020-0272 [Crossref] [ Google Scholar]
- Sharma D, Singh S, Kumar P, Jain GK, Aggarwal G, Almalki WH, et al. Mechanisms of photodynamic therapy. In: Kesharwani P, ed. Nanomaterials for Photodynamic Therapy. Woodhead Publishing; 2023. p. 41-54. 10.1016/b978-0-323-85595-2.00017-7.
- Hu X, Zhang H, Wang Y, Shiu B-C, Lin JH, Zhang S. Synergistic antibacterial strategy based on photodynamic therapy: progress and perspectives. Chem Eng J 2022; 450(Pt 3):138129. doi: 10.1016/j.cej.2022.138129 [Crossref] [ Google Scholar]
- Li X, Bai H, Yang Y, Yoon J, Wang S, Zhang X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv Mater 2019; 31(5):e1805092. doi: 10.1002/adma.201805092 [Crossref] [ Google Scholar]
- Furuyama T, Ishii T, Ieda N, Maeda H, Segi M, Uchiyama M. Cationic axial ligands on sulfur substituted silicon(iv) phthalocyanines: improved hydrophilicity and exceptionally red-shifted absorption into the NIR region. Chem Commun (Camb) 2019; 55(51):7311-4. doi: 10.1039/c9cc03022k [Crossref] [ Google Scholar]
- de Oliveira de Siqueira LB, da Silva Cardoso V, Rodrigues IA, Vazquez-Villa AL, Dos Santos EP, da Costa Leal Ribeiro Guimarães B. Development and evaluation of zinc phthalocyanine nanoemulsions for use in photodynamic therapy for Leishmania spp. Nanotechnology 2017; 28(6):065101. doi: 10.1088/1361-6528/28/6/065101 [Crossref] [ Google Scholar]
- de Oliveira de Siqueira LB, Dos Santos Matos AP, da Silva Cardoso V, Villanova JCO, da Costa Leal Ribeiro Guimarães B, Dos Santos EP. Clove oil nanoemulsion showed potent inhibitory effect against Candida spp. Nanotechnology 2019; 30(42):425101. doi: 10.1088/1361-6528/ab30c1 [Crossref] [ Google Scholar]
- Mahpishanian S, Fatemi M, Ghandehari F. The effect of Lactobacillus plantarum and selenium-enriched Lactobacillus plantarum on Staphylococcus aureus-induced osteomyelitis. J Kerman Univ Med Sci 2022; 29(6):520-8. doi: 10.34172/jkmu.2022.63 [Crossref] [ Google Scholar]
- Adams DM, Yakubu MT. Aqueous extract of Digitariaexilis grains ameliorate diabetes in streptozotocin-induced diabetic male Wistar rats. J Ethnopharmacol 2020; 249:112383. doi: 10.1016/j.jep.2019.112383 [Crossref] [ Google Scholar]
- Pence MA, Liesman R. Clinical microbiology. In: Clarke W, Marzinke MA, eds. Contemporary Practice in Clinical Chemistry. 4th ed. Academic Press; 2020. p. 985-1006. 10.1016/b978-0-12-815499-1.00055-7.
- Yazdanpanah L, Shahbazian H, Nazari I, Arti HR, Ahmadi F, Mohammadianinejad SE. Prevalence and related risk factors of diabetic foot ulcer in Ahvaz, south west of Iran. Diabetes Metab Syndr 2018; 12(4):519-24. doi: 10.1016/j.dsx.2018.03.018 [Crossref] [ Google Scholar]
- Medberry CJ, Tottey S, Jiang H, Johnson SA, Badylak SF. Resistance to infection of five different materials in a rat body wall model. J Surg Res 2012; 173(1):38-44. doi: 10.1016/j.jss.2010.08.035 [Crossref] [ Google Scholar]
- Zheng BD, Li SL, Huang ZL, Zhang L, Liu H, Zheng BY. A non-aggregated zinc(II) phthalocyanine with hexadeca cations for antitumor and antibacterial photodynamic therapies. J Photochem Photobiol B 2020; 213:112086. doi: 10.1016/j.jphotobiol.2020.112086 [Crossref] [ Google Scholar]
- Kashef N, Ravaei Sharif Abadi G, Djavid GE. Phototoxicity of phenothiazinium dyes against methicillin-resistant Staphylococcus aureus and multi-drug resistant Escherichia coli. Photodiagnosis Photodyn Ther 2012; 9(1):11-5. doi: 10.1016/j.pdpdt.2011.11.004 [Crossref] [ Google Scholar]
- Heydarnia E, Taghian F, Jalali Dehkodi K, Moghadasi M. Effects of eight weeks of combined training with antioxidant vitamins E and C on glutathione, glutathione peroxidase, and superoxide dismutase in the heart tissue of streptozotocin-induced diabetic rats. Gene Cell Tissue 2021; 8(3):e111277. doi: 10.5812/gct.111277 [Crossref] [ Google Scholar]
- Sulaiman GM, Tawfeeq AT, Jaaffer MD. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: an in vivo and in vitro study. Biotechnol Prog 2018; 34(1):218-30. doi: 10.1002/btpr.2568 [Crossref] [ Google Scholar]
- Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol 2011; 89(6):873-91. doi: 10.1189/jlb.1010550 [Crossref] [ Google Scholar]
- Peluso I, Raguzzini A. Salivary and urinary total antioxidant capacity as biomarkers of oxidative stress in humans. Patholog Res Int 2016; 2016:5480267. doi: 10.1155/2016/5480267 [Crossref] [ Google Scholar]
- Adwas AA, Elsayed A, Azab AE, Quwaydir FA. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng 2019; 6(1):43-7. doi: 10.15406/jabb.2019.06.00173 [Crossref] [ Google Scholar]
- Salih Ağırtaş M, Karataş C, Özdemir S. Synthesis of some metallophthalocyanines with dimethyl 5-(phenoxy)-isophthalate substituents and evaluation of their antioxidant-antibacterial activities. Spectrochim Acta A Mol Biomol Spectrosc 2015; 135:20-4. doi: 10.1016/j.saa.2014.06.139 [Crossref] [ Google Scholar]
- Doina D, Filip A, Clichici S, Suciu S, Muresan A, Decea N. Oxidative effects after photodynamic therapy in rats. Vet Med (Auckl) 2008; 65(1):364-9. [ Google Scholar]