Avicenna Journal of Clinical Microbiology and Infection. 10(2):65-69.
doi: 10.34172/ajcmi.3472
Original Article
Variability on the Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ruppia cirrhosa Extract Using Two Different Methods of Extraction
Emna Chaabani 1, 2  , Iness Bettaieb Rebey 1
, Iness Bettaieb Rebey 1  , Wissem Aidi Wannes 1, *
, Wissem Aidi Wannes 1, *  , Riadh Ksouri 1
, Riadh Ksouri 1  , Abdessalem Shili 2
, Abdessalem Shili 2 
Author information:
1Laboratory of Medicinal and Aromatic Plants, Biotechnology Center of Borj-Cedria, BP 901, 2050, Hammam-Lif, Tunisia
2Département Génie Halieutique et Environnement, Unité de Recherche Ecosystèmes et Ressources Aquatiques, Université de Carthage, Institut National Agronomique de Tunisie, 43 Avenue Charles Nicolle, 1082 Tunis, Tunisia
Abstract
Background: Nowadays, there is increasing attention to the discovery of new bioactive substances from marine sources. This research aimed to characterize the phytochemical composition as well as antioxidant and antimicrobial activities of Tunisian Ruppia cirrhosa extracts (RCEs) using two different extraction methods.
Methods: RCEs were obtained by two different extraction methods: maceration and successive extraction. The determination of polyphenolic contents and antioxidant activity was made by calorimetric assay, and the effect of RCE was observed against pathogenic bacteria and fungi using the solid diffusion method.
Results: The successive extraction of R. cirrhosa extract relatively showed higher total phenol (38.1 mg GAE/g) and condensed tannin (18.07 mg CE/g) contents than the maceration extraction (35.43 mg EAG/g and 12.99 mg CE/g, respectively). However, the total flavonoid amount of RCE was higher in the maceration extraction (33.09 mg CE/g) than in the successive extraction (21.27 mg CE/g). The total antioxidant capacity of RCE indicated a decrease in this activity after fractionation. Indeed, the activity of RCE decreased from 47.8 to 37.83 mg GAE/g, and RCE obtained by the two extraction methods showed moderate antioxidant activity using reducing power (IC50=380-490 µg/mL) and β-carotene bleaching (IC50=110-310 μg/mL) assays. Furthermore, RCEs obtained by maceration had the greatest antibacterial activity against all tested strains (IZ=3.33-9.33 mm) except Salmonella typhimurium (IZ=2 mm), Enterococcus faecalis (IZ=6 mm), and Streptococcus aureus (3.67 mm) as compared to those obtained by successive extraction. The strains of Candida had a sensitivity for R. cirrhosa extracts obtained by maceration. Indeed, R. cirrhosa extracts obtained by successive extraction had higher inhibitory activity against Candida krusei deduced through an inhibition diameter of 6 mm.
Conclusion: It can be concluded that R. cirrhosa extract is rich in bioactive molecules, and it has an extremely promising biological potential.
Keywords: Ruppia cirrhosa, Maceration, Successive extraction, Phytochemical characterization, Antioxidant activity, Antimicrobial activity
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Chaabani E, Rebey IB, Wannes WA, Ksouri R, Shili A. Variability on the phytochemical composition, antioxidant and antimicrobial activities of Ruppia cirrhosa extract using two different methods of extraction. Avicenna J Clin Microbiol Infect. 2023; 10(2):65-69. doi:10.34172/ajcmi.3472
Introduction
Natural products have been applied to cure human diseases since the beginning of mankind (1). The aquatic ecosystems are an important resource for a broad range of natural bioactive products (2), and the attention to the discovery of new bioactive substances from marine sources is increasing (3). Polyphenols from marine organisms are far less studied than those from terrestrial sources since their structural diversity and variability require powerful analytical tools. However, both their biological relevance and potential properties make them an attractive group deserving of increasing scientific interest. The marine seagrasses are known as magnoliophytes, and they are flowering plants, namely, angiosperms (4). Nowadays, marine seagrasses give rise to dense formations called “herbariums”, which are found in almost all coastal environments around the world (5). These beds characterize the infralittoral stage where they preferentially colonize soft substrates. Five species of marine seagrasses are reported in the Mediterranean, including Cymodocea nodosa, Halophila stipulacea, Posidonia oceanica, Zostera marina, and Nanozostera noltii(6). For Ruppia cirrhosa and Ruppia maritima, these two species are widespread in lagoon environments (7). These two species belong to the widgeon grass family (Ruppiaceae), and they can exist in single populations with no other vascular plants present, and they are barely ever found together (2).
Polyphenols from marine organisms are far less studied than those from terrestrial sources since their structural diversity and variability require powerful analytical tools (8). R. cirrhosa was found to be rich in chicoric acid ( ≤ 30.20 mg/g) (2), has promising pharmacological effects in antiviral, contain anti-inflammatory, antihyperglycemic, antihyperlipidemic, antimicrobial, antioxidant, and anti-aging properties, and impacts digestive system illness (9). The findings concerning secondary metabolites of R. cirrhosa, especially polyphenolic contents are limited (2,10). Additionally, the determination of the biological properties of R. cirrhosais still scarce (2,11). Therefore, this research aimed to characterize the phytochemical composition as well as antioxidant and antimicrobial activities of Tunisian RCE using two different extraction methods.
Materials and Methods
Seagrass material
Ruppia cirrhosawas manually taken from the North Lake of Tunis and was put in an icebox. It was thoroughly washed using tap water and then sterile distilled water to be air dried at room temperature for one week. Afterward, dried R. cirrhosawasground with a blender into powder to be analyzed.
Extract Preparation
Extraction by Maceration
For this extraction, 2 g of dry R. cirrhosapowder was mixed with 20 mL of polar solvent (80% acetone) for 30 minutes. The mixture was stirred with a magnetic stirrer, standing for 24 hours at 4°C in the dark and then was filtered through filter Watman paper No. 4 (12).
Extraction by Successive Solvents
This extraction was done using 3 solvents with different polarities, namely, hexane (Polarity: 0), petroleum ether, and acetone (Polarity: 5.4) at 80%. Weigh 5 g of the magnoliophyte R. cirrhosa using a precision balance. Before proceeding with the actual extraction, successive washes with hexane and petroleum ether were considered to remove pigments and lipids. For this, 50 mL of hexane was added to each sample. The mixture was then stirred for 1 hour and then filtered through ashless filter paper (Wattman No. 4); afterward, another 50 mL of petroleum ether was added to each sample. Once a clear and transparent supernatant was obtained, 50 mL of 80% acetone was added to the various algae samples which were stirred for 30 minutes and reserved at rest for 24 hours at 4 °C in the dark. Subsequently, the solution was filtered through ashless filter paper (Whatman No. 4).
Phytochemical Contents
The assay of polyphenols and the total flavonoid assay were done according to Dewanto et al (13), and the assay of condensed tannins was determined following Sun et al (14).
Antioxidant Activity
The total antioxidant measurement was based on the reduction of Mo6+ ions to Mo5+ by the seagrass extract to form the green-colored phosphate-Mo5+ complex at an acid pH (15). The antiradical activity was measured by 2,2’-diphenyl-1-picrylhydrazyl (DPPH). A test sample of 1 mL of the extract at different concentrations (10, 50, 75, 100, 500, 1000, and 1500 µg/mL) was placed in the presence of 250 µL of a DPPH solution (0.2 mM in methanol). The mixture remained at rest for 30 minutes and in the dark for incubation (16).
For reducing power assay, samples at different concentrations (from 10 to 1500 μg/mL) were mixed with 1 mL K3[Fe(CN)6 and 1 mL tryptone phosphate water (0.2 M, pH 6.6) (1%), incubated in a water bath at 50°C for 20 minutes, then 1 mL of tricarboxylic acid (10%) was added to each reaction. Afterward, 1 mL of this solution was added to 1 mL of distilled water and 0.2 mL of 0.1% FeCl3 solution (16).
The iron chelating power assay involved mixing 100 µL of the extract of known concentrations with 50 µL of FeCl26H2O solution (2mM: 0.00379g/100mL) to leave them to rest for 5 minutes at room temperature. A dose of 100 µL of a solution of Ferrozine (5 mmol/L) was added afterward to trigger the reaction. Finally to adjust the mixture to 3mL, 2750 µL of distilled water must be added (17).
For β-carotene bleaching activity, a sample of 2 mg of β-carotene was dissolved in 20 mL of chloroform. Then, 2 mL of the mixture was added to 20 mg of linoleic acid and 200 mg of Tween 40. After the evaporation of the chloroform at 40 °C, 100 mL of hydrogen peroxide was added to the residue. After vigorous stirring, 150 µL of the β-carotene/linoleic acid mixture was added to 10 µL of the samples and prepared in the extraction solvent at different concentrations. Then, three repetitions were carried out for each concentration (16).
Antimicrobial Activity
Enterococcus faecalis (ATCC 29212), Micrococcus luteus (NCIMB 8166), Staphylococcus aureus (ATCC 29213), Escherichia coli (ATCC 35218), Shigella flexneri (ATCC 29203), Pseudomonas aeruginosa (ATCC 27853), Klebsiella sp. IP Tunis, and Salmonella typhimurium (ATCC 14028) were the bacterial strains used for the study of antibacterial activity. For antifungal activity, C. glabrata (ATCC 90030), C. albicans (ATCC 2091), C. tropicalis (ATCC 06-085), and C. Krusei (ATCC 6258) were used.
The antibacterial and antifungal activities of seagrass extracts against pathogenic bacteria and fungi were carried out by the solid diffusion method (18). To fix the strains of bacteria and yeasts on the agar medium, an incubation was carried out for 15 minutes at 37 °C. The boxes were then removed and the excess liquid was discarded under sterile conditions. Another incubation of these boxes in the oven at 37 °C for a quarter of an hour allows them to dry, and thus a carpet of bacteria or yeasts is obtained. Sterile Whatman paper discs 6 mm in diameter were placed on the bacteria and yeast mats. Then,10 μL doses of the extracts (0.5 mg/mL) were put on the discs. The effect of RCE on the growth of the strains around the disc was observed after incubation at 37 °C for 24 hours. The formation of a halo around each disk reflects the inhibition of the growth of bacteria and yeasts (19). This activity consisted of measuring the diameter of inhibition, and the positive controls used for these activities were antibiotics (Gentamicin).
Results
Phytochemical Characterization
The analysis of the obtained results concerning the amounts of polyphenols showed significant variability between the two extraction methods (Figure 1). The successive extraction of R. cirrhosaextract relatively indicated higher total phenol (38.1 mg GAE/g) and condensed tannin (18.07 mg CE/g) contents than the extraction of maceration (35.43 mg EAG/g and12.99 mg CE/g, respectively). However, the total flavonoid content of RCE was higher in the maceration extraction (33.09 mg CE/g) than in the successive extraction (21.27 mg CE/g).
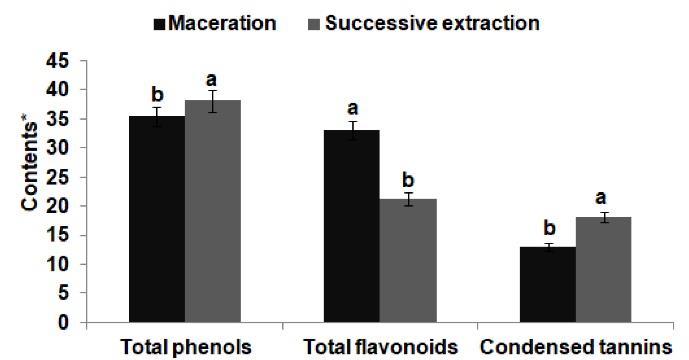
Figure 1.
Total Contents of Total Phenolics, Total Flavonoids, and Condensed Tannins of Ruppia cirrhosa Extracts Obtained by Maceration and Successive Extraction. Note. *Total phenolic contents were expressed as milligrams of gallic acid equivalents per gram of dry extract (mg GAE/g DE); Total flavonoid contents were expressed as milligrams of catechin equivalents per gram of dry extract (mg CE/g DE); Condensed tannin contents were expressed as milligrams catechin equivalents per gram of dry extract (mg CE/g DE). Values are given as mean ± S.D (n = 3). Means followed by the same small letter (a, b) shared significant differences at P< 0.05(Duncan test)
.
Total Contents of Total Phenolics, Total Flavonoids, and Condensed Tannins of Ruppia cirrhosa Extracts Obtained by Maceration and Successive Extraction. Note. *Total phenolic contents were expressed as milligrams of gallic acid equivalents per gram of dry extract (mg GAE/g DE); Total flavonoid contents were expressed as milligrams of catechin equivalents per gram of dry extract (mg CE/g DE); Condensed tannin contents were expressed as milligrams catechin equivalents per gram of dry extract (mg CE/g DE). Values are given as mean ± S.D (n = 3). Means followed by the same small letter (a, b) shared significant differences at P< 0.05(Duncan test)
Antioxidant Activity
Table 1 presents the antioxidant activities of RCE obtained by maceration and successive extraction using different assays. The total antioxidant capacity of RCEshowed a decrease in this activity after fractionation. Indeed, this activity decreased RCE from 47.8 to 37.83 mg GAE/g, and the positive control quercetin had the lowest total antioxidant capacity (28.15 mg GAE/g). Furthermore, the results indicated a decrease in the antiradical activity using DDPH assay after fractionation as well as an increase of 2.5 folds for IC50 ranging from 6.34 to 16.04 μg/mL. RCE obtained by the two extraction methods showed moderate antioxidant activity using reducing power and β-carotene bleaching assays. Additionally, a decrease in the reducing power and β-carotene bleaching effect of RCE was highlighted after fractionation. For reducing power, IC50 increased from 380 μg/mL to 490 μg/mL, while for the β-carotene bleaching effect, IC50 increased from 110 μg/mL to 310 μg/mL.
Table 1.
Antioxidant Activities of Ruppia Cirrhosa Extracts Obtained by Maceration and Successive Extraction
|
|
TAC
(mg GAE/g)
|
DPPH Activity
IC
50
(μg/mL)
|
Reducing Power
EC
50
(μg/mL)
|
β-carotene Bleaching
IC
50
(μg/mL)
|
|
Ruppia cirrhosaextract
|
| Maceration |
47.82 ± 1.25b
|
6.37 ± 0.35b
|
380 ± 0.23b
|
110 ± 0.33a
|
| Successive extraction |
37.83 ± 1.11b
|
16.04 ± 0.75b
|
490 ± 0.55a
|
310 ± 0.11b
|
| Positive control |
| Quercetin |
28.15 ± 0.80a
|
-
|
- |
- |
| BHT |
- |
11 ± 0.00a
|
- |
75 ± 0.01c
|
| Ascorbic acid |
- |
- |
45 ± 0.01c
|
- |
| EDTA |
- |
- |
- |
- |
Note. TAC: Total antioxidant capacity was expressed as milligrams of gallic acid equivalents per gram (mg GAE/g); DPPH: 1,1-diphenyl-2-picrylhydrazyl; BHT: Butylated hydroxytoluene; EDTA: ethylenediaminetetraacetic acid. Values are given as mean ± SD. (n = 3) followed by different letters (a-c) imply the significant differences (P < 0.05) according to the Tukey HSD test.
Antimicrobial Activity
Table 2 illustrates the antimicrobial activities of RCE obtained by maceration and successive extraction. RCE obtained by maceration had the greatest antibacterial activity against all tested strains (IZ = 3.33-9.33 mm) except for S. typhimurium (IZ = 2 mm), E. faecalis (IZ = 6 mm), and S. aureus (3.67 mm) as compared to those obtained by successive extraction. The positive control Gentamicin generally exhibited the best antibacterial activity (IZ = 8.65-13.55 mm) against all bacterial strains as compared to RCE using the two extraction methods (IZ = 2-9.33 mm). The strains of Candida were sensitive to RCE obtained by maceration. In other words, RCE obtained by successive extraction had higher inhibitory activity against C. krusei deduced through an inhibition diameter of 6 mm.
Table 2.
Antimicrobial Activities of Ruppia Cirrhosa Extracts Obtained by Maceration and Successive Extraction
|
Microorganisms
|
Inhibition Zone (mm)
|
|
Maceration
|
Successive Extraction
|
Gentamicin
(10 μg/disc)
|
|
Bacteria strains
|
| Gram-positive |
|
|
|
|
Entrococcus feacalis
|
6 ± 1.13c
|
8 ± 1.13b
|
12 ± 0.95a
|
|
Micrococcus luteus
|
3.33 ± 0.65b
|
2.33 ± 0.65c
|
13.55 ± 0.35a
|
|
Staphylococcus aureus
|
3.67 ± 0.65b
|
4 ± 00a
|
10.37 ± 0.25a
|
| Gram-negative |
|
|
|
|
Escherichia coli
|
9 ± 1.13a
|
1.33 ± 0.65c
|
9.47 ± 0.45a
|
|
Shigella flexneri
|
9.33 ± 0.65b
|
4.33 ± 0,65c
|
12.33 ± 0.35a
|
|
Pseudomonas aeruginosa
|
3 ± 00b
|
- |
8.65 ± 0.65a
|
|
Klebsiella sp. IP Tunis
|
3 ± 00b
|
3 ± 00b
|
10.83 ± 0.75b
|
|
Salmonella typhimirium
|
2 ± 00b
|
2.66 ± 0,65b
|
12.55 ± 0.55a
|
|
Yeast Strains
|
|
Candida glabrata
|
2 ± 0.00b
|
5 ± 00a
|
- |
|
Candida albicans
|
2.67 ± 0.65a
|
2.67 ± 0.65a
|
- |
|
Candida tropicalis
|
1.33 ± 0.65b
|
2.67 ± 0.65a
|
- |
|
Candida Krusei
|
4.67 ± 0.65b
|
6 ± 0.00a
|
- |
Note. Activity is absent (-) if the inhibition zone (IZ) was < 1 mm, low if IZ = 1 mm, slight if IZ between 2-3 mm, moderate if IZ between 4-5 mm, high if IZ between 6-9 mm, strong if IZ > 9 mm. Values are given as mean ± S.D. (n = 3) followed by different letters (a-c) imply the significant differences (P < 0.05) according to the Tukey HSD test.
Discussion
This study investigated the variability of the phytochemical, antioxidant, and antimicrobial activities using two different extraction methods. Indeed, the amounts of total phenols and condensed tannins increased using successive extraction, but total flavonoid content decreased by 1.5 times. The presence of phenols, flavonoids, and tannins in seagrasses may indicate that their extracts have antioxidant activity. This activity was believed to help prevent several diseases through free-radical scavenging activity (20).
In this study, a decrease was observed in reducing power and β-carotene bleaching activities after fractionation. This study was the first account of findings on reducing power and β-carotene bleaching activities of RCE. In our study, there was also a decrease in the antiradical activity using DDPH assay after fractionation with an increase of 2.5 folds for IC50 ranging from 6.34 to 16.04 μg/mL. However, Hasle Enerstvedt et al (2) noted that RCE exhibits an IC50 = 152.9-175.7 µg/mL, showing a low radical scavenging activity. However, after partition with ethyl acetate, the hydraulic R. cirrhosa phase had a very strong antiradical scavenging activity (IC50 = 31.8 μg/ mL). The differences recorded between our results and those of these authors can be explained by the fact that the extraction method was different, the solvents were not of the same nature (polarity), and the extraction process (continuous extraction, magnetic stirring, supercritical fluid extraction, and the like) and the extraction conditions (extraction time, temperature) were varied. That is why there was no uniform solvent or extraction method to determine the biological capacity of terrestrial plants let alone aquatic macrophytes. This shows the importance of screening and fractionation to look for interesting and recoverable species at an industrial scale. From RCE, Hasle Enerstvedt et al (2) isolated one phenolic acid (chicoric acid), and 8 flavonoids (four quercetin derivatives and four isorhamnetin derivatives) had a strong antiradical DPPH activity (12.1-88.4 μg/mL). Several studies have reported that polyphenols of seagrass extracts are mainly responsible for antioxidant activities (21-25).
Regarding the antimicrobial activity, the two extraction methods of RCE showed modest to extremely appreciable inhibitory activities of bacterial and fungal strains with IZ varying from 1.33 to 9.33 mm. These inhibitory effects could be due to the presence of active molecules (alkaloids, glycosides, phenolic acids, tannins, flavonoids, saponins, and Terpenes) of RCE. Moreover, Hasle Enerstvedt et al (2) reported that RCE is rich in phenolic chicoric acid ( ≤ 30.20 mg/g) which has a potent antimicrobial activity (26). Abd El-Hady et al (11) found that RCE is not active against the fungus Aspergillus flavus and A. fumigates, but it is only active against Alternaria alternata (IZ = 10 mm). This ethanol RCE also had active inhibitory action against B. subtilis (IZ = 10.50 mm), S. aureus (IZ = 10 mm), E. coli (IZ = 19.50 mm), and P. aeruginosa (IZ = 11 mm). In fact, the ethanol extract effect was significant against both tested Gram-positive and negative bacteria. This can be explicated by the presence of a cell wall with a single layer in Gram-positive bacteria but with a multi-layered structure bordered by an outer cell membrane in Gram-negative bacteria (27).
Conclusion
Since RCE is rich in bioactive molecules and has highly promising biological potential, other investigations can be carried out on this seagrass to identify and characterize these bioactive molecules by advanced chromatographic techniques for possible valorization in various fields such as cosmetics, foods, and pharmaceuticals.
Authors’ Contribution
Conceptualization: Emna Chaabani.
Data curation: Emna Chaabani.
Formal analysis: Wissem Aidi Wannes.
Funding acquisition: Emna Chaabani.
Investigation: Iness Bettaieb Rebey.
Methodology: Emna Caabani.
Project administration: Riadh Ksouri.
Resources: Emna Chaabani.
Supervision: Abdessalem Shili.
Validation: Emna Chaabani, Abdessalem Shili.
Visualization: Wissem Aidi Wannes, Iness Bettaieb Rebey.
Writing–original draft: Emna Chaabani.
Writing–review & editing: Emna Chaabani, Abdessalem Shili.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
This study was approved by the Research Committee of the National Institute of Agronomic Research of Tunisia (UR03AGRO1).
References
- Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol 2022; 20(1):14. doi: 10.1186/s43141-021-00290-4 [Crossref] [ Google Scholar]
- Hasle Enerstvedt K, Lundberg A, Jordheim M. Characterization of polyphenolic content in the aquatic plants Ruppiacirrhosa and Ruppia maritima - a source of nutritional natural products. Molecules 2017; 23(1):16. doi: 10.3390/molecules23010016 [Crossref] [ Google Scholar]
- Khairy HM, El-Sheikh MA. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi J Biol Sci 2015; 22(5):623-30. doi: 10.1016/j.sjbs.2015.01.010 [Crossref] [ Google Scholar]
- Kuo J, den Hartog C. Seagrasses: a profile of an ecological group. Biol Mar Mediterr 2000; 7(2):3-17. [ Google Scholar]
- Pergent-Martini C, Le Ravallec C. Guidelines for Impact Assessment on Seagrass Meadows. United Nations Environment Programme; 2007.
- Green EP, Short FT. World Atlas of Seagrasses. Berkeley: University of California Press; 2003.
- Shili A, Maïz NB, Boudouresque CF, Trabelsi EB. Abrupt changes in Potamogeton and Ruppia beds in a Mediterranean lagoon. Aquat Bot 2007; 87(3):181-8. doi: 10.1016/j.aquabot.2007.03.010 [Crossref] [ Google Scholar]
- Mateos R, Pérez-Correa JR, Domínguez H. Bioactive properties of marine phenolics. Mar Drugs 2020; 18(10):501. doi: 10.3390/md18100501 [Crossref] [ Google Scholar]
- Peng Y, Sun Q, Gao R, Park Y. AAK-2 and SKN-1 are involved in chicoric-acid-induced lifespan extension in Caenorhabditis elegans. J Agric Food Chem 2019; 67(33):9178-86. doi: 10.1021/acs.jafc.9b00705 [Crossref] [ Google Scholar]
- Harborne JB, Williams CA. Occurrence of sulphated flavones and caffeic acid esters in members of the fluviales. Biochem Syst Ecol 1976; 4(1):37-41. doi: 10.1016/0305-1978(76)90007-7 [Crossref] [ Google Scholar]
- Abd El-Hady HH, Daboor SM, Ghoniemy AE. Nutritive and antimicrobial profiles of some seagrasses from Bardawil Lake, Egypt. Egypt J Aquat Res 2007; 33(3):103-10. [ Google Scholar]
- Mau JL, Chao GR, Wu KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J Agric Food Chem 2001; 49(11):5461-7. doi: 10.1021/jf010637h [Crossref] [ Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 2002; 50(10):3010-4. doi: 10.1021/jf0115589 [Crossref] [ Google Scholar]
- Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 1998; 46(10):4267-74. doi: 10.1021/jf980366j [Crossref] [ Google Scholar]
-
Mejri H, Ouerghemi I, Aidi Wannes W, Haddada FM, Tlili N, Hammami M, et al. Phytochemical analysis, antioxidant, anticancer and anti-inflammatory activities of Lyciumeuropaeum fruits. Int J Environ Health Res. 2022:1-10. 10.1080/09603123.2022.2115469.
- Wannes WA, Marzouk B. Characterization of myrtle seed (Myrtus communis var baetica) as a source of lipids, phenolics, and antioxidant activities. J Food Drug Anal 2016; 24(2):316-23. doi: 10.1016/j.jfda.2015.11.001 [Crossref] [ Google Scholar]
- Zhao J, Fan X, Wang S, Li S, Shang S, Yang Y. Bromophenol derivatives from the red alga Rhodomelaconfervoides. J Nat Prod 2004; 67(6):1032-5. doi: 10.1021/np030546+ [Crossref] [ Google Scholar]
- Graham DR, Dixon RE, Hughes JM, Thornsberry C. Disk diffusion antimicrobial susceptibility testing for clinical and epidemiologic purposes. Am J Infect Control 1985; 13(6):241-9. doi: 10.1016/0196-6553(85)90024-0 [Crossref] [ Google Scholar]
- Baran EJ, Peterson LR, Finegold SM. Methods for testing antimicrobial effectiveness. In: Bailey and Scott’s Diagnostic Microbiology. 9th ed. St. Louis, Missouri: Mosby; 1994. p. 168-88.
- Ragupathi Raja Kannan R, Arumugam R, Grignon-Dubois M, Anantharaman P. Antioxidant activity of seagrasses of the Mandapam coast, India. Pharm Biol 2012; 50(2):182-7. doi: 10.3109/13880209.2011.591807 [Crossref] [ Google Scholar]
- Ragupathi Raja Kannan R, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalusacoroides (LF) Royle. Asian Pac J Trop Med 2010; 3(11):898-901. doi: 10.1016/s1995-7645(10)60216-7 [Crossref] [ Google Scholar]
- Ragupathi Raja Kannan R, Arumugam R, Thangaradjou T, Anantharaman P. Phytochemical constituents, antioxidant properties and p-coumaric acid analysis in some seagrasses. Food Res Int 2013; 54(1):1229-36. doi: 10.1016/j.foodres.2013.01.027 [Crossref] [ Google Scholar]
- Girija K, Parthiban C, Hemalatha A, Saranya C, Anantharaman P. Evaluation of antioxidant activities and preliminary phytochemical analysis of seagrasses Halodulepinifolia, Halophila ovalis and Syringodiumisoetifolium. Res J Phytochem 2013; 114:181-7. [ Google Scholar]
- Styshova ON, Popov AM, Artyukov AA, Klimovich AA. Main constituents of polyphenol complex from seagrasses of the genus Zostera, their antidiabetic properties and mechanisms of action. Exp Ther Med 2017; 13(5):1651-9. doi: 10.3892/etm.2017.4217 [Crossref] [ Google Scholar]
- Kalaivani P, Kavitha D, Amudha P. In vitro antioxidant activity and phytochemical composition of Syringodiumisoetifolium. Res J Pharm Technol 2021; 14(12):6201-6. doi: 10.52711/0974-360x.2021.01073 [Crossref] [ Google Scholar]
- Kagkli DM, Corich V, Bovo B, Lante A, Giacomini A. Antiradical and antimicrobial properties of fermented red chicory (Cichorium intybus L) by-products. Ann Microbiol 2016; 66(4):1377-86. doi: 10.1007/s13213-016-1225-3 [Crossref] [ Google Scholar]
- Yoa J, Moellering R. Antibacterial agents. In: Manual of Clinical Microbiology. Washington, DC: ASM; 1995. p. 1281-90.