Avicenna Journal of Clinical Microbiology and Infection. 9(3):119-123.
doi: 10.34172/ajcmi.2022.3393
Original Article
Evaluation of Anti-leishmanial Effect of Selenium Nanoparticles on Leishmania major Promastigotes In Vitro
Roghayeh Norouzi 1  , Farzaneh Mirzaei 2
, Farzaneh Mirzaei 2  , Abolghasem Siyadatpanah 3
, Abolghasem Siyadatpanah 3  , Seyed Jafar Adnani Sadati 4, 5, *
, Seyed Jafar Adnani Sadati 4, 5, * 
Author information:
1Department of Pathobiology, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran
2Department of Parasitology and Mycology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3Ferdows School of Paramedical and Health, Birjand University of Medical Sciences, Birjand, Iran
4Department of Microbiology Immunology and Parasitology, School of Medicine, Qom University of Medical Sciences, Qom, Iran
5Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran
Abstract
Background:
Cutaneous leishmaniasis (CL) remains a major health threatening disease in Iran and many countries around the world. Antimony compounds are currently used to treat CL. Due to the side effects and high resistance, the use of alternative therapies, especially the use of nanoparticles, has been considered by researchers. The aim of this study was to investigate the anti-leishmanial activity of selenium nanoparticles (SeNPs) on Leishmania major in vitro.
Methods: In this experimental study, the anti-leishmanial activity of the SeNPs was evaluated at concentrations of 1.25, 2.5, 5, 10, 25, 50, and 100 µg/mL at exposure times of 24, 48, and 72 hours on 106 live parasites. Then, the number of live parasites was counted by trypan blue using a neobar slide and light microscope (Hemocytometer method). Glucantime and distilled water were considered positive and negative controls, respectively. Then, 50% inhibitory concentration (IC50) values were calculated by SigmaPlotTM 13 software. All reactions were performed in triplicate, and the results were considered as average.
Results: The results of this study revealed that all concentrations of SeNPs have anti-leishmanial activity. The concentration of 100 µg/mL of SeNPs had the highest anti-leishmanial effect (100%) after 72 hours of exposure. Further, the IC50 content of SeNPs on L. major after 24, 48, and 72 hours was calculated to be 42.76, 34.53, and 22.69 µg/mL, respectively.
Conclusions: The results indicated that SeNPs in different concentrations has an inhibitory effect on the growth of L. major. However, further investigations are required to determine the efficacy of SeNPs in vivo.
Keywords: Selenium nanoparticles, Anti-leishmanial activity, Leishmania major, In vitro
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Norouzi R, Mirzaei F, Siadatpanah A, Adnani Sadati SJ. Evaluation of anti-leishmanial effect of selenium nanoparticles on Leishmania major promastigotes In Vitro. Avicenna J Clin Microbiol Infect. 2022; 9(3):130-134. doi:10.34172/ajcmi.2022.3393
Introduction
Leishmania strains cause a range of human diseases in tropical and subtropical regions around the world (1), which are manifested as cutaneous leishmaniasis (CL), muco-cutaneous leishmaniasis, and visceral leishmaniasis (2). The disease is caused by an obligatory intracellular protozoan called Leishmania of the order Kinetoplastida. Approximately 350 million people worldwide are at risk for the disease, and 2 million new cases are reported annually (3,4).Antimony compounds have been at the forefront for the treatment of this disease for decades, but these drugs have toxic properties on the liver, heart, and kidneys, require repeated injections, and cause resistance to the parasite (5-7). Therefore, it seems necessary to provide a more effective drug with fewer side effects and faster wound healing. Accordingly, researchers have turned to the use of nanoparticles to treat infectious diseases in recent years. Nanotechnology is currently being proposed as a new way to combat parasites and their diseases (8,9). In recent years, some nanoparticles (e.g., gold, selenium, silver, and sulfide) have been used to treat leishmaniasis.
According to some studies, selenium nanoparticles (SeNPs) are more effective in treating diseases than selenium itself. SeNPs with antioxidant and equally pro-oxidant properties provide different ways to explore different pathological conditions (10). SeNPs have different applications including acting as an antioxidant, improving learning, enhancing hair growth being anti-bacterial and anti-cancer aiding digestion, modulating the immune system, and improving reproductive and growth functions (11).Due to the different properties of these nanoparticles, this study was carried out to investigate the anti-leishmanial activity of SeNPs on Leishmania major parasite in vitro.
Materials and Methods
Parasite Culture
The standard strain of L. major promastigote (MRHO/IR/75/ER) was prepared from Pasteur Institute of Iran and cultured in Novy–MacNeal–Nicolle two-phase culture medium. Then, it was transferred to RPMI-1640 culture medium with antibiotics, including penicillin (100 units/mL), streptomycin (100 μg/mL), and 20% fetal bovine serum for mass propagation. Afterwards, flasks of culture medium were incubated at 25 ± 1°C and examined daily by reverse microscopy (invert).
Characterization of SeNPs
SeNPs nanopowder was purchased from Pishgaman Iran Nanomaterials Company. The average particle size of SeNPs was 30 nm, true density was 3.89 g/cm3, specific surface area was 30-50 m2/g, and purity was 99.95%. Its properties were determined by scanning electron microscopy (SEM, EM3200, KYKY Technology Development Ltd, Beijing, China) and transmission electron microscopy (TEM, Leo 906, Zeiss 100 KV, Germany). Concentrations of 1.25, 2.5, 5, 10, 25, 50, and 100 µg/mL were prepared from SeNPs by dissolving it in distilled water. The diluted extracts were sterilized by a 0.22 μm syringe filter with a diameter of 25 mm made by Sartreus, Germany.
Challenging the SeNPs With Leishmania Parasite and Determining the IC50
To investigate the lethal effect of SeNPs on promastigotes of L. major parasite, 106 parasites in logarithmic phase were exposed to concentrations of 1.25, 2.5, 5, 10, 25, 50, and 100 µg/mL. Then, 40 µg/mL glucantime and distilled water were used as positive and negative controls, respectively. Three flasks were considered for each concentration as well as for positive and negative controls. All groups were incubated at 25 ± 1°C, and after 24, 48, and 72 hours, the number of live parasites was counted by Trypan Blue using a neobar slide and light microscopy (Hemocytometer). The mortality rate for each of the different concentrations of SeNPs on the parasitic promastigotes was calculated by the following formula:
GI = (a-b / a)
where a is the number of live parasites in the negative control sample, and b is regarded as the number of live parasites in the sample containing SeNPs. Then, the amount of 50% inhibitory concentration (IC50) for the above extract was calculated using SigmaPlotTM 13 software.
Statistical Analyses
In this study, all data were entered into Excel software to report the results as mean and standard deviation, and SigmaPlotTM 13 software (Systat Software Inc., San Jose, CA, USA) was used to calculate IC50.
Results
The effect of different concentrations of SeNPs (1.25, 2.5, 5, 10, 25, 50, and 100 µg/mL) after 24, 48, and 72 hours of exposure to L. major promastigotes were studied in vitro. The IC50 content of SeNPs after 24, 48, and 72 hours on L. major was calculated to be 42.76, 34.53, and 22.69 µg/mL, respectively. The highest anti-leishmanial effect(100%) was observed at a concentration of 100 µg/mL after 72 hours of exposure. Table 1 presents the amount of Leishmania promastigotes being exposed to different concentrations of SeNPs after 24, 48, and 72 hours of incubation, and Table 2 illustrates the rate of inhibition of Leishmania promastigotes. Moreover, Figure 1 displays the average lethal percentage of SeNPs on L. major promastigotes, and Figures 2 and 3 represent the TEM and SEM images of SeNPs, respectively.
Table 1.
Number of Leishmania Promastigotes Against Different Concentrations of SeNPs After 24, 48, and 72 Hours of Incubation
|
Concentration(µg/mL)
|
24 Hours
|
48 Hours
|
72 Hours
|
| 1.25 |
2182 |
3554 |
5086 |
| 2.5 |
2088 |
3350 |
4596 |
| 5 |
1900 |
3064 |
4105 |
| 10 |
1759 |
2819 |
3247 |
| 25 |
1525 |
2206 |
2818 |
| 50 |
1102 |
1593 |
1715 |
| 100 |
187 |
123 |
0 |
| Positive control |
0 |
0 |
0 |
| Negative control |
2346 |
4086 |
6128 |
Note. SeNPs: Selenium nanoparticles.
Table 2.
The Effect of Different Concentrations of SeNPs on Promastigotes of Leishmania major Parasites After 24, 48 and 72 Hours of Incubation
|
Concentration(µg/mL)
|
24 Hours (%)
|
48 Hours (%)
|
72 Hours (%)
|
| 1.25 |
7 |
13 |
17 |
| 2.5 |
11 |
18 |
25 |
| 5 |
19 |
25 |
33 |
| 10 |
25 |
31 |
47 |
| 25 |
35 |
46 |
54 |
| 50 |
53 |
61 |
72 |
| 100 |
92 |
97 |
100 |
| Positive control |
100 |
100 |
100 |
| Negative control |
0 |
0 |
0 |
Note. SeNPs: selenium nanoparticles.
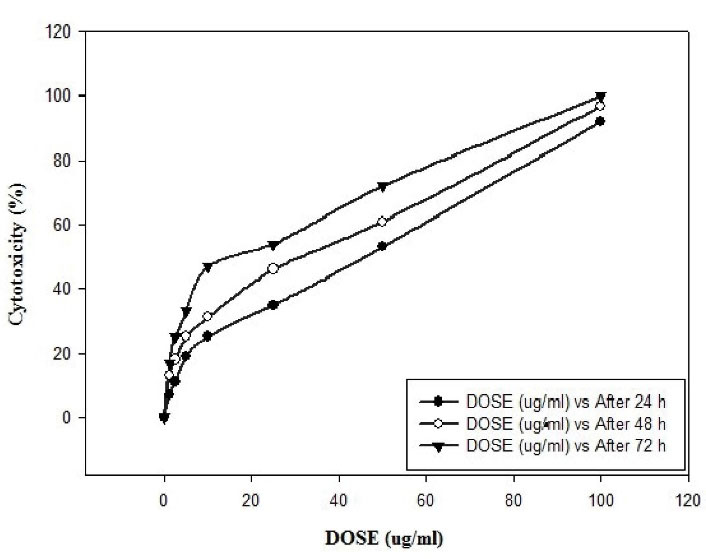
Figure 1.
Mean Mortality of SeNPs on Promastigotes of Leishmania major. Note. SeNPs: Selenium nanoparticles
.
Mean Mortality of SeNPs on Promastigotes of Leishmania major. Note. SeNPs: Selenium nanoparticles
Figure 2.
TEM Image of SeNPs in 25 nm. Note. TEM: Transmission electronic microscopy; SeNPs: Selenium nanoparticles
.
TEM Image of SeNPs in 25 nm. Note. TEM: Transmission electronic microscopy; SeNPs: Selenium nanoparticles

Figure 3.
SEM Image of SeNPs in 100 nm. Note. SEM: Scanning electronic microscopy;SeNPs: Selenium nanoparticles.
.
SEM Image of SeNPs in 100 nm. Note. SEM: Scanning electronic microscopy;SeNPs: Selenium nanoparticles.
Discussion
According to the World Health Organization (WHO), approximately 14 million people in Africa, Asia, Europe, and the US are directly affected by leishmaniasis (12). Antimony is recommended as the main drug to treat leishmaniasis, but there are limitations such as high costs, side effects, repeated injections, and ineffectiveness; therefore, the preparation of a new anti-leishmanial drug is one of the most essential needs. Therefore, the aim of this study was to investigate the anti-leishmanial activity of SeNPs on L. major in vitro. The highest lethality (100%) was observed at a concentration of 100 µm/mL after 72 hours of exposure. One of the mechanisms by which selenium exerts its beneficial effects on the health is through selenoprotein. In addition, selenium replaces sulfur in methionine in the form of selenomethionine, which can be incorporated into non-specific proteins (13).
Mayelifar et al incubated L. major promastigotes with silver NPs for 1 hour and then applied 8 electrical pulses with an electroporation device. The results showed that the presence of silver NPs and the simultaneous application of electrical pulses lead to a significant decrease in parasite survival (14). Akhzari et al used activated melittin along with liposomes and albumin NPs to treat leishmaniasis wounds caused by L. major, and they found that the size of wounds in this group significantly reduced (15). Further, a study by Casa et al revealed that albumin NPs containing amphotericin B reduced the toxicity of the drug and reduced the tissue toxicity of the drug to the tissues of the liver, spleen, heart, and kidney as well (16). In another study, Haddad et al reviewed the anti-leishmaniasis effect of curcumin-laden chitosan NPs on L. major and Leishmania infantum under in vitro conditions, and counting the number of parasites indicated that the synthesized NPs have a favorable effect in growth inhibition (17). The results of another study by Torabi et al demonstrated that gold NPs reduce the number of L. major amastigotes in Leishmaniasis wounds and reduce the mortality rate of mice (18).
The effects of SeNPs on L. infantum were investigated by Soflaei et al, and IC50 NPs against amastigotes and promastigotes of L. infantum were reported to be 10 and 25 μg/mL, respectively (19). Excretory-secretory L. major on macrophage apoptosis represented that chitosan can increase the ability of infected macrophages to remove parasites by reducing apoptosis (20). Sazgarnia et al surveyed the effect of gold NPs and microwave radiation on the survival of L. major promastigotes and amastigotes, concluding that the presence of gold NPs during microwave irradiation is more lethal to promastigotes and amastigotes; therefore, it could be suggested as a new approach for treating leishmaniasis (21). In another study, Jameii et al (22) examined the anti-leishmaniasis effect of selenium and silver NPs on L. major wound healing and found that the diameter of nano-selenium group wounds does not show a significant difference with the control group, while the diameter of nano-silver group wounds exhibited a significant difference with the control group, which is larger than the group treated with glucantime (positive control).
In the study by Baiocco et al which evaluated the lethal effect of silver NPs against visceral leishmaniasis, the results displayed that, compared to antimony compounds, silver NPs have a greater effect on Leishmania mortality (23). In a review study, Elmi et al exhibited that NPs of silver, gold, chitosan, and metal oxides have a lethal or inhibitory effect on Leishmania (24). In another study, Jebali and Kazemi examined the effects of NPs of silver, gold, titanium dioxide, zinc dioxide, and magnesium dioxide under ultraviolet light, infrared, and dark conditions, observing the highest anti-leishmanial activity for silver NPs; further, both ultraviolet and infrared light were found to have anti-leishmaniasis activity (25). El-Khadragy et al biosynthesized silver NPs using Moringa oleifera leaf extract and investigated the anti-leishmaniasis activity of these NPs in a mouse model with L. major infection. They concluded that treatment with biosynthesized silver NPs using M. oleifera extract have a higher and faster clinical effect than treatment with standard pentavalent antimony possibly due to increased antioxidant activity (26). Different results obtained from various studies are due to different NPs and the differences in measurement units such as micrograms, milligrams, and the like.
The results of this study indicated that all concentrations of SeNPs have anti-leishmanial activity, and a concentration of 100 µg/mL of SeNPs has the highest anti-leishmanial effect (100%) at 72 hours of exposure. However, further in vivo conditions are required to determine the performance of SeNPs.
Acknowledgments
This manuscript is a part of the thesis (No. IR.MUQ.REC.1400.185) approved by the School of Medicine, Qom University of Medical Sciences, Qom, Iran. Authors of this study appreciate Qom University of Medical Sciences for their cooperation and financial support.
Conflict of Interests
The authors declared no conflict of interests.
Ethical Approval
This study was approved by the research committee of Qom University of Medical Sciences (IR.MUQ.REC.1400.185).
References
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M. The genome of the kinetoplastid parasite, Leishmania major. Science 2005; 309(5733):436-42. doi: 10.1126/science.1112680 [Crossref] [ Google Scholar]
- Mohammadi Azni S, Nokandeh Z, Khorsandi A, Sanei Dehkordi AR. Epidemiology of cutaneous leishmaniasis in Damghan district. Journal Mil Med 2010; 12(3):131-5. [ Google Scholar]
- Savoia D. Recent updates and perspectives on leishmaniasis The Journal of Infection in Developing Countries. J Infect Dev Ctries 2015; 9(06):588-96. doi: 10.3855/jidc.6833 [Crossref] [ Google Scholar]
- Katakura K. Molecular epidemiology of leishmaniasis in Asia (focus on cutaneous infections). Curr Opin Infect Dis 2009; 22(2):126-30. doi: 10.1097/QCO.0b013e3283229ff2 [Crossref] [ Google Scholar]
- Mirzaei F, Norouzi R, Siyadatpanah A, Mitsuwan W, Nilforoushzadeh M, Maleksabet A. Butanol fraction of Kelussia odoratissima Mozaff inhibits the growth of Leishmania major promastigote and amastigote. World 2020; 10(2):254-9. doi: 10.36380/scil.2020.wvj33 [Crossref] [ Google Scholar]
- Firooz A, Mortazavi H, Khamesipour A, Ghiasi M, Abedini R, Balighi K. Old world cutaneous leishmaniasis in Iran: clinical variants and treatments. J Dermatolog Treat 2021; 32(7):673-83. doi: 10.1080/09546634.2019.1704214 [Crossref] [ Google Scholar]
- Sampaio RN, Lucas IC, Costa Filho AV. The use of azythromycin and N-methyl glucamine for the treatment of cutaneous Leishmaniasis caused by Leishmania (Leishmania) amazonensis in C57BL6 mice. An Bras Dermatol 2009; 84(2):125-8. doi: 10.1590/s0365-05962009000200004 [Crossref] [ Google Scholar]
- Khan MA, Maruno M, Khaskhely NM, Ramzi ST, Hosokawa A, Uezato H. Inhibition of intracellular proliferation of Leishmania parasites in vitro and suppression of skin lesion development in BALB/c mice by a novel lipid A analog (ONO-4007). Am J Trop Med Hyg 2002; 67(2):184-90. doi: 10.4269/ajtmh.2002.67.184 [Crossref] [ Google Scholar]
- Underwood C, Van Eps A. Nanomedicine and veterinary science: The reality and the practicality. Vet J 2012; 193(1):12-23. doi: 10.1016/j.tvjl.2012.01.002 [Crossref] [ Google Scholar]
- Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol 2006; 69(5):485-92. doi: 10.1007/s00253-005-0179-3 [Crossref] [ Google Scholar]
- Tapiero H, Townsend D, Tew K. The antioxidant role of selenium and seleno-compounds. Biomed Pharmacother 2003; 57(3-4):134-44. doi: 10.1016/s0753-3322(03)00035-0 [Crossref] [ Google Scholar]
- Igbineweka O, Aghedo F, Idusuyi O, Hussain N. Evaluating the efficacy of topical silver nitrate and intramuscular antimonial drugs in the treatment of cutaneous leishmaniasis in sokoto, Nigeria. Afr J Clin Exp Microbiol 2012; 13(2):90-7. doi: 10.4314/ajcem.v13i2.6 [Crossref] [ Google Scholar]
- Behne D, Kyriakopoulos A. Mammalian selenium-containing proteins. Annu Rev Nutr 2001; 21:453. doi: 10.1146/annurev.nutr.21.1.453 [Crossref] [ Google Scholar]
- Mayelifar K, Sazgarnia A, Yadegari Dehkordi S, Eshghi H, Attaran N, Soudmand Salarabadi S. Inhibitory effect of electroporation and silver nanoprticles on the growth of leishmania major promastigotes: Influence of pulse duration. Medical Journal of Mashhad University of Medical Sciences 2013; 56(4):247-54. doi: 10.22038/mjms.2013.1762 [Crossref] [ Google Scholar]
- Akhzari S, Nabian S, Shayan P, Taheri M. Evaluation of the effect of liposome carriers and albumin nanoparticles containing activated melittin on inhibiting the growth of Leishmania major amastigote in vivo. Journal of Ilam University of Medical Sciences 2022; 29(6):36-47. [ Google Scholar]
- Casa D, Scariot D, Khalil N, Nakamura C, Mainardes R. Bovine serum albumin nanoparticles containing amphotericin B were effective in treating murine cutaneous leishmaniasis and reduced the drug toxicity. Exp Parasitol 2018; 192:12-8. doi: 10.1016/j.exppara.2018.07.003 [Crossref] [ Google Scholar]
- Haddad A, Delavari M, Arbabi M. Evaluation of anti-leishmaniasis activity of curcumin-loaded chitosan nanoparticles on Leishmania major and L infantum in vitro. Feyz 2021; 25(4):1040-6. [ Google Scholar]
- Torabi N, Mohebali M, Shahverdi AR, Rezayat SM, Edrissian GH, Esmaeili J. Nanogold for the treatment of zoonotic cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an animal trial with methanol extract of Eucalyptus camaldulensis. Journal of Pharmaceutical & Health Sciences 2011; 1:15-8. [ Google Scholar]
- Soflaei S, Dalimi A, Abdoli A, Kamali M, Nasiri V, Shakibaie M. Anti-leishmanial activities of selenium nanoparticles and selenium dioxide on Leishmania infantum. Comp Clin Path 2014; 23(1):15-20. doi: 10.1007/s00580-012-1561-z [Crossref] [ Google Scholar]
- Feizabadi E, Zavaran Hosseini A, Soudi S, Khosrojerdi A. Studying the role of chitosan nanoparticle loaded with Leishmania major Secretory and excretory antigens on the number of apoptotic macrophages in parasite sensitive mouse. Daneshvar Medicine: Basic and Clinical Research Journal 2020; 26(6):9-18. [ Google Scholar]
- Sazgarnia A, Taheri AR, Soudmand S, Parizi AJ, Rajabi O, Darbandi MS. Antiparasitic effects of gold nanoparticles with microwave radiation on promastigotes and amastigotes of Leishmania major. Int J Hyperthermia 2013; 29(1):79-86. [ Google Scholar]
- Jameii F, Dalimi AA, Karimi M, Ghaffarifar F. Healing effect comparison of selenium and silver nanoparticles on skin leishmanial lesions in mice. Avicenna J Clin Med 2015; 22(3):217-223. doi: 10.3109/02656736.2012.758875 [Crossref] [ Google Scholar]
- Baiocco P, Ilari A, Ceci P, Orsini S, Gramiccia M, Di Muccio T. Inhibitory effect of silver nanoparticles on trypanothione reductase activity and Leishmania infantum proliferation. ACS Med Chem Lett 2011; 2(3):230-3. doi: 10.1021/ml1002629 [Crossref] [ Google Scholar]
- Elmi T, Gholami S, Fakhar M, Azizi F. A review on the use of nanoparticles in the treatment. Journal of Mazandaran University of Medical Sciences 2013; 23(102):126-33. [ Google Scholar]
- Jebali A, Kazemi B. Nano-based antileishmanial agents: a toxicological study on nanoparticles for future treatment of cutaneous leishmaniasis. Toxicology in vitro 2013; 27(6):1896-904. [ Google Scholar]
- El-Khadragy M, Alolayan EM, Metwally DM, El-Din MFS, Alobud SS, Alsultan NI. Clinical efficacy associated wi134th enhanced antioxidant enzyme activities of silver nanoparticles biosynthesized using Moringa oleifera leaf extract, against cutaneous leishmaniasis in a murine model of Leishmania major. Int J Environ Res Public Health 2018; 15(5):1037. doi: 10.3390/ijerph15051037 [Crossref] [ Google Scholar]