Avicenna Journal of Clinical Microbiology and Infection. 9(1):31-40.
doi: 10.34172/ajcmi.2022.06
Review Article
Current Perspectives on Viable but Non-culturable Bacteria in Food Safety and Public Health
Sharareh Shamloei 1  , Ali Nabavi-Rad 2
, Ali Nabavi-Rad 2  , Habibollah Nazem 1, Abbas Yadegar 2, *
, Habibollah Nazem 1, Abbas Yadegar 2, * 
Author information:
1Department of Biochemistry, Payame Noor University, Isfahan, Iran
2Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
*
Corresponding author: Abbas Yadegar, PhD; Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Shahid Arabi Avenue, Yemen Street, Velenjak, Tehran, Iran, Tel: +98-21-22432518, Fax: +98-21-22432527, Email:
a.yadegar@sbmu.ac.ir,
babak_y1983@yahoo.com
Abstract
The viable but non-culturable (VBNC) state is defined as an adaptive mechanism for microorganisms adjusting to stressful conditions. Although VBNC bacteria are alive and metabolically active, they are unable to grow on routine culture media. Nevertheless, the potential capacity of VBNC pathogens to retain virulence activity and further resuscitate into the culturable state in favorable conditions constitutes a major hazard to food safety and public health. Food processing, transformation, and storage, as well as non-thermal techniques, can provoke pathogens toward VBNC induction. The distinct characteristic of VBNC bacteria led to the emergence of novel culture-independent techniques to prevent the misinterpretation of food safety. To deepen our knowledge of the molecular aspect of the VBNC state, several mechanism-oriented studies investigated the metabolic activity of VBNC bacteria and their correlation with different stressful conditions. This review aims to discuss the molecular mechanisms and genomic factors underlying the induction and resuscitation of the VBNC state. The study will further highlight innovative detection methods to provide a comprehensive perspective for future studies in the emerging fields of research concerning VBNC state, food safety, and public health.
Keywords: VBNC, Food safety, Public health, Waterborne pathogen, Foodborne bacteria
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Shamloei S, Nabavi-Rad A, Nazem H, Yadegar A. Current perspectives on viable but non-culturable bacteria in food safety and public health. Avicenna J Clin Microbiol Infect. 2022; 9(1):31-40. doi:10.34172/ajcmi.2022.06
Background
Escherichia coli and foodborne pathogen Vibrio cholera were the first bacteria demonstrated to enter the viable but non-culturable (VBNC) state, forcing the researchers to revisit some fundamental notions regarding pathogen survival and viable counting methods (1). Stressful conditions lead to a biologically inactive form of life termed the VBNC state that is undetectable by typical bacteriological media culture. However, VBNC bacteria preserve their membrane integrity, low levels of gene expression, and traceable metabolic functionality (2). The presence of detectable metabolic activity distinguishes the VBNC state from dormancy, which is a convertible characteristic of a metabolic shutdown. This state will further allow bacteria to utilize nutrients and maintain their genome and virulence activity. Thus, bacterial capacity for colonization and growth can be recovered by the resuscitation process under certain favorable conditions (3). The regain of virulence capacity following the VBNC state might be independent of the resuscitation ability of bacteria and relies on both factors stimulating the VBNC state or the resuscitation process. Furthermore, the results of recovery methods are strain-specific and not all VBNC strains can undergo resuscitation (4).
Foodborne disease, including diarrhea, nausea, vomiting, and kidney and liver dysfunction, has raised a substantial concern in every region globally. Traditional culture-based strategies for quantifying microorganisms and determining the viability of bacterial cells through colony formation have faced challenges owing to the presence of dormancy and VBNC state (5). The potential capacity of VBNC bacteria to cause infection emphasizes the great significance of the VBNC state in food safety and public health (6). The frequent exposure of food to a variety of environmental conditions may provide adequate stress for VBNC induction. Moreover, the previous detection of VBNC bacteria during the food process and the fact that VBNC pathogens can maintain toxin production ability and recover within the human body, have attracted widespread attention to VBNC in the food industry (7,8). Several microbial pathogens can enter the VBNC state in a diversity of food products, including vegetables, fruit juice (9), chicken (10), and seafood (11). Further, not only VBNC bacteria have been detected in drinking water, but also it is suggested that conventional disinfection methods in water treatment systems can stimulate VBNC induction (12). It might be more practical to grasp an ecological perspective regarding the VBNC state and consider it as a typical defensive strategy for non-spore-forming bacteria.
This review focuses on discussing the characteristics and persistence of VBNC bacteria and their importance in food safety and public health. Accordingly, the most recent studies are considered to further highlight recent advances in detecting the techniques of VBNC cells to provide a perspective for future investigations in this dynamic field of research.
VBNC State
Induction of VBNC State
The main characteristic of the VBNC state is the ability of bacterial cells to tolerate stressful environmental conditions. Although large-scale studies are yet to determine the comprehensive impacts of different conditions on VBNC induction, several physical and chemical stress factors are suggested to disturb the natural balance of bacterial growth conditions and lead the bacteria to induce the VBNC state (13). Starvation (14), extreme or low temperature (15), osmotic (16) and oxidative stress (17), UV irradiation (18), pulsed light, pulsed electric field (19), heavy metal (20), and acute alteration of pH or salinity (21), as well as food processing and preservation strategies (22), are the main uncovered factors stressing the bacteria to VBNC state as shown in Figure 1.
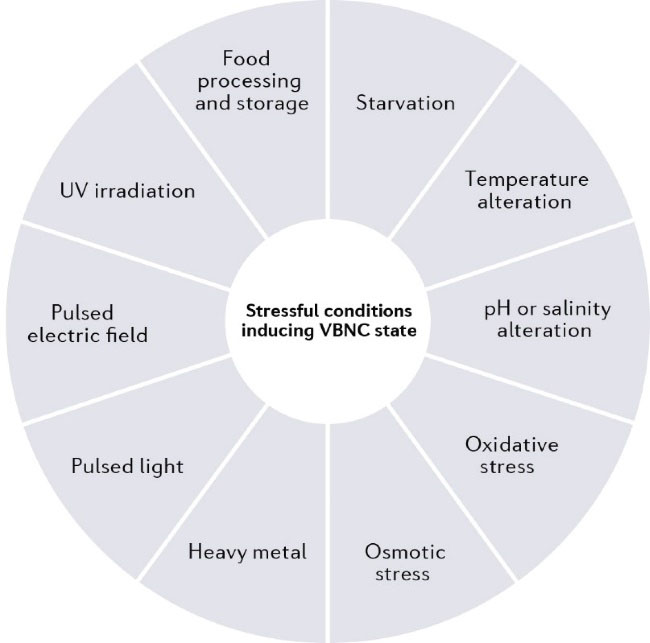
Figure 1.
Main Stress Factors Stimulating Particular Bacteria Toward VBNC Induction.
.
Main Stress Factors Stimulating Particular Bacteria Toward VBNC Induction.
Although stress conditions for VBNC induction have been well studied, the molecular mechanism and genetic control underlying the VBNC state have received little attention. Due to the presence of a vast diversity of VBNC bacterial species, regulatory strategies are suggested to be strain-specific. Several studies uncovered the involvement of different mechanisms in VBNC induction, including RelA promotion and ToxR reduction in V. cholera (23), RpoS, MarA, YgfA, RelE (24), VapC, and HipA upregulation, and VapB downregulation in E. coli (25), and ClpP accumulation in Legionella pneumophila (26). In stress conditions, Campylobacter jejuniencodes high-temperature response protein B, polyphosphate kinase (PPK), and GltD and GlnA, which encode the required proteins for glutamine and glutamate production (27). The key enzymatic activity of PPK is the modulation of polyphosphate accumulation that mediates stress response, survival, colonization, and the virulence of several bacteria. Given that the PPK mutant C. jejuni demonstrated a reduced capacity of VBNC formation, PPK regulatory genes might be potential therapeutic targets in ceasing C. jejuni reinfection (28). Furthermore, a stringent response following amino acid starvation leads to RelA-dependent ppGpp synthesis, which might provoke VBNC formation. Additionally, it has been indicated that E. coli mutants on ppGpp production exhibited a reduction in VBNC induction (29). It is also reported that in Salmonella enterica, deletion in the C-terminal domain of ClpX will delay the induction of the VBNC state (30).
Characteristics of VBNC Bacteria
Despite the loss of culturability on conventional media, VBNC bacteria retain the absorptive capacity and express particular genetic materials; thereby, it is suggested that VBNC cells have several common properties with culturable cells. Nonetheless, a number of physiological conversions such as deceleration in nutrient absorption, protein synthesis, and macromolecular metabolism occur through VBNC induction (31). Collectively, major variations underlying the VBNC state include cellular morphology, metabolic activity, stress toleration, and genetic regulation.
The alteration in cellular morphology during the transition toward the VBNC state leads to cell dwarfing and rounding. Additionally, a reduction in the cell size is a possible strategy in VBNC cells to restrict the energy requirement (32). In contrast to Gram-negative bacteria, some Gram-positive bacteria such as Enterococcus faecalis demonstrated an expansion in the cell size (33). It is further indicated that Gram-positive bacteria are more susceptible to the induction of the VBNC state (34). The VBNC cells of Helicobacter pylori represented a coccoid appearance while preserving their virulence capacity with limited metabolic activity (35). Rod-shaped Vibrio parahaemolyticus in the exponential phase also transforms to a cocci form in the VBNC state and becomes more flexible (36). Nevertheless, similar morphological changes are reported in non-VBNC cells, indicating the unreliability of cellular appearance to reveal the VBNC state (37). Considering the major part of the cell wall in shaping cellular morphology, the aforementioned changes might be the consequences of cell wall conversion. It is proposed that resuscitation-promoting factors (Rpfs) can recover the culturable form of bacteria by lysing cell wall peptidoglycan (38). Moreover, the cell wall of E. faecalis was reported to be less prone to physical interruption, and the chemical analysis of its peptidoglycan exhibited an enhancement in cross-linking and lipoteichoic acids (33). In addition to the cell wall, VBNC cells might further modify membrane composition to preserve membrane fluidity with unsaturated and short-chain fatty acids. The prevention of fatty acid production in Vibrio vulnificus is associated with reduced survival and VBNC induction (39).
Modification of metabolic activity in VBNC cells is responsible for alterations in the synthesis of proteins, fatty acids, and peptidoglycans, which eventually determines the composition of cell walls and membranes (32). In this state, following the reduction in the concentration of nucleic acid, a dense cytoplasm will surround the compact chromosomal DNA (40). Despite the reduction in total energy production in the VBNC state, the promotion in electron transfer and adenosine triphosphate (ATP) level can provide the limited energy required to preserve cellular homeostasis (41). Furthermore, some studies indicated an enhancement in protein production as protein aggregation is a defensive strategy that facilitates bacterial adaptation to environmental stress and antibiotic intervention through VBNC induction (42). However, several metabolic pathways maintain their activity during VBNC induction. In Rhodococcus biphenylivorans, esterase, esterase lipase, leucine arylamidase, valine arylamidase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, α-glucosidase, and β-glucosidase are reported with similar activities in culturable and non-culturable forms (43). Additionally, Bacillus stratosphericusis demonstrated by XTT cell viability assay to preserve its respiration, which is a pivotal metabolic process for cell survival (44).
Resuscitation of VBNC Bacteria
Salmonella enteritidis was the first bacterial model used to represent resuscitation from the VBNC state, in which the bacteria recovered culturability following the addition of heart infusion broth (45). Resuscitation is a remarkable capacity of the VBNC state, giving a reversible characteristic to VBNC cells to regain normal levels of physiological and metabolic activities (46). A number of strategies are developed for detecting true resuscitation and excluding the regrowth of culturable bacteria such as the extreme dilution of VBNC cells with artificial seawater to remove nutrients and prevent the presence of culturable bacteria (47). Nonetheless, there is only a period of time following VBNC induction termed as “resuscitation window”, in which the bacteria can undergo resuscitation. Thereafter, the number of resuscitation will significantly reduce and bacteria die ultimately (37). The term the resuscitation window mostly relies on bacterial species and incubation period, as well as the conditions of entering and recovering from the VBNC state (48).
Resuscitation from the VBNC state requires particular conditions, including the removal of stress conditions, addition of rich nutrients, stabilization of osmotic pressure, degradation of hydrogen peroxide, and the presence of a host (49). Arcobacter butzleri can enter this state in seawater at 4 °C and recover the normal state within 9 days of nutrient addition (50). Although the vital nutrients for resuscitation are poorly understood, it is suggested that a combination of methionine, glutamine, threonine, serine, and asparagine can accelerate E. coli recovery from the VBNC state (51). It is further indicated that owing to the reduction in superoxide dismutase functionality in the VBNC state, the addition of reactive oxygen species scavengers such as pyruvate can stimulate resuscitation (52). Moreover, biological stimuli are natural promoters for resuscitation, in which particular VBNC species including but not limited to E. coli, Francisella tularensis, and C. jejuni can resuscitate and grow in the presence of eukaryotic cells, mammal cells, and embryonated eggs, respectively (49). In addition to environmental inducers, the cell-free supernatant of bacteria can promote resuscitation probably by quorum sensing autoinducer AI-2 (53). A Micrococcus luteus cytokine Rpf is also reported to induce the resuscitation of the specific species of the Mycobacterium genus (54). Nevertheless, due to the presence of a vast variety of bacterial species and stimulus factors, the molecular mechanism of the recovering process is far from full elucidation.
Detection Methods for VBNC Bacteria
Distinct characteristics of VBNC bacteria have made the conventional microbiological detection techniques inauthentic. Hence, the unique aspect of this state, as well as the importance of VBNC bacteria in food safety and public health encouraged the scientists to the development of novel detection methods for VBNC cells. As a result, a number of innovative strategies are established to detect VBNC bacteria mainly based on their metabolic activity (Table 1).
Table 1.
Different Detection Methods for VBNC Bacteria
|
Method
|
Platform
|
Elements
|
Advantages
|
Limitations
|
| Direct viable count |
Based on bacterial elongation (55) |
Antibiotics such as nalidixic acid, aztreonam, and ciprofloxacin (55) |
Simple protocol, direct examination (55) |
Low sensitivity (56) |
| CTC staining |
Based on bacterial respiratory reaction (57) |
CTC (57) |
Simple protocol and typical equipment (58) |
Low sensitivity and CTC toxicity (59) |
| LIVE/DEAD®BacLightTM assay |
Based on the integrity of bacterial membrane (60) |
SYTO 9, PI (61) |
High sensitivity, accuracy, and rapid results (62) |
Different affinities of SYTO 9 with different states of cells, not specific (62) |
| Molecular methods |
Based on the presence of bacterial mRNA (63) |
RT-qPCR (64) |
High sensitivity, accuracy, specific, and rapid results (63) |
Selection of suitable target genes (65) |
Note. CTC: 5-cyano-2,3-ditolyl-tetrazolium chloride; PI: Propidium iodide; RT-qPCR: Reverse transcription-quantitative polymerase chain reaction; SYTO 9: A blue-excited, green-fluorescent nucleic acid stain that is cell-permeable; VBNC: Viable but non-culturable.
Direct Viable Count
The direct viable count (DVC) of bacterial cells was first proposed based on the recognition of elongated cells by direct microscopy or epifluorescence microscopy (66). This method is based on the sensitivity of viable bacteria to antibiotics such as nalidixic acid, aztreonam, and ciprofloxacin, which lead VBNC cells toward elongation, while dead cells demonstrate no alteration in this regard. Consequently, nalidixic acid as a DNA replication inhibitor may not be convenient in this method (55); however, aztreonam is suitable for Cytophaga allerginae and Serratia marcescens (67), while ciprofloxacin is suitable for Listeria monocytogenes (68). Considering different bacterial responses to antibiotic treatments regarding their species and nutrient uptake capacity, this method is significantly limited, thereby it is not a commonly used technique (56).
5-Cyano-2,3-ditolyl-tetrazolium Chloride (CTC) Staining Method
The electron transportation of the bacterial respiratory chain can reduce CTC to CTC-formazan, which is detectable by the epifluorescence microscopic technique (57). This method is frequently applied with DVC to give a comprehensive enumeration of viable cells as DVC demonstrates elongation and CTC staining detects cellular respiratory activity (69). Although the simple protocol and typical equipment of CTC staining resulted in a widespread application, it cannot detect extremely low levels of metabolic activity (58). Furthermore, the toxicity of CTC staining can suppress bacterial metabolic activity and thereby underestimates the authentic count of viable cells (59).
LIVE/DEAD® BacLight TM Assay
The LIVE/DEAD BacLight is a commercially available kit, consisting of two DNA-binding dyes, the green fluorescent dye, the red fluorescent stain, namely, propidium iodide (PI), and SYTO 9 (61). While the SYTO 9 can penetrate intact, as well as damaged cell membranes and mark all cells, PI can only mark damaged cell membranes and therefore distinguish viable from injured and membrane-permeabilized cells (60). Under fluorescence microscopy or flow cytometry, VBNC bacteria would appear green, while the injured or dead cells would appear red. Although this method established more accuracy in counting VBNC cells than DVC and CTC staining methods, several factors such as the bleaching effect of green fluorescent staining, different affinities of viable and dead bacteria with SYTO 9, and background fluorescence should be considered when performing LIVE/DEAD assay (62).
Molecular Methods
Recent advances in molecular techniques have facilitated the detection of gene expression and metabolic activity by the reverse transcription-quantitative polymerase chain reaction (RT-qPCR), quantitative real-time PCR (qRT-PCR), and loop-mediated isothermal amplification. The incapability of the PCR to determine DNA from viable or dead cells has led to the application of DNA inhibitors such as propidium monoazide (PMA) previous to DNA extraction, which penetrates through the damaged membrane and prevents DNA amplification (70). However, a limitation of the PMA-qPCR method is the presence of dead cells with intact membranes such as particular UV-killed bacteria (71). Hence, bacterial mRNA, owing to its short half-life within the cell, is suggested as an accurate marker for determining gene expression and bacterial viability (63). Although a continuously expressed gene is targeted based on bacterial species, housekeeping and virulence genes are primary markers to be detected by the RT-qPCR and thereby determining cell viability (64). Nonetheless, the limitation of RT-qPCR is the selection of suitable target genes, which are transcribed independent of physiological alterations (65).
VBNC Pathogens
Following the discovery of VBNC cells in 1982, several researchers expressed the intention of seeking different bacterial species capable of entering the VBNC state. Further, some studies have reviewed and presented various lists of human pathogens reported to induce the VBNC state (13,37,72,73), which are further discussed as follows.
Foodborne Pathogens
The increasing population of VBNC pathogens in a vast majority of environments has a substantial impact on food safety. The frequent exposure of food to a narrow diversity of environments throughout processing, transference, and storage can provide several opportunities for VBNC induction. Freezing or refrigeration, cooking, fermentation, and additive addition (e.g., salt) are different types of stress conditions to induce the VBNC state in foodborne pathogens (74). The addition of acidic additives such as citric acid and acetic acid during the food process can lead to VBNC induction in Staphylococcus aureus. It is reported that the presence of sufficient nutrients in the acidic condition can stimulate S. aureus to induce the VBNC state, whereas the acidic condition with nutrient starvation can damage and eliminate the bacteria (75,76). Furthermore, various non-thermal techniques, including ultrasonication, cold plasma technology, irradiation, supercritical technology, pulsed electric field, high hydrostatic pressure, pulsed ultraviolet technology, and ozone are used in the sanitization of food products without heat application (77). Several studies demonstrated the formation of the VBNC state for pathogen microorganisms by non-thermal technologies such as electrolyzed water (78), high-pressure CO2 (41), pulsed light inactivation (79), thermosonication (18), and non-thermal plasma (80).
The potential capacity of several VBNC pathogens to preserve the expression of virulence factors and toxins has caused major concerns in food safety (81). Multiple studies indicated the development of disorders in the host following inoculation with VBNC pathogens. The injection of VBNC V. vulnificus into animal models can cause mortality through in vivo resuscitation (82). The isolation of VBNC V. cholerae from rabbit intestinal loops represented the enterotoxigenicity of this VBNC pathogen (83). Moreover, a rabbit ileal loop assay was applied to exhibit the preservation of enterotoxin production in VBNC E. coli H10407 (84). The detection of VBNC bacteria in mouse and human urine samples, previously considered sterile, further revealed the hazardous characteristic of VBNC pathogens in developing infectious diseases (85).
Waterborne Pathogens
Obligate and opportunistic pathogens are the two major groups of waterborne pathogens that can cause diseases in the host regardless of the health condition and in a subgroup of sensitive individuals, respectively. These microorganisms can grow as primary colonizers by attaching to solid surfaces and forming multiple- or single-strain biofilms. Similarly, they might multiply as secondary colonizers by integrating with the preformed biofilm (86). Microorganisms within mature biofilms tend to morphological heterogeneity by inducing the VBNC state and persisted formation in stressful conditions. Consequently, the flow and pressure in the drinking water distribution system can detach the biofilm and facilitate the transmission of waterborne VBNC pathogens (87). The major risk for developing waterborne diseases is related to the consumption of fecal contaminated water such as in the case of discharging wastewater in fresh water and coastal seawater (88). S. enterica, Shigella spp., V. cholerae, E. coli, Yersinia enterocolitica, Campylobacter spp., and H. pylori are among major water-related pathogens (86). Primary disorders following infection with waterborne pathogens are cholera, bacillary dysentery, gastroenteritis, typhoid fever, salmonellosis, and acute diarrhea (88).
A major threat to human health is the development of gastrointestinal diseases such as peptic ulcers and gastric cancer following the H. pylori infection. This pathogen can survive unfavorable conditions in drinking water through biofilm formation and VBNC induction (89). Although VBNC induction leads to a reduction in metabolic activity, the coccoid form of H. pylori can colonize and stimulate inflammation within the host gastric mucosa by expressing virulence factors (90). As a result, researchers have declared that the acquisition and transmission of H. pylori are closely related to the quality of drinking water (91).
Persistence of VBNC State
Virulence of VBNC Pathogens
Despite the contrary reports regarding the virulence capacity of VBNC pathogens, it is suggested that microorganisms can preserve their virulence through the VBNC state and cause infection following resuscitation. Vibrio harveyi is reported to cease the expression of the hemolysin gene in the VBNC state, while the resuscitated bacteria are lethal and their injection into zebrafish is reported to cause death within a week (92). As regards V. vulnificus, it is demonstrated that its virulence capacity is reduced to some extent by VBNC induction. However, non-culturable V. vulnificus can resuscitate within the mouse and thereby lead to the fatal infection of the host (82).
Several studies further investigated the correlation between the stress condition and regulation of virulence genes as some virulence genes are required for the tolerance and survival of bacteria within the host. The L. monocytogenes LO28 mutation in the induction of the acid tolerance response represented diminished virulence in a murine model (93). The acid adaptation of L. monocytogenes strains by short-term exposure of bacteria to low pH may increase the expression of virulence genes such as inlA, opuC, and sigB; consequently, it may promote the survival and invasiveness of these microbes (94). Additionally, the combination of different stress conditions can influence the growth and virulence of the pathogens; therefore, extreme alterations in temperature and pH of the environment might increase pathogen invasiveness and lead to outbreaks (95).
Biofilm Formation
The capacity of biofilm formation is a common attribute of several microorganisms, suggesting a dominant stage of bacterial colonization. The developmental process of biofilm formation is triggered by the aggregation and/or surface attachment of planktonic bacteria and is fulfilled by the dispersion of microorganisms from the biofilm structure (96). Due to the complex architecture of the biofilm, interior areas within biofilms are hypoxic, nutrient-limited, and acidic owing to the accumulation of waste products (97). Considering that these stress conditions can independently induce the VBNC state, pathogens deep within the biofilm structure tend to VBNC induction. In addition, the culturability of bacteria in multispecies biofilms is influenced by the metabolic activity of other strains (98). It is noteworthy that the active starvation response of biofilm bacteria is reported to establish antibiotic tolerance (99); thereby, biofilm bacteria are highly resistant to antibiotic interventions and more difficult to eradicate compared to planktonic cells (100).
Approximately 95% of the waterborne pathogens in drinking water distribution systems are located at biofilm structures, whereas only 5% are detectable through sampling the water phase (101). Drinking water is substantially associated with the contamination of food and the development of multiple diseases, thus several studies are conducted on VBNC bacteria within biofilms. Foodborne pathogen C. jejuni induces the VBNC state inside biofilms to persist for 4 months and resist biocide (102). Furthermore, the reduced inflammatory activity of VBNC cells and the lower activation of macrophages can facilitate the immune evasion of biofilm bacteria (103). In addition to stress tolerance, biofilm formation is a concentration mechanism for H. pylori bacteria through which a small fraction of the biofilm can escape conventional microbiological sampling while containing sufficient pathogens to infect the host (104).
Antibiotic Resistance
The VBNC state by definition is the adaptation of cells to stressful conditions to be less influenced by exogenous stressors. The aforementioned biofilm formation is additional protection, in which microorganisms form an extracellular matrix to escape the host immune defense and establish antibiotic resistance. Moreover, the combination of morphological alterations with a low metabolic rate in VBNC cells will reduce the effectiveness of antibiotics targeting substances or metabolic pathways of culturable bacteria (105). Despite the morphological similarity between the VBNC and culturable forms of particular bacteria, the microorganism can withstand antimicrobial substances owing to epigenetic alterations. In VBNC E. coli, significantly expressed genes are reported to be involved in substance transportation such as efflux pumps to reduce the accumulation of toxic compounds and gene regulators such as transcriptional regulatory factors, stress regulators, and virulence genes (106). Further, VBNC induction can be the consequence of antibiotic treatment as various concentrations of ciprofloxacin might lead to quinolone-induced VBNC E. coli (107). Similarly, this might be an explanation for the frequent reinfection of H. pylori-infected individuals who undergo remission following an antibiotic intervention.
Food Safety and Public Health
As a worldwide challenging issue, food safety is the protection of the food supply chain from contamination by pathogens and chemical compounds (108). Various documents have confirmed the presence of VBNC pathogens in food and drinking water (109). The diversity of the surrounding environment during food processing and the detection of VBNC pathogens in food have widely challenged public health. Experimental studies have indicated the capacity of VBNC bacteria to resuscitate and lead the host toward a lethal state (82). Accordingly, solid evidence suggests that VBNC pathogens might be responsible for foodborne outbreaks. In 1998, contamination of salted salmon roe with E. coli O157 caused an outbreak in Japan. The underestimated number of viable pathogens due to the presence of VBNC E. coli was proposed as the main reason for the outbreak (110). The induction of VBNC Salmonella oranienburg following osmotic stress in dried processed squids led to another foodborne outbreak in Japan (111). The E. coli O104:H4 strain expressing genes characteristics of enterohemorrhagic E. coli (EHEC) and enteroaggregative E. coli (EAEC) resulted in an outbreak and several cases of bloody diarrhea and hemolytic uremic syndrome by encountering copper ions or particular tap water and thereby entering the VBNC state (112). Nevertheless, the presence of favorable conditions for bacterial resuscitation is of great significance for determining the pathogenicity of VBNC cells. VBNC S. typhimurium LT4 was demonstrated to recover only through oral administration, suggesting the gastrointestinal tract as a favorable environment for resuscitation (113). However, S. typhimurium ATCC 14028 failed to recover into a culturable cell by transmission through the digestive tract (114).
Limitations and Future Perspective
Decades of research have shed light on the characteristics and potential capacity of VBNC cells to provide novel strategies for eliminating VBNC pathogens. Despite advances in the biotechnology and promotion of detection techniques, limitations in the study design prevent us to deepen our knowledge regarding the induction and resuscitation of the VBNC state. Heterogeneous cultivation conditions encompassing culturable, non-culturable, injured, and dead bacteria can interfere with study analyses concerning morphological properties and metabolic activities associated with the induction and recovery of VBNC bacteria. Complete isolation of VBNC cells from heterogeneous systems and application of microfluidic technology and time-lapse microscopy may allow us to establish a more accurate platform for studying VBNC bacteria at the single-cell level. Furthermore, the development of sensitive and easily operated detection methods for overcoming the shortcoming of current methods should be a priority in the modern field of food sciences. Finally, genomic and proteomic studies need to address common and strain-specific mechanisms by which pathogens can transform from a culturable into a VBNC state and vice versa.
Conclusions
The VBNC state has been indicated as a pivotal characteristic of bacteria to survive stressful conditions and further develop infectious diseases in the host. The complexity of the VBNC concept as a corresponding mechanism of adaptation has drawn substantial attention. Nonetheless, given the surreptitious characteristic of this state, little is known regarding the induction and resuscitation of VBNC cells. Fast and accurate recognition of VBNC pathogens is of great significance in tackling possible contaminants and foodborne outbreaks. Owing to the distinct features of VBNC cells, conventional microbiological plate count techniques might result in underestimating the abundance of viable microorganisms. Despite the development of innovated culture-independent methods, strain-specific characteristics and induction conditions extremely challenge food safety and public health. Therefore, a universal classification system and platform, as well as general biomarkers are critical to be established to accelerate further investigations.
Acknowledgments
We gratefully thank the staff of the Foodborne and Waterborne Diseases Research Center in the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran for the support and contribution to conducting this work.
Conflict of Interests
None declared.
Ethical Approval
Not applicable.
References
- Xu HS, Roberts N, Singleton FL, Attwell RW, Grimes DJ, Colwell RR. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol 1982; 8(4):313-23. doi: 10.1007/bf02010671 [Crossref] [ Google Scholar]
- Schottroff F, Fröhling A, Zunabovic-Pichler M, Krottenthaler A, Schlüter O, Jäger H. Sublethal injury and viable but non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Front Microbiol 2018; 9:2773. doi: 10.3389/fmicb.2018.02773 [Crossref] [ Google Scholar]
- Wagley S, Morcrette H, Kovacs-Simon A, Yang ZR, Power A, Tennant RK. Bacterial dormancy: a subpopulation of viable but non-culturable cells demonstrates better fitness for revival. PLoS Pathog 2021; 17(1):e1009194. doi: 10.1371/journal.ppat.1009194 [Crossref] [ Google Scholar]
- Kan Y, Jiang N, Xu X, Lyu Q, Gopalakrishnan V, Walcott R. Induction and resuscitation of the viable but non-culturable (VBNC) state in Acidovorax citrulli, the causal agent of bacterial fruit blotch of cucurbitaceous crops. Front Microbiol 2019; 10:1081. doi: 10.3389/fmicb.2019.01081 [Crossref] [ Google Scholar]
- Gao R, Liao X, Zhao X, Liu D, Ding T. The diagnostic tools for viable but nonculturable pathogens in the food industry: current status and future prospects. Compr Rev Food Sci Food Saf 2021; 20(2):2146-75. doi: 10.1111/1541-4337.12695 [Crossref] [ Google Scholar]
- Ashbolt NJ. Microbial contamination of drinking water and human health from community water systems. Curr Environ Health Rep 2015; 2(1):95-106. doi: 10.1007/s40572-014-0037-5 [Crossref] [ Google Scholar]
- Ayrapetyan M, Oliver JD. The viable but non-culturable state and its relevance in food safety. Curr Opin Food Sci 2016; 8:127-33. doi: 10.1016/j.cofs.2016.04.010 [Crossref] [ Google Scholar]
- Giagnoni L, Arenella M, Galardi E, Nannipieri P, Renella G. Bacterial culturability and the viable but non-culturable (VBNC) state studied by a proteomic approach using an artificial soil. Soil Biol Biochem 2018; 118:51-8. doi: 10.1016/j.soilbio.2017.12.004 [Crossref] [ Google Scholar]
- Nicolò MS, Gioffrè A, Carnazza S, Platania G, Silvestro ID, Guglielmino SP. Viable but nonculturable state of foodborne pathogens in grapefruit juice: a study of laboratory. Foodborne Pathog Dis 2011; 8(1):11-7. doi: 10.1089/fpd.2009.0491 [Crossref] [ Google Scholar]
- Chen H, Zhao YY, Shu M, Zhang TT, Bi Y, Gao YY. Detection and evaluation of viable but non-culturable Escherichia coli O157:H7 induced by low temperature with a BCAC-EMA-Rti-LAMP assay in chicken without enrichment. Food Anal Methods 2019; 12(2):458-68. doi: 10.1007/s12161-018-1377-9 [Crossref] [ Google Scholar]
- Zhong Q, Wang B, Wang J, Liu Y, Fang X, Liao Z. Global proteomic analysis of the resuscitation state of Vibrio parahaemolyticus compared with the normal and viable but non-culturable state. Front Microbiol 2019; 10:1045. doi: 10.3389/fmicb.2019.01045 [Crossref] [ Google Scholar]
- Zhang S, Guo L, Yang K, Zhang Y, Ye C, Chen S. Induction of Escherichia coli into a VBNC state by continuous-flow UVC and subsequent changes in metabolic activity at the single-cell level. Front Microbiol 2018; 9:2243. doi: 10.3389/fmicb.2018.02243 [Crossref] [ Google Scholar]
- Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol 2014; 5:258. doi: 10.3389/fmicb.2014.00258 [Crossref] [ Google Scholar]
- Gray DA, Dugar G, Gamba P, Strahl H, Jonker MJ, Hamoen LW. Extreme slow growth as alternative strategy to survive deep starvation in bacteria. Nat Commun 2019; 10(1):890. doi: 10.1038/s41467-019-08719-8 [Crossref] [ Google Scholar]
- Wei C, Zhao X. Induction of viable but nonculturable Escherichia coli O157:H7 by low temperature and its resuscitation. Front Microbiol 2018; 9:2728. doi: 10.3389/fmicb.2018.02728 [Crossref] [ Google Scholar]
- Wasfi R, Abdellatif GR, Elshishtawy HM, Ashour HM. First-time characterization of viable but non-culturable Proteus mirabilis: induction and resuscitation. J Cell Mol Med 2020; 24(5):2791-801. doi: 10.1111/jcmm.15031 [Crossref] [ Google Scholar]
- Liao X, Liu D, Ding T. Nonthermal plasma induces the viable-but-nonculturable state in Staphylococcus aureus via metabolic suppression and the oxidative stress response. Appl Environ Microbiol 2020; 86(5):e02216-19. doi: 10.1128/aem.02216-19 [Crossref] [ Google Scholar]
- Liao H, Jiang L, Zhang R. Induction of a viable but non-culturable state in Salmonella typhimurium by thermosonication and factors affecting resuscitation. FEMS Microbiol Lett 2018; 365(2):fnx249. doi: 10.1093/femsle/fnx249 [Crossref] [ Google Scholar]
- Emanuel E, Dubrovin I, Pogreb R, Pinhasi GA, Cahan R. Resuscitation of pulsed electric field-treated Staphylococcus aureus and Pseudomonas putida in a rich nutrient medium. Foods 2021; 10(3):660. doi: 10.3390/foods10030660 [Crossref] [ Google Scholar]
- Maertens L, Matroule JY, Van Houdt R. Characteristics of the copper-induced viable-but-non-culturable state in bacteria. World J Microbiol Biotechnol 2021; 37(3):37. doi: 10.1007/s11274-021-03006-5 [Crossref] [ Google Scholar]
- Wong HC, Wang P. Induction of viable but nonculturable state in Vibrio parahaemolyticus and its susceptibility to environmental stresses. J Appl Microbiol 2004; 96(2):359-66. doi: 10.1046/j.1365-2672.2004.02166.x [Crossref] [ Google Scholar]
- Li Y, Huang T, Bai C, Fu J, Chen L, Liang Y. Reduction, prevention, and control of Salmonella enterica viable but non-culturable cells in flour food. Front Microbiol 2020; 11:1859. doi: 10.3389/fmicb.2020.01859 [Crossref] [ Google Scholar]
- Jayakumar JM, Balasubramanian D, Reddi G, Almagro-Moreno S. Synergistic role of abiotic factors driving viable but non-culturable Vibrio cholerae. Environ Microbiol Rep 2020; 12(4):454-65. doi: 10.1111/1758-2229.12861 [Crossref] [ Google Scholar]
- Lin H, Ye C, Chen S, Zhang S, Yu X. Viable but non-culturable E coli induced by low level chlorination have higher persistence to antibiotics than their culturable counterparts. Environ Pollut 2017; 230:242-9. doi: 10.1016/j.envpol.2017.06.047 [Crossref] [ Google Scholar]
- Ayrapetyan M, Williams T, Oliver JD. Relationship between the viable but nonculturable state and antibiotic persister cells. J Bacteriol 2018; 200(20):e00249-18. doi: 10.1128/jb.00249-18 [Crossref] [ Google Scholar]
- Alleron L, Khemiri A, Koubar M, Lacombe C, Coquet L, Cosette P. VBNC Legionella pneumophila cells are still able to produce virulence proteins. Water Res 2013; 47(17):6606-17. doi: 10.1016/j.watres.2013.08.032 [Crossref] [ Google Scholar]
- Lv R, Wang K, Feng J, Heeney DD, Liu D, Lu X. Detection and quantification of viable but non-culturable Campylobacter jejuni. Front Microbiol 2019; 10:2920. doi: 10.3389/fmicb.2019.02920 [Crossref] [ Google Scholar]
- Gangaiah D, Kassem Kassem, II II, Liu Z, Rajashekara G. Importance of polyphosphate kinase 1 for Campylobacter jejuni viable-but-nonculturable cell formation, natural transformation, and antimicrobial resistance. Appl Environ Microbiol 2009; 75(24):7838-49. doi: 10.1128/aem.01603-09 [Crossref] [ Google Scholar]
- Ayrapetyan M, Williams TC, Oliver JD. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol 2015; 23(1):7-13. doi: 10.1016/j.tim.2014.09.004 [Crossref] [ Google Scholar]
- Kusumoto A, Miyashita M, Kawamoto K. Deletion in the C-terminal domain of ClpX delayed entry of Salmonella enterica into a viable but non-culturable state. Res Microbiol 2013; 164(4):335-41. doi: 10.1016/j.resmic.2013.01.011 [Crossref] [ Google Scholar]
- Zhao X, Zhong J, Wei C, Lin CW, Ding T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front Microbiol 2017; 8:580. doi: 10.3389/fmicb.2017.00580 [Crossref] [ Google Scholar]
- Progulske-Fox A, Chukkapalli SS, Getachew H, Dunn WA, Oliver JD. VBNC, previously unrecognized in the life cycle of Porphyromonas gingivalis?. J Oral Microbiol 2022; 14(1):1952838. doi: 10.1080/20002297.2021.1952838 [Crossref] [ Google Scholar]
- Signoretto C, Lleò MM, Tafi MC, Canepari P. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl Environ Microbiol 2000; 66(5):1953-9. doi: 10.1128/aem.66.5.1953-1959.2000 [Crossref] [ Google Scholar]
- Robben C, Fister S, Witte AK, Schoder D, Rossmanith P, Mester P. Induction of the viable but non-culturable state in bacterial pathogens by household cleaners and inorganic salts. Sci Rep 2018; 8(1):15132. doi: 10.1038/s41598-018-33595-5 [Crossref] [ Google Scholar]
- Ierardi E, Losurdo G, Mileti A, Paolillo R, Giorgio F, Principi M. The puzzle of coccoid forms of Helicobacter pylori: beyond basic science. Antibiotics (Basel) 2020; 9(6):293. doi: 10.3390/antibiotics9060293 [Crossref] [ Google Scholar]
- Su CP, Jane WN, Wong HC. Changes of ultrastructure and stress tolerance of Vibrio parahaemolyticus upon entering viable but nonculturable state. Int J Food Microbiol 2013; 160(3):360-6. doi: 10.1016/j.ijfoodmicro.2012.11.012 [Crossref] [ Google Scholar]
- Pinto D, Santos MA, Chambel L. Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit Rev Microbiol 2015; 41(1):61-76. doi: 10.3109/1040841x.2013.794127 [Crossref] [ Google Scholar]
- Jia Y, Yu C, Fan J, Fu Y, Ye Z, Guo X. Alterations in the cell wall of Rhodococcus biphenylivorans under norfloxacin stress. Front Microbiol 2020; 11:554957. doi: 10.3389/fmicb.2020.554957 [Crossref] [ Google Scholar]
- Day AP, Oliver JD. Changes in membrane fatty acid composition during entry of Vibrio vulnificus into the viable but nonculturable state. J Microbiol 2004; 42(2):69-73. [ Google Scholar]
- Trevors JT, van Elsas JD, Bej AK. The molecularly crowded cytoplasm of bacterial cells: dividing cells contrasted with viable but non-culturable (VBNC) bacterial cells. Curr Issues Mol Biol 2013; 15:1-6. [ Google Scholar]
- Zhao F, Wang Y, An H, Hao Y, Hu X, Liao X. New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2. mBio 2016; 7(4):e00961-16. doi: 10.1128/mBio.00961-16 [Crossref] [ Google Scholar]
- Bollen C, Dewachter L, Michiels J. Protein aggregation as a bacterial strategy to survive antibiotic treatment. Front Mol Biosci 2021; 8:669664. doi: 10.3389/fmolb.2021.669664 [Crossref] [ Google Scholar]
- Su X, Sun F, Wang Y, Hashmi MZ, Guo L, Ding L. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci Rep 2015; 5:18590. doi: 10.1038/srep18590 [Crossref] [ Google Scholar]
- Cooper M, Fridman G, Fridman A, Joshi SG. Biological responses of Bacillus stratosphericus to floating electrode-dielectric barrier discharge plasma treatment. J Appl Microbiol 2010; 109(6):2039-48. doi: 10.1111/j.1365-2672.2010.04834.x [Crossref] [ Google Scholar]
- Roszak DB, Grimes DJ, Colwell RR. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol 1984; 30(3):334-8. doi: 10.1139/m84-049 [Crossref] [ Google Scholar]
- Baffone W, Casaroli A, Citterio B, Pierfelici L, Campana R, Vittoria E. Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int J Food Microbiol 2006; 107(1):83-91. doi: 10.1016/j.ijfoodmicro.2005.08.015 [Crossref] [ Google Scholar]
- Whitesides MD, Oliver JD. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol 1997; 63(3):1002-5. doi: 10.1128/aem.63.3.1002-1005.1997 [Crossref] [ Google Scholar]
- Senoh M, Ghosh-Banerjee J, Ramamurthy T, Hamabata T, Kurakawa T, Takeda M. Conversion of viable but nonculturable Vibrio cholerae to the culturable state by co-culture with eukaryotic cells. Microbiol Immunol 2010; 54(9):502-7. doi: 10.1111/j.1348-0421.2010.00245.x [Crossref] [ Google Scholar]
- Dong K, Pan H, Yang D, Rao L, Zhao L, Wang Y. Induction, detection, formation, and resuscitation of viable but non-culturable state microorganisms. Compr Rev Food Sci Food Saf 2020; 19(1):149-83. doi: 10.1111/1541-4337.12513 [Crossref] [ Google Scholar]
- Fera MT, Maugeri TL, Gugliandolo C, La Camera E, Lentini V, Favaloro A. Induction and resuscitation of viable nonculturable Arcobacter butzleri cells. Appl Environ Microbiol 2008; 74(10):3266-8. doi: 10.1128/aem.00059-08 [Crossref] [ Google Scholar]
- Pinto D, Almeida V, Almeida Santos M, Chambel L. Resuscitation of Escherichia coli VBNC cells depends on a variety of environmental or chemical stimuli. J Appl Microbiol 2011; 110(6):1601-11. doi: 10.1111/j.1365-2672.2011.05016.x [Crossref] [ Google Scholar]
- Imazaki I, Nakaho K. Temperature-upshift-mediated revival from the sodium-pyruvate-recoverable viable but nonculturable state induced by low temperature in Ralstonia solanacearum: linear regression analysis. J Gen Plant Pathol 2009; 75(3):213-26. doi: 10.1007/s10327-009-0166-0 [Crossref] [ Google Scholar]
- Ayrapetyan M, Williams TC, Oliver JD. Interspecific quorum sensing mediates the resuscitation of viable but nonculturable vibrios. Appl Environ Microbiol 2014; 80(8):2478-83. doi: 10.1128/aem.00080-14 [Crossref] [ Google Scholar]
- Su X, Chen X, Hu J, Shen C, Ding L. Exploring the potential environmental functions of viable but non-culturable bacteria. World J Microbiol Biotechnol 2013; 29(12):2213-8. doi: 10.1007/s11274-013-1390-5 [Crossref] [ Google Scholar]
- Byrd JJ, Xu HS, Colwell RR. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol 1991; 57(3):875-8. doi: 10.1128/aem.57.3.875-878.1991 [Crossref] [ Google Scholar]
- Rice SA, McDougald D, Kjelleberg S. Vibrio vulnificus: a physiological and genetic approach to the viable but nonculturable response. J Infect Chemother 2000; 6(2):115-20. doi: 10.1007/pl00012150 [Crossref] [ Google Scholar]
- Wideman NE, Oliver JD, Crandall PG, Jarvis NA. Detection and potential virulence of viable but non-culturable (VBNC) Listeria monocytogenes: a review. Microorganisms 2021; 9(1):194. doi: 10.3390/microorganisms9010194 [Crossref] [ Google Scholar]
- Créach V, Baudoux AC, Bertru G, Rouzic BL. Direct estimate of active bacteria: CTC use and limitations. J Microbiol Methods 2003; 52(1):19-28. doi: 10.1016/s0167-7012(02)00128-8 [Crossref] [ Google Scholar]
- Ullrich S, Karrasch B, Hoppe H, Jeskulke K, Mehrens M. Toxic effects on bacterial metabolism of the redox dye 5-cyano-2,3-ditolyl tetrazolium chloride. Appl Environ Microbiol 1996; 62(12):4587-93. doi: 10.1128/aem.62.12.4587-4593.1996 [Crossref] [ Google Scholar]
- Hurst A. Bacterial injury: a review. Can J Microbiol 1977; 23(8):935-44. doi: 10.1139/m77-139 [Crossref] [ Google Scholar]
- Asadishad B, Ghoshal S, Tufenkji N. Method for the direct observation and quantification of survival of bacteria attached to negatively or positively charged surfaces in an aqueous medium. Environ Sci Technol 2011; 45(19):8345-51. doi: 10.1021/es201496q [Crossref] [ Google Scholar]
- Stiefel P, Schmidt-Emrich S, Maniura-Weber K, Ren Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol 2015; 15:36. doi: 10.1186/s12866-015-0376-x [Crossref] [ Google Scholar]
- Sheridan GE, Masters CI, Shallcross JA, MacKey BM. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol 1998; 64(4):1313-8. doi: 10.1128/aem.64.4.1313-1318.1998 [Crossref] [ Google Scholar]
- Lothigius A, Sjöling A, Svennerholm AM, Bölin I. Survival and gene expression of enterotoxigenic Escherichia coli during long-term incubation in sea water and freshwater. J Appl Microbiol 2010; 108(4):1441-9. doi: 10.1111/j.1365-2672.2009.04548.x [Crossref] [ Google Scholar]
- Fey A, Eichler S, Flavier S, Christen R, Höfle MG, Guzmán CA. Establishment of a real-time PCR-based approach for accurate quantification of bacterial RNA targets in water, using Salmonella as a model organism. Appl Environ Microbiol 2004; 70(6):3618-23. doi: 10.1128/aem.70.6.3618-3623.2004 [Crossref] [ Google Scholar]
- Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol 1979; 25(3):415-20. doi: 10.1139/m79-063 [Crossref] [ Google Scholar]
- Heidelberg JF, Shahamat M, Levin M, Rahman I, Stelma G, Grim C. Effect of aerosolization on culturability and viability of gram-negative bacteria. Appl Environ Microbiol 1997; 63(9):3585-8. doi: 10.1128/aem.63.9.3585-3588.1997 [Crossref] [ Google Scholar]
- Besnard V, Federighi M, Cappelier JM. Evidence of viable but non-culturable state in Listeria monocytogenes by direct viable count and CTC-DAPI double staining. Food Microbiol 2000; 17(6):697-704. doi: 10.1006/fmic.2000.0366 [Crossref] [ Google Scholar]
- Cappelier JM, Besnard V, Roche S, Garrec N, Zundel E, Velge P. Avirulence of viable but non-culturable Listeria monocytogenes cells demonstrated by in vitro and in vivo models. Vet Res 2005; 36(4):589-99. doi: 10.1051/vetres:2005018 [Crossref] [ Google Scholar]
- Nocker A, Cheung CY, Camper AK. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs dead bacteria by selective removal of DNA from dead cells. J Microbiol Methods 2006; 67(2):310-20. doi: 10.1016/j.mimet.2006.04.015 [Crossref] [ Google Scholar]
- Nocker A, Camper AK. Novel approaches toward preferential detection of viable cells using nucleic acid amplification techniques. FEMS Microbiol Lett 2009; 291(2):137-42. doi: 10.1111/j.1574-6968.2008.01429.x [Crossref] [ Google Scholar]
- Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev 2010; 34(4):415-25. doi: 10.1111/j.1574-6976.2009.00200.x [Crossref] [ Google Scholar]
- Oliver JD. The viable but nonculturable state in bacteria. J Microbiol 2005; 43 Spec No:93-100. [ Google Scholar]
- Begley M, Hill C. Stress adaptation in foodborne pathogens. Annu Rev Food Sci Technol 2015; 6:191-210. doi: 10.1146/annurev-food-030713-092350 [Crossref] [ Google Scholar]
- Bai H, Zhao F, Li M, Qin L, Yu H, Lu L. Citric acid can force Staphylococcus aureus into viable but nonculturable state and its characteristics. Int J Food Microbiol 2019; 305:108254. doi: 10.1016/j.ijfoodmicro.2019.108254 [Crossref] [ Google Scholar]
- Li HT, Wang HF, Wang Y, Pan JZ, Fang Q. A minimalist approach for generating picoliter to nanoliter droplets based on an asymmetrical beveled capillary and its application in digital PCR assay. Talanta 2020; 217:120997. doi: 10.1016/j.talanta.2020.120997 [Crossref] [ Google Scholar]
- Jadhav HB, Annapure US, Deshmukh RR. Non-thermal technologies for food processing. Front Nutr 2021; 8:657090. doi: 10.3389/fnut.2021.657090 [Crossref] [ Google Scholar]
- Han D, Hung YC, Wang L. Evaluation of the antimicrobial efficacy of neutral electrolyzed water on pork products and the formation of viable but nonculturable (VBNC) pathogens. Food Microbiol 2018; 73:227-36. doi: 10.1016/j.fm.2018.01.023 [Crossref] [ Google Scholar]
- Rowan NJ, Valdramidis VP, Gómez-López VM. A review of quantitative methods to describe efficacy of pulsed light generated inactivation data that embraces the occurrence of viable but non culturable state microorganisms. Trends Food Sci Technol 2015; 44(1):79-92. doi: 10.1016/j.tifs.2015.03.006 [Crossref] [ Google Scholar]
- Liao X, Hu W, Liu D, Ding T. Stress resistance and pathogenicity of nonthermal-plasma-induced viable-but-nonculturable Staphylococcus aureus through energy suppression, oxidative stress defense, and immune-escape mechanisms. Appl Environ Microbiol 2021; 87(2):e02380-20. doi: 10.1128/aem.02380-20 [Crossref] [ Google Scholar]
- Dinu LD, Bach S. Induction of viable but nonculturable Escherichia coli O157:H7 in the phyllosphere of lettuce: a food safety risk factor. Appl Environ Microbiol 2011; 77(23):8295-302. doi: 10.1128/aem.05020-11 [Crossref] [ Google Scholar]
- Oliver JD, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol 1995; 61(7):2620-3. doi: 10.1128/aem.61.7.2620-2623.1995 [Crossref] [ Google Scholar]
- Amel BK, Amine B, Amina B. Survival of Vibrio fluvialis in seawater under starvation conditions. Microbiol Res 2008; 163(3):323-8. doi: 10.1016/j.micres.2006.06.006 [Crossref] [ Google Scholar]
- Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell RR, Cormier M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol 1996; 62(12):4621-6. doi: 10.1128/aem.62.12.4621-4626.1996 [Crossref] [ Google Scholar]
- Anderson M, Bollinger D, Hagler A, Hartwell H, Rivers B, Ward K. Viable but nonculturable bacteria are present in mouse and human urine specimens. J Clin Microbiol 2004; 42(2):753-8. doi: 10.1128/jcm.42.2.753-758.2004 [Crossref] [ Google Scholar]
- Wingender J, Flemming HC. Biofilms in drinking water and their role as reservoir for pathogens. Int J Hyg Environ Health 2011; 214(6):417-23. doi: 10.1016/j.ijheh.2011.05.009 [Crossref] [ Google Scholar]
- Zhang J, Li W, Chen J, Qi W, Wang F, Zhou Y. Impact of biofilm formation and detachment on the transmission of bacterial antibiotic resistance in drinking water distribution systems. Chemosphere 2018; 203:368-80. doi: 10.1016/j.chemosphere.2018.03.143 [Crossref] [ Google Scholar]
- Cabral JP. Water microbiology Bacterial pathogens and water. Int J Environ Res Public Health 2010; 7(10):3657-703. doi: 10.3390/ijerph7103657 [Crossref] [ Google Scholar]
- Linke S, Lenz J, Gemein S, Exner M, Gebel J. Detection of Helicobacter pylori in biofilms by real-time PCR. Int J Hyg Environ Health 2010; 213(3):176-82. doi: 10.1016/j.ijheh.2010.03.006 [Crossref] [ Google Scholar]
- Monstein HJ, Jonasson J. Differential virulence-gene mRNA expression in coccoid forms of Helicobacter pylori. Biochem Biophys Res Commun 2001; 285(2):530-6. doi: 10.1006/bbrc.2001.5179 [Crossref] [ Google Scholar]
- Ahmed KS, Khan AA, Ahmed I, Tiwari SK, Habeeb A, Ahi JD. Impact of household hygiene and water source on the prevalence and transmission of Helicobacter pylori: a South Indian perspective. Singapore Med J 2007; 48(6):543-9. [ Google Scholar]
- Sun F, Chen J, Zhong L, Zhang XH, Wang R, Guo Q. Characterization and virulence retention of viable but nonculturable Vibrio harveyi. FEMS Microbiol Ecol 2008; 64(1):37-44. doi: 10.1111/j.1574-6941.2008.00442.x [Crossref] [ Google Scholar]
- Marron L, Emerson N, Gahan CG, Hill C. A mutant of Listeria monocytogenes LO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Appl Environ Microbiol 1997; 63(12):4945-7. doi: 10.1128/aem.63.12.4945-4947.1997 [Crossref] [ Google Scholar]
- Conte MP, Petrone G, Di Biase AM, Ammendolia MG, Superti F, Seganti L. Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microb Pathog 2000; 29(3):137-44. doi: 10.1006/mpat.2000.0379 [Crossref] [ Google Scholar]
- Garner MR, James KE, Callahan MC, Wiedmann M, Boor KJ. Exposure to salt and organic acids increases the ability of Listeria monocytogenes to invade Caco-2 cells but decreases its ability to survive gastric stress. Appl Environ Microbiol 2006; 72(8):5384-95. doi: 10.1128/aem.00764-06 [Crossref] [ Google Scholar]
- Rumbaugh KP, Sauer K. Biofilm dispersion. Nat Rev Microbiol 2020; 18(10):571-86. doi: 10.1038/s41579-020-0385-0 [Crossref] [ Google Scholar]
- Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol 2008; 6(3):199-210. doi: 10.1038/nrmicro1838 [Crossref] [ Google Scholar]
- Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW. Interaction of Legionella pneumophila and Helicobacter pylori with bacterial species isolated from drinking water biofilms. BMC Microbiol 2011; 11:57. doi: 10.1186/1471-2180-11-57 [Crossref] [ Google Scholar]
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 2011; 334(6058):982-6. doi: 10.1126/science.1211037 [Crossref] [ Google Scholar]
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 2016; 14(9):563-75. doi: 10.1038/nrmicro.2016.94 [Crossref] [ Google Scholar]
- Wingender J, Flemming HC. Contamination potential of drinking water distribution network biofilms. Water Sci Technol 2004; 49(11-12):277-86. [ Google Scholar]
- Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol 2003; 69(8):4343-51. doi: 10.1128/aem.69.8.4343-4351.2003 [Crossref] [ Google Scholar]
- Cerca F, Andrade F, França Â, Andrade EB, Ribeiro A, Almeida AA. Staphylococcus epidermidis biofilms with higher proportions of dormant bacteria induce a lower activation of murine macrophages. J Med Microbiol 2011; 60(Pt 12):1717-24. doi: 10.1099/jmm.0.031922-0 [Crossref] [ Google Scholar]
- Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW. Persistence of Helicobacter pylori in heterotrophic drinking-water biofilms. Appl Environ Microbiol 2008; 74(19):5898-904. doi: 10.1128/aem.00827-08 [Crossref] [ Google Scholar]
- Fleischmann S, Robben C, Alter T, Rossmanith P, Mester P. How to evaluate non-growing cells-current strategies for determining antimicrobial resistance of VBNC bacteria. Antibiotics (Basel) 2021; 10(2):115. doi: 10.3390/antibiotics10020115 [Crossref] [ Google Scholar]
- Ye C, Lin H, Zhang M, Chen S, Yu X. Characterization and potential mechanisms of highly antibiotic tolerant VBNC Escherichia coli induced by low level chlorination. Sci Rep 2020; 10(1):1957. doi: 10.1038/s41598-020-58106-3 [Crossref] [ Google Scholar]
- Mason DJ, Power EG, Talsania H, Phillips I, Gant VA. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother 1995; 39(12):2752-8. doi: 10.1128/aac.39.12.2752 [Crossref] [ Google Scholar]
- Gizaw Z. Public health risks related to food safety issues in the food market: a systematic literature review. Environ Health Prev Med 2019; 24(1):68. doi: 10.1186/s12199-019-0825-5 [Crossref] [ Google Scholar]
- Fakruddin M, Mannan KS, Andrews S. Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol 2013; 2013:703813. doi: 10.1155/2013/703813 [Crossref] [ Google Scholar]
- Makino SI, Kii T, Asakura H, Shirahata T, Ikeda T, Takeshi K. Does enterohemorrhagic Escherichia coli O157:H7 enter the viable but nonculturable state in salted salmon roe?. Appl Environ Microbiol 2000; 66(12):5536-9. doi: 10.1128/aem.66.12.5536-5539.2000 [Crossref] [ Google Scholar]
- Asakura H, Makino S, Takagi T, Kuri A, Kurazono T, Watarai M. Passage in mice causes a change in the ability of Salmonella enterica serovar Oranienburg to survive NaCl osmotic stress: resuscitation from the viable but non-culturable state. FEMS Microbiol Lett 2002; 212(1):87-93. doi: 10.1111/j.1574-6968.2002.tb11249.x [Crossref] [ Google Scholar]
- Aurass P, Prager R, Flieger A. EHEC/EAEC O104:H4 strain linked with the 2011 German outbreak of haemolytic uremic syndrome enters into the viable but non-culturable state in response to various stresses and resuscitates upon stress relief. Environ Microbiol 2011; 13(12):3139-48. doi: 10.1111/j.1462-2920.2011.02604.x [Crossref] [ Google Scholar]
- Amel D, Amina B. Resuscitation of seventeen-year stressed Salmonella typhimurium. Oceanol Hydrobiol Stud 2008; 37(1):69-82. doi: 10.2478/v10009-007-0038-x [Crossref] [ Google Scholar]
- Habimana O, Nesse LL, Møretrø T, Berg K, Heir E, Vestby LK. The persistence of Salmonella following desiccation under feed processing environmental conditions: a subject of relevance. Lett Appl Microbiol 2014; 59(5):464-70. doi: 10.1111/lam.12308 [Crossref] [ Google Scholar]