Avicenna Journal of Clinical Microbiology and Infection. 10(1):32-37.
doi: 10.34172/ajcmi.2023.3367
Original Article
Cryptococcus neoformans var. grubii: The In Vitro Antifungal Susceptibility Pattern in Addition to the Quantification of Phospholipase and Proteinase Enzymatic Activities
Munesh K Gupta 1  , Ragini Tilak 1, *
, Ragini Tilak 1, *  , Namrata Pal 1, Ashish Kumar Singh 2, Jaya Chakravarty 3, Bhupendra Kumar 4
, Namrata Pal 1, Ashish Kumar Singh 2, Jaya Chakravarty 3, Bhupendra Kumar 4
Author information:
1Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University Varanasi, India
2Scientist C, MRHRU Datia, ICMR NIRTH Jabalpur, India
3Department of General medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
4Department of Zoology, Institute of Sciences, Banaras Hindu University Varanasi, India
Abstract
Background: Cryptococcal meningoencephalitis is a life-threatening fungal infection in human immunodeficiency virus (HIV)-infected patients. Cryptococcus neoformans var. grubii and neoformans are the causative agents that usually respond well to fluconazole and amphotericin B. However, resistance/ non-responding cryptococcal meningitis cases to fluconazole and amphotericin B have been reported globally.
Methods: The causative Cryptococcus was identified by phenotypic and singleplex polymerase chain reaction (PCR) targeting the putative sugar transporter (STR1) gene. In addition, the phospholipase and proteinase enzymatic activities of the isolates were determined by the plate method using egg yolk agar and bovine serum albumin agar plates, respectively. Finally, the in-vitro minimal inhibitory concentration (MIC) of fluconazole, voriconazole, and amphotericin B against isolated C. neoformans strains was determined by the broth microdilution method.
Results: A total of 50 C. neoformans strains were isolated from the cerebrospinal fluid of HIV-infected patients, which were further identified as variety grubii by simplex polymerase chain reaction (PCR). All the isolated strains producing phospholipase and proteinase enzymes were determined by the calculation of Pz, a ratio of colony diameter and diameter of colony plus the precipitation zone. A comparative high proteinase enzyme activity was observed, and these strains produced medium to high phospholipase (mean Pz 0.3720±0.082, range 0.23-0.56) and proteinase activity (Mean Pz 0.3069±0.086, range 0.012- 0.54). A varied antifungal MIC was detected, and voriconazole had the lowest MIC50 and MIC90 (0.03 & 0.06 µg/mL) in comparison to fluconazole and amphotericin B.
Conclusion:Cryptococcus neoformans var. grubii is the commonest cause of cryptococcal meningoencephalitis in HIV-infected patients. The isolates had varied extracellular hydrolytic enzyme activities. The emergence of C. neoformans strains with higher fluconazole MIC (≥4 mcg/mL) could have resulted in treatment failure.
Keywords: Amphotericin B, Fluconazole, Var. grubii, HIV, str1 gene, MIC
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Gupta MK, Tilak R, Pal N, Singh AK, Chakravarty J, Kumar B. Cryptococcus neoformans var. grubii: The In Vitro antifungal susceptibility pattern in addition to the quantification of phospholipase and proteinase enzymatic activities. Avicenna J Clin Microbiol Infect. 2023; 10(1):32-37. doi:10.34172/ajcmi.2023.3367
Introduction
Cryptococcus neoformans is a common cause of meningoencephalitis in human immunodeficiency virus (HIV)-infected patients (1). Two species of Cryptococcus named neoformans and gattii cause human infections. C. neoformans usually causes meningoencephalitis in immunocompromised patients, whereas C. gattii is manifested as cryptococcoma in immunocompetent individuals. Based on the serotypes whether A or D, C. neoformans is further distinguished as two varieties called grubii and neoformans. Among these two, grubii is reported to be the most common cause of cryptococcal infections in immunocompromised individuals (2).
Cryptococcus neoformans, an environmental saprotroph, enters the human body through inhalation and results in self-limiting localized respiratory infections in the immunocompetent individual as the alveolar macrophages not only phagocytose the inhaled yeast cells promptly but also recruit other inflammatory cells, viz. neutrophils, macrophages, and natural killer cells at the site of infection. However, in immunocompromised patients, HIV-infected patients having CD4 + T cell < 100 cells/µL, patients on chemotherapy, radiotherapy, corticosteroids, and the like, Cryptococcus escapes the immune system and disseminates hematogenously where it involves the distant body organs, brain parenchyma, genitourinary tract, and skin (1,3). Different factors such as polysaccharide capsule, extracellular enzymes (e.g., urease, proteinase, phenoloxidase, and phospholipase), and the ability to grow at 37°C accentuate its adhesion, invasion, dissemination, and survival from the innate immune system (4). The identification of such factors helps in understanding its pathogenesis.
Different groups of antifungal agents, including azoles, polyene, and antimetabolites, are used to treat cryptococcal meningitis. Fluconazole and amphotericin B are widely employed in developing countries. However, C. neoformans strains, resistant/non-responding to amphotericin B, fluconazole, and 5-flucytosine have been reported around the globe, resulting in treatment failure/relapse (5-7). Keeping these facts in mind, a prospective study was performed to determine the variety of Cryptococcus causing human infections and their antifungal susceptibility for fluconazole, amphotericin B, and voriconazole. In addition, the study quantified the extracellular phospholipase and proteinase hydrolytic enzyme activities.
Materials and Methods
The prospective study was approved by the Ethics Committee of the Institute of medical Sciences, BHU Varanasi (Dean/2019/EC/1021). Cryptococcus strains were isolated from the cerebrospinal fluid of HIV-infected patients, anti-retroviral therapy (ART) Clinic, Sir Sundarlal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
We isolated and identified the yeast causing meningoencephalitis in HIV-infected patients, presented to the ART Clinic, Sir Sundarlal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, as per the standard mycology procedures, including the capsule demonstration by India ink wet mount, culture characteristics, urea hydrolysis test, and the growth at 37°C. The isolated Cryptococcus was speciated by the singleplex polymerase chain reaction (PCR) using the primers targeted to the putative sugar transporter (STR1) gene. Briefly, the cryptococcal strains were cultivated on the yeast extract peptone dextrose (YPD) broth, Himedia, India medium overnight. The cells were centrifuged and suspended in 400 µL lysis buffer containing TE buffer and sodium dodecyl sulfate. The suspension was boiled twice with intermittent vortexing. Subsequently, lyticase was added to break the fungal cell wall. The CTAB extraction buffer was then added, and purification with phenol [chloroform: Isoamyl alcohol (25:24:1)] was performed after incubation at 65°C. Finally, the extracted DNA was precipitated with chilled isopropanol. DNA was dissolved in the TE buffer (pH: 8.6), and the quantity of the extracted DNA was measured by a nanodrop spectrophotometer. Next, the putative sugar transporter (str1) gene was amplified by using str1F (5’GAGATTCGGCAGGAAGAAGC3’) and str1R (5’CGTAAGGGATGACGAAAAGGTA3’) primers in a reaction volume of 25 µL, having 2.5 µL of 10X buffer, 2.5 µL of 10 nM dNTP mixture, 1 unit Taq Polymerase, and 1 µL each primer with 5 µL of extracted DNA.8 Then, thermal conditions were followed for the PCR for 30 cycles of amplification, including initial denaturation at 94°C for 5 minutes, annealing at 55°C for 30 seconds, and elongation at 72°C for 30 seconds with a final extension step at 72°C for 6 minutes. The amplicon was subsequently electrophoresed on 1.2% agarose gel, and the results were compared with previous reports (8).
All the isolated strains were assessed for polysaccharide capsule, urease production, growth at 37°C, and the presence of the phenoloxidase enzyme by standard mycological procedures. Phospholipase and proteinase activities were quantified by the plate method (9,10). The Sabouraud dextrose agar medium supplemented with 1 mol/L sodium chloride, 0.005 mol/L calcium chloride, and 8% sterile egg yolk suspension was used to determine the phospholipase activity, whereas bovine serum albumin (BSA) agar containing yeast carbon base (1.17%), 0.2% BSA, and 2% agar was employed to quantitate the proteinase enzyme (9,10). The 10 µL thick suspension of the isolated strains was placed in the centre of the above-mentioned media. The plates were incubated at 37°C for five successive days in a biochemical oxygen demand incubator. The tests for enzyme activities were executed in triplicates where an average value of the colony diameter (a) and the diameter of the colony plus precipitation zone (b) underwent measurement. The enzyme activities of these isolated strains were expressed as Pz value (a/b) as described by Price et al (11). According to this definition, a low Pz value implies high enzyme production, while a high Pz value indicates low enzyme production. All the isolated strains were grouped into different classes such as high (Pz < 0.4), medium (Pz 0.41-0.60), low (Pz 0.61-0.80), very low (Pz 0.81-0.99), and none (Pz 1) according to previous research (12).
The antifungal susceptibility of fluconazole, amphotericin B, and voriconazole against isolated Cryptococcus was executed by the broth microdilution method using the Clinical and Laboratory Standards Institute (CLSI) M27A3 guidelines. Briefly, 0.5 McFarland suspension of the strains having a concentration of 0.5-1.5 × 106 cells/mL was prepared, which was further diluted 20 times in RPMI1640 and 50 times in normal saline to have a final concentration of 0.5-2.5 × 103 yeast cells/mL. Fluconazole, voriconazole, and amphotericin B drug powder were procured from Sigma Pharmaceuticals, India. Fluconazole was dissolved in distilled water to make a master stock of 6.4 mg/mL, whereas voriconazole and amphotericin B were dissolved in DMSO to make a master stock of 1.6 mg/mL. These master stock solutions were further diluted (1:50) in RPMI 1640 to make a working solution of 128 µg/mL for fluconazole and 32 µg/mL for amphotericin B and voriconazole. These dilutions were prepared by two-fold drug dilution using RPMI1640 broth (13).
Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 strains were used to standardize the broth microdilution assay. RPMI 1640 broth, without bicarbonate, buffered to a pH rate of 7.0 with morpholinepropanesulfonic acid was employed to determine the minimum inhibitory concentration of the above-mentioned drugs. Microdilution assay was performed in 96-well, flat-bottom microtitre plates in which 100 µL volume of the diluted drug and 100 µL of yeast suspension were placed, and these plates were incubated at 35°C for 72 hours and examined for growth. For amphotericin B, the minimal inhibitory concentration (MIC) was determined where a 100% growth reduction was observed, whereas 80% growth inhibition was noted as the MIC for fluconazole, and voriconazole compared to the growth control (13).
Statistical Analysis
The distributions of datasets obtained in the study were checked for normality using the Kolmogorov-Smirnoff test. The means were separated using Tukey’s test when data were normally distributed, and variances were homogeneous (Bartlett’s test for equal variances). The data were presented as the mean ± standard deviation (SD), along with their confidence interval values. Student’s t-test was conducted to analyze and compare the phospholipase and proteinase enzyme activities of C. neoformans. However, the chi-square test was performed for the percent analysis of the performance of both enzymes. The obtained P values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SPSS software, version 23.0.
Results
A total of 50 Cryptococcus strains were isolated from the cerebrospinal fluid of HIV-infected patients in 2 years. These strains were identified by standard mycology procedures, including culture characteristics, urease enzyme production, and growth at 37°C. Further characterization of these Cryptococcus strains was performed by the singleplex PCR, targeting the STR1 gene, which revealed C. neoformans var. grubii, a sole causative agent in all 50 cases (Figure 1). All these 50 strains determined virulence factors such as the polysaccharide capsule, urease enzyme, phenoloxidase enzyme, phospholipase, and proteinase enzyme.
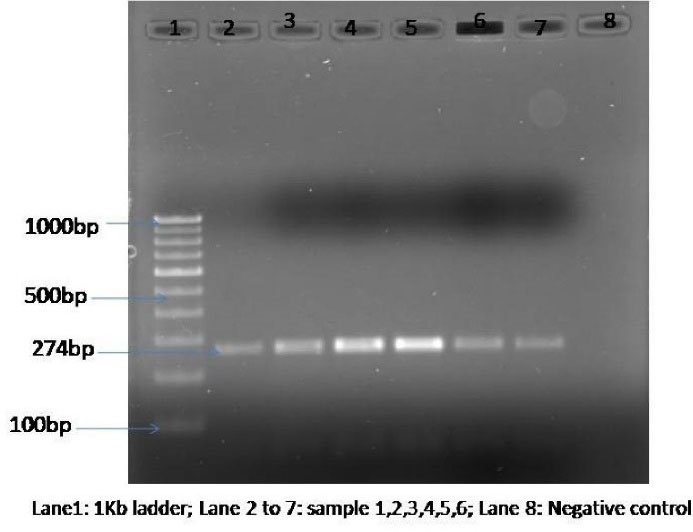
Figure 1.
Gel Electrophoresis Image Showing the Band Size of The Polymerase Chain Reaction Products of Cryptococcus neoformans
.
Gel Electrophoresis Image Showing the Band Size of The Polymerase Chain Reaction Products of Cryptococcus neoformans
The quantification of the phospholipase and proteinase enzyme activities of the isolated C. neoformans strains was done by the plate method. A significantly high proteinase enzyme activity was observed compared to phospholipase activity (t-value = 3.874 and P = 0.0002). All 50 C. neoformans strains had medium to strong phospholipase activity and proteinase enzyme activities with Pz value [mean ( ± SD): 0.3720 (0.082), range 0.23-0.56] and [Mean ( ± SD) 0.3069 ( ± 0.086), range 0.012-0.54], respectively (Table 1). Moreover, when enzyme activity was categorized into strong and medium types by taking the cutoff enzyme activity at the Pz value of ≤ 0.4 and 0.41-0.60 as strong and medium and analyzed, a significantly stronger proteinase enzymatic activity was found than that of phospholipase (Table 2).
Table 1.
Comparison of Mean Phospholipase and Proteinase Enzyme Activities Produced by Isolated Cryptococcus neoformans var. grubii Strains
|
Enzyme Activity
|
Mean Pz Value
|
Standard Deviation
|
95% CI
|
t-value
|
P
Value
|
| Phospholipase |
0.3720 |
0.82 |
-0.0984 to
-0.0318 |
-3.874 |
0.0002 |
| Proteinase |
0.3069 |
0.086 |
Note. CI: Confidence interval.
Table 2.
Comparison of Phospholipase and Proteinase Enzyme Activities of Cryptococcus neoformans var. grubii Strains
|
Enzyme Activity
|
Strong
No. (%)
|
Medium
No. (%)
|
Total
|
Chi-square Value
|
P
Value
|
| Phospholipase |
30 (60%) |
20 (40%) |
50 |
10.1871 |
0.001414 |
| Proteinase |
44 (88%) |
6 (12%) |
50 |
The MIC of fluconazole, voriconazole, and amphotericin B against the isolated C. neoformans strains was determined by the broth microdilution method. The MIC of the tested antifungals against C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 was within the susceptibility range. A varied antifungal MIC was observed against the tested strains (Table 3). A higher fluconazole MIC than that of amphotericin B and voriconazole was observed as well. The mean, MIC50, and MIC90 of fluconazole were 1.0175 ± 0.799, 1, and 2 µg/mL; whereas 0.0455 ± 0.029, 0.03, and 0.125 µg/mL were the mean, MIC50, and MIC90 of voriconazole against all 50 isolates. The 0.1643 ± 0.131, 0.125, and 0.25 µg/mL were also observed as the mean, MIC50, and MIC90 of amphotericin B against all isolates (Figure 2, Table 4).
Table 3.
Number of C. neoformans var. grubii Strains Having Specific Antifungal MIC
|
Drug
|
MIC (µg/mL)
|
|
0.03
|
0.06
|
0.125
|
0.25
|
0.5
|
1
|
2
|
4
|
8
|
16
|
32
|
64
|
| Fluconazole |
0 |
0 |
1 |
3 |
18 |
19 |
7 |
2 |
0 |
0 |
0 |
0 |
| Voriconazole |
35 |
10 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Amphotericin B |
4 |
12 |
19 |
10 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Note. MIC: Minimal inhibitory concentration.
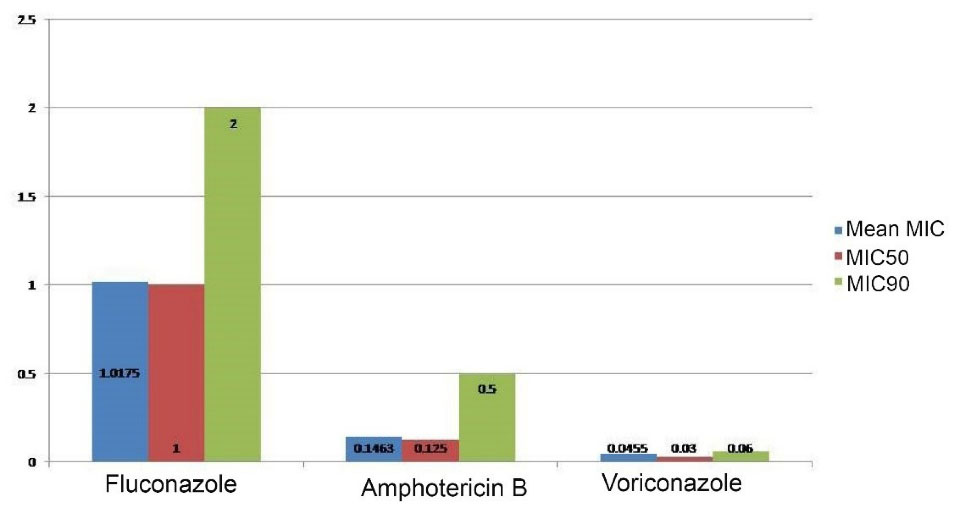
Figure 2.
Mean, MIC50, and MIC90 of Antifungal Agents Against Isolated Cryptococcus neoformans Strains. Note. MIC: Minimal inhibitory concentration
.
Mean, MIC50, and MIC90 of Antifungal Agents Against Isolated Cryptococcus neoformans Strains. Note. MIC: Minimal inhibitory concentration
Table 4.
Mean, MIC50, and MIC90 of Tested Antifungal Agents Against Cryptococcus neoformans var. grubii Strains
|
Antifungal Agent
|
MIC Range (µg/mL)
|
MIC50
(µg/mL)
|
MIC90
(µg/mL)
|
Mean MIC±SD
|
| Fluconazole |
0.125-4 |
1 |
2 |
1.017 ± 0.79 |
| Voriconazole |
0.03-0.125 |
0.03 |
0.06 |
0.0455 ± 0.029 |
| Amphotericin B |
0.03-0.5 |
0.125 |
0.25 |
0.1463 ± 0.114 |
Note. MIC: Minimal inhibitory concentration; SD: Standard deviation.
Discussion
Cryptococcus neoformans is the opportunistic yeast, which is manifested as meningoencephalitis among HIV-infected patients having CD4 + T cells < 100/µL. C. neoformans is divided into three serotypes, A, D, and AD hybrid. Serotype A is known as grubii, whereas serotype D is known as neoformans. These two varieties of C. neoformans primarily result in these central nervous system (CNS) manifestations worldwide. The grubii variety is the most common cause of about 95% of cases of cryptococcal infection worldwide, whereas the neoformans variety causes ~5% of the cryptococcal infection in western countries (3). In the present study, grubii(n = 50) is the sole causative agent of all cryptococcal meningitis cases among HIV-infected patients. A similar study in North India has reported C. neoformans var grubii as a causative agent in all 46 cases of cryptococcal meningitis (14). The causative variety is identified by phenotypic, serological, and molecular methods. Phenotypic and serological methods are time-taking and laborious; thus, this differentiation can be executed by the singleplex PCR targeting the str1 gene by which we differentiate the two varieties of the C. neoformans and identify the C. gattii species (8).
Being environmental saprotrophs, the basidiospores of Cryptococcus aerosolize and deposit in the host alveoli, where active cell-mediated immunity impedes transmission. However, in HIV-infected patients and immunocompromised individuals, the virulence factors of Cryptococcus, the polysaccharide capsule, phenoloxidases, urease, phospholipase, and proteinase act together and lead to a disease state by mechanisms such as adhesion, invasion, and dissemination (2). The polysaccharide capsule and urease enzyme production are the major virulence factors of Cryptococcus. The capsule prevents phagocytosis, whereas the urease enzyme helps in the intracellular survival of C. neoformans by increasing pH (4,15). Nonetheless, the phenoloxidase enzyme makes it a neurotrophic yeast (2,4). Enzymes urease, phenoloxidase, proteinase, and phospholipase were produced by all 50 isolated strains, but in the present study, we only quantified phospholipase and proteinase enzymes.
The phospholipase enzyme demonstrates phospholipase B, lysophospholipase hydrolase, and lysophospholipase transacylase activities which support fungal cell attachment. This enzyme also degrades the phospholipid component of the cell membrane, accentuating its invasion (16). Cryptococcal proteinase enzyme degrades different cellular proteins, including collagen, elastin, fibrinogen, complement factors, and immunoglobulin, disrupting the host tissue barriers (17). Thus, all these enzymes, along with the capsule, accentuate the pathogenesis. The roles of phospholipase and proteinase enzymes in the invasion and dissemination of Cryptococcus have also been reported in other studies (16-18).
Cryptococcal meningoencephalitis cases are managed by combination therapy, including amphotericin B, fluconazole, and 5-flucytosine. Amphotericin B binds to the sterol component of a fungal cell wall, resulting in pore formation with subsequent lysis. In contrast, fluconazole inhibits the synthesis of ergosterol, an important component of the fungal cell membrane. In addition, 5-flucytosine inhibits the synthesis of fungal nucleic acid. C. neoformans strains usually respond well to the above-mentioned drugs. However, fluconazole resistance/non-susceptibility/increasing MIC has been reported from different parts of the globe (5,6,7). Inadequate doses of fluconazole with widespread use in agriculture as a preventive tool for black banana rot disease may attribute the emergence (19). ARTEMIS DISK Global Antifungal Surveillance Study has reported constantly increasing fluconazole resistance among C. neoformans from 7.3% to 11.7% (20). Non-responding/increasing fluconazole MIC against C. neoformans strains has been reported from Taiwan and Uganda. In the present study, 1 and 2 µg/mL fluconazole MIC50 and MIC90 were observed, respectively, against 50 isolates of C. neoformans, whereas it was 8 and 32 µg/mL in Uganda (21). Our results are in line with the findings of a study by Chaudhary et al, reporting 2 and 4 µg/mL fluconazole MIC50 and MIC90, respectively, against the isolated C. neoformans var. grubii strains (22). Another study from AIIMS, New Delhi reported a higher fluconazole MIC50 and MIC90 values of 4 and 16 µg/mL, respectively (23). This variability may be attributed to the AIIMS, an apex centre of health care in India, where the most referred patients are treated.
Fluconazole is widely used in the management of cryptococcal meningoencephalitis. For effective management, the adequate drug concentration is to be achieved at the site of action (brain parenchyma). However, a lower fluconazole cerebellum AUC/plasma AUC of 46.9% has been reported earlier by Sudan et al (24,25). Thus, conventional fluconazole doses can easily tackle the strains with a low fluconazole MIC. In contrast, strains with a higher MIC have to be managed either by high drug doses or the drug combination. Accordingly, treatment failure is a common occurrence with a higher fluconazole MIC, as strains having a lower fluconazole MIC are eliminated at conventional dosing, leaving behind the subpopulation with a higher MIC, subsequently resulting in treatment failure/relapse (26,27). CLSI recommends an 8 µg/mL fluconazole epidemiological cutoff against C. neoformans (28). However, Sudan et al reported 2 µg/mL MIC as a fluconazole breakpoint against C. neoformans isolated from meningoencephalitis cases (24).
Voriconazole has a similar mechanism of action to fluconazole. Espinel-Ingroff et al reported epidemiological cutoff (ECOFF) for voriconazole (0.25 µg/mL) against C. neoformans and C. gattii (28). Our study results revealed a lower (0.03 µg/mL) voriconazole MIC50. Conversely, Thompson et al found a higher voriconazole antifungal activity against C. neoformans strains (29). Thus, a lower voriconazole MIC against isolated C. neoformans may provoke clinicians for its use in treatment as it can easily cross the blood-brain barrier.
Amphotericin B binds the sterol component of the fungal cell wall, resulting in pore formation with subsequent leakage of cytoplasmic content and cell death. Therefore, amphotericin B, along with fluconazole, is used in the continuous phases of the treatment. Espinel-Ingroffet al reported (0.5 µg/mL) an epidemiological cutoff for amphotericin B against C. neoformans (28). All the isolated strains had a MIC within epidemiological cutoff values in our study. These results conform to the findings of the study by Chaudharyet al, demonstrating 0.235, 0.25, and 0.5 µg/mL amphotericin B mean, MIC50, and MIC90 values, respectively, against 160 clinical isolates of C. neoformans var. grubii (22).
Although the MIC of the tested antifungal agents is within the epidemiological cut-off, a close watch on the treatment response is the need of time as in the last decade. Numerous cases of relapse/ treatment failure have been reported globally. Sudanet al found a higher 96.8% success rate in patients infected with C. neoformans with 2 µg/mL fluconazole MIC compared to a 52% success rate in patients infected with strains having 4 µg/mL MIC (24). However, in the present study, we only determined the in-vitro MIC of fluconazole, voriconazole, and amphotericin B against isolated Cryptococcus strains, without any correlation of clinical responses with the antifungal MIC. Thus, there is a need for a prospective study to determine the correlation of fluconazole and amphotericin B MICs with treatment outcomes in cryptococcal meningoencephalitis.
Conclusion
Cryptococcus neoformans var. grubii is the sole causative agent of meningoencephalitis among enrolled patients. All the isolates have urease, proteinase, and phospholipase enzymes. Moreover, the emergence of C. neoformans strains having a higher fluconazole MIC might result in treatment failure/relapse in meningoencephalitis cases.
Authors’ Contribution
Conceptualization: Munesh K Gupta, Ragini Tilak, Jaya Chakravarty.
Data curation: Munesh K Gupta, Namrata Pal, Bhupendra Kumar.
Formal analysis: Munesh K Gupta, Namrata Pal, Ashish Kumar Singh, Bhupendra Kumar.
Investigation: Munesh K Gupta, Namrata Pal, Ashish Kumar Singh.
Methodology: Munesh K Gupta, Namrata Pal, Ragini Tilak, Jaya Chakravarty, Bhupendra Kumar, Ashish Kumar Singh.
Resources: Munesh K Gupta, Ragini Tilak, Jaya Chakravarty.
Software: Ashish Kumar Singh, Bhupendra Kumar, Munesh K Gupta.
Supervision: Ragini Tilak, Jaya Chakravarty, Munesh K Gupta.
Validation: Ragini Tilak, Munesh K Gupta.
Visualization: Ragini Tilak, Jaya Chakravarty.
Writing–original draft: Namrata Pal, Ashish Kumar Singh, Bhupendra Kumar, Munesh K Gupta.
Writing–review & editing: Munesh K Gupta, Ragini Tilak, Jaya Chakravarty, Bhupendra Kumar.
Competing Interests
There is no conflict of interest.
Funding
There is no source of funding.
References
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 2017; 13(1):13-24. doi: 10.1038/nrneurol.2016.167 [Crossref] [ Google Scholar]
- Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol 2014; 6:169-82. doi: 10.2147/clep.s38850 [Crossref] [ Google Scholar]
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am 2016; 30(1):179-206. doi: 10.1016/j.idc.2015.10.006 [Crossref] [ Google Scholar]
- O’Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 2012; 25(3):387-408. doi: 10.1128/cmr.00001-12 [Crossref] [ Google Scholar]
- Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungal. J Antimicrob Chemother 2013; 68(11):2435-44. doi: 10.1093/jac/dkt221 [Crossref] [ Google Scholar]
- Bongomin F, Oladele RO, Gago S, Moore CB, Richardson MD. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018; 61(5):290-7. doi: 10.1111/myc.12747 [Crossref] [ Google Scholar]
- Singhal S, Gupta P, Lamba BS, Singh P, Chouhan MI, Meher D. Rare case of amphotericin-B resistant cryptococcal meningitis in HIV non reactive patient. Int J Infect Dis 2016; 45 Suppl 1:199-200. doi: 10.1016/j.ijid.2016.02.459 [Crossref] [ Google Scholar]
- Feng X, Fu X, Ling B, Wang L, Liao W, Yao Z. Development of a singleplex PCR assay for rapid identification and differentiation of Cryptococcus neoformans var grubii, Cryptococcus neoformans var neoformans, Cryptococcus gattii, and hybrids. J Clin Microbiol 2013; 51(6):1920-3. doi: 10.1128/jcm.00064-13 [Crossref] [ Google Scholar]
- Costa CR, Passos XS, e Souza LK, de Andrade Lucena P, de Fátima Lisboa Fernandes O, do Rosário Rodrigues Silva M. Differences in exoenzyme production and adherence ability of Candida spp isolates from catheter, blood and oral cavity. Rev Inst Med Trop Sao Paulo 2010; 52(3):139-43. doi: 10.1590/s0036-46652010000300005 [Crossref] [ Google Scholar]
- Rüchel R, Tegeler R, Trost M. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia 1982; 20(3):233-44. doi: 10.1080/00362178285380341 [Crossref] [ Google Scholar]
- Pandey N, Gupta MK, Tilak R. Extracellular hydrolytic enzyme activities of the different Candida spp isolated from the blood of the intensive care unit-admitted patients. J Lab Physicians 2018; 10(4):392-6. doi: 10.4103/jlp.jlp_81_18 [Crossref] [ Google Scholar]
- Galán-Ladero MA, Blanco MT, Sacristán B, Fernández-Calderón MC, Pérez-Giraldo C, Gómez-García AC. Enzymatic activities of Candida tropicalis isolated from hospitalized patients. Med Mycol 2010; 48(1):207-10. doi: 10.3109/13693780902801242 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. CLSI Document M27-A2. Wayne, PA: CLSI; 2002.
- Duggal S, Duggal N, Hans C, Duggal AK. Epidemiology of cryptococcal meningitis associated with HIV in an Indian hospital. Epidemiology (Sunnyvale) 2014; 4(4):166. doi: 10.4172/2161-1165.1000166 [Crossref] [ Google Scholar]
- Fu MS, Coelho C, De Leon-Rodriguez CM, Rossi DCP, Camacho E, Jung EH. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog 2018; 14(6):e1007144. doi: 10.1371/journal.ppat.1007144 [Crossref] [ Google Scholar]
- Almeida F, Wolf JM, Casadevall A. Virulence-associated enzymes of Cryptococcus neoformans. Eukaryot Cell 2015; 14(12):1173-85. doi: 10.1128/ec.00103-15 [Crossref] [ Google Scholar]
- Zaragoza O. Basic principles of the virulence of Cryptococcus. Virulence 2019; 10(1):490-501. doi: 10.1080/21505594.2019.1614383 [Crossref] [ Google Scholar]
- Vidotto V, Melhem M, Pukinskas S, Aoki S, Carrara C, Pugliese A. Extracellular enzymatic activity and serotype of Cryptococcus neoformans strains isolated from AIDS patients in Brazil. Rev Iberoam Micol 2005; 22(1):29-33. doi: 10.1016/s1130-1406(05)70003-6 [Crossref] [ Google Scholar]
- Smith KD, Achan B, Hullsiek KH, McDonald TR, Okagaki LH, Alhadab AA. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother 2015; 59(12):7197-204. doi: 10.1128/aac.01299-15 [Crossref] [ Google Scholar]
- Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 105-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 2010; 48(4):1366-77. doi: 10.1128/jcm.02117-09 [Crossref] [ Google Scholar]
- Chen YC, Chang TY, Liu JW, Chen FJ, Chien CC, Lee CH. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: a 12-year longitudinal study. BMC Infect Dis 2015; 15:277. doi: 10.1186/s12879-015-1023-8 [Crossref] [ Google Scholar]
- Chowdhary A, Randhawa HS, Sundar G, Kathuria S, Prakash A, Khan Z. In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var grubii and Cryptococcus gattii serotype B from north-western India. J Med Microbiol 2011; 60(Pt 7):961-7. doi: 10.1099/jmm.0.029025-0 [Crossref] [ Google Scholar]
- Datta K, Jain N, Sethi S, Rattan A, Casadevall A, Banerjee U. Fluconazole and itraconazole susceptibility of clinical isolates of Cryptococcus neoformans at a tertiary care centre in India: a need for care. J Antimicrob Chemother 2003; 52(4):683-6. doi: 10.1093/jac/dkg399 [Crossref] [ Google Scholar]
- Rizk ML, Zou L, Savic RM, Dooley KE. Importance of drug pharmacokinetics at the site of action. Clin Transl Sci 2017; 10(3):133-42. doi: 10.1111/cts.12448 [Crossref] [ Google Scholar]
- Sudan A, Livermore J, Howard SJ, Al-Nakeeb Z, Sharp A, Goodwin J. Pharmacokinetics and pharmacodynamics of fluconazole for cryptococcal meningoencephalitis: implications for antifungal therapy and in vitro susceptibility breakpoints. Antimicrob Agents Chemother 2013; 57(6):2793-800. doi: 10.1128/aac.00216-13 [Crossref] [ Google Scholar]
- Stott KE, Beardsley J, Whalley S, Kibengo FM, Mai NTH, Tùng NLN. Population pharmacokinetic model and meta-analysis of outcomes of amphotericin B deoxycholate use in adults with cryptococcal meningitis. Antimicrob Agents Chemother 2018; 62(7):e02526-17. doi: 10.1128/aac.02526-17 [Crossref] [ Google Scholar]
- Mpoza E, Rhein J, Abassi M. Emerging fluconazole resistance: implications for the management of cryptococcal meningitis. Med Mycol Case Rep 2018; 19:30-2. doi: 10.1016/j.mmcr.2017.11.004 [Crossref] [ Google Scholar]
- Espinel-Ingroff A, Aller AI, Canton E, Castañón-Olivares LR, Chowdhary A, Cordoba S. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 2012; 56(11):5898-906. doi: 10.1128/aac.01115-12 [Crossref] [ Google Scholar]
- Thompson GR 3rd, Wiederhold NP, Fothergill AW, Vallor AC, Wickes BL, Patterson TF. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob Agents Chemother 2009; 53(1):309-11. doi: 10.1128/aac.01216-08 [Crossref] [ Google Scholar]