Avicenna Journal of Clinical Microbiology and Infection. 7(1):15-21.
doi: 10.34172/ajcmi.2020.03
Original Article
Synthesis and Antimicrobial Evaluation of the Potassium Salts of Benzhydrazine Dithiocarbamates
Hamid Beyzaei 1, *  , Sedigheh Esmaeilzadeh Bahabadi 2, *, Shahla Najafi 2, Fahime Heidari Sadegh 2
, Sedigheh Esmaeilzadeh Bahabadi 2, *, Shahla Najafi 2, Fahime Heidari Sadegh 2
Author information:
1Department of Chemistry, Faculty of Science, University of Zabol, Zabol, Iran.
2Department of Biology, Faculty of Science, University of Zabol, Zabol, Iran.
*
Corresponding author: Hamid Beyzaei, Associate Professor, Department of Chemistry, Faculty of Science, University of Zabol, P.O. Box 538-98615, Zabol, Iran, Tel: +98 54 31232186, Fax: +98 54 31232180, E-mail:
hbeyzaei@uoz.ac.ir, Sedigheh Esmaeilzadeh Bahabadi, Associate Professor, Department of Biology, Faculty of Science, University of Zabol, P.O. Box 538-98615, Zabol, Iran, Tel: +98 54 31232187, Fax: +98 54 31232180, E-mail:
esmaeilzadeh@uoz.ac.ir
Abstract
Background: New antimicrobial agents must be designed and synthesized for treating infectious diseases. In this study, antibacterial and antifungal activities of 6 potassium dithiocarbamates including three newly synthesized products were assessed on 10 bacterial and 3 fungal pathogens.
Methods: To this end, some benzhydrazine derivatives were reacted with carbon disulfide to afford dithiocarbamates, followed by applying diethyl ether and potassium hydroxide as solvent and base. Then, antimicrobial susceptibility tests were used to determine minimum inhibitory concentration, the minimum bactericidal concentration, and minimum fungicidal concentration values.
Results: The chemical structure of all synthesized dithiocarbamates were characterized with 1 H-, 13C-NMR (hydrogen-1 and 13-carbon nuclear magnetic resonance) and Fourier-transform infrared spectra. A variety of inhibitory effects was observed by the synthesized salts. Most synthetic dithiocarbamates affected bacterial strains and could efficiently block the proliferation of pathogenic fungi.
Conclusions: In general, prepared dithiocarbamates as potent chelating agents are able to interact with cell wall sulfur-containing compounds and the essential enzymes of microorganisms. In addition, the design of new hydrazine-based ligands and their corresponding complexes in future research can improve therapeutic properties. The evaluation of the cytotoxic effects of synthesized dithiocarbamates can also help their antimicrobial usages. Thus, these sulfur-rich and water-soluble salts are potential agents for combating plant pests.
Keywords: Antibacterial activity, Antifungal effect, Broth microdilution, Potassium dithiocarbamate, Streak plate
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
The lack of personal and social hygiene has led to the spread of infectious diseases in society (1). In addition, drug-resistant bacterial and fungal pathogenic strains have increasingly spread because of the inappropriate and unnecessary use of antibiotics and antifungal agents (2). As a result, the discovery, design, and synthesis of new antimicrobial compounds have become an inevitable necessity in the field of hygiene and healthcare (3). Dithiocarbamates are biologically active organic compounds containing the -N(C=S)S- moiety, which are usually prepared in the presence of a base from the reaction of amines or their equivalent derivatives with carbon disulfide (4). According to (5), numerous available functional groups in dithiocarbamates have given them diverse therapeutic properties (Figure 1). Thiram is a potent scabicidal drug, which is especially prescribed for the treatment of skin diseases. It is also used as a fungicide in agriculture (6). In addition, dithiocarb as a chelating agent helps to remove toxic metals from body tissues (7). Further, (8) confirmed the inhibitory effects of brassinin derivatives on cancer cell lines, especially human clones (Caco-2). Furthermore, the blocking properties of valine dithiocarbamate zinc(II) complex against breast cancer cells (MCF-7) were more pronounced compared to cisplatin (9). Moreover, the excellent inhibitory effects against two β-carbonic anhydrase enzymes belonging to pathogenic Mycobacterium tuberculosis were observed with N-mono- and N-,N-disubstituted dithiocarbamates (10).
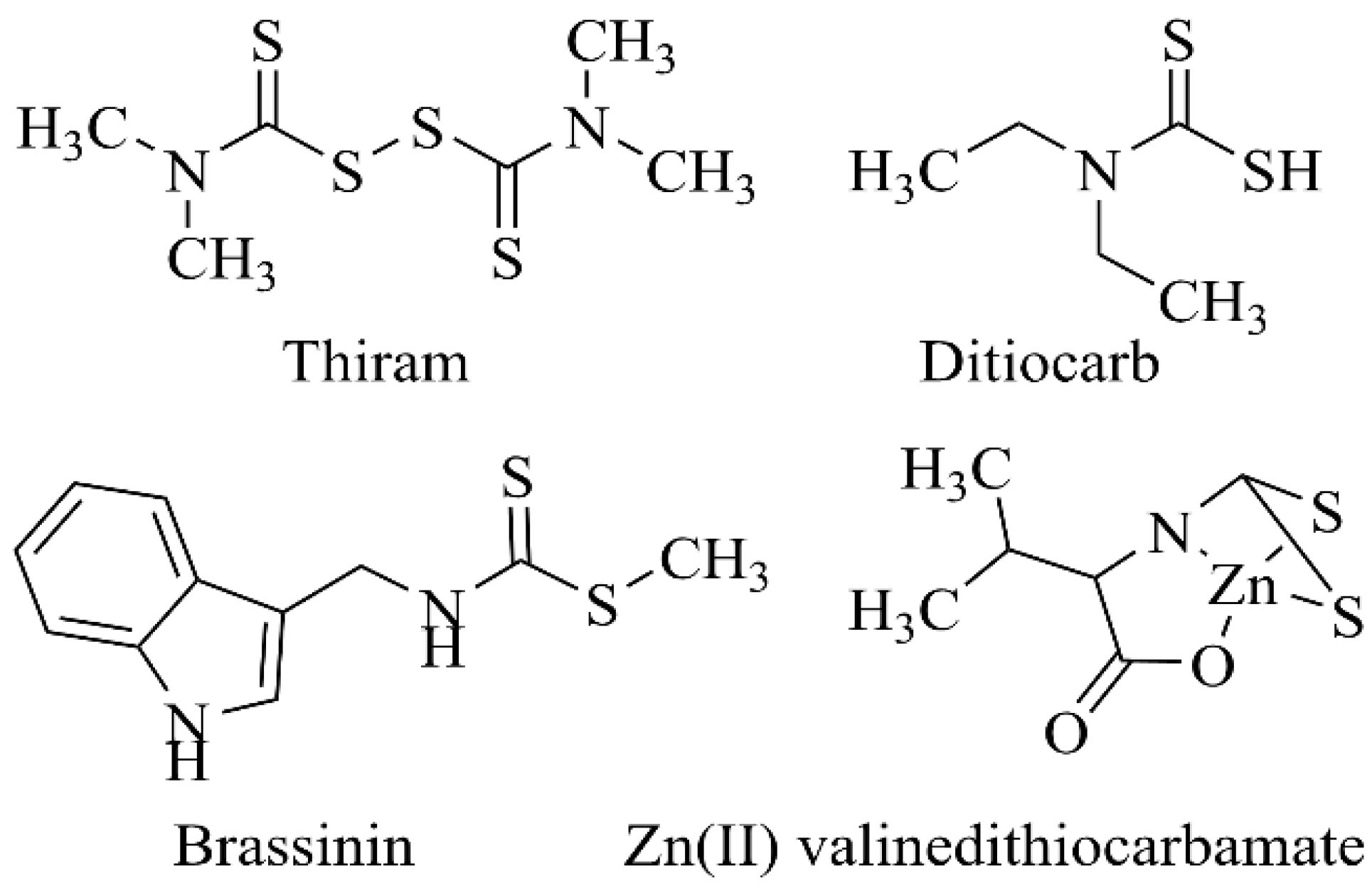
Figure 1.
Biologically Active Dithiocarbamate Derivatives (6-9).
.
Biologically Active Dithiocarbamate Derivatives (6-9).
The varied biological properties of dithiocarbamates encouraged us to synthesize several derivatives of this family via the reaction of different benzhydrazines and carbon disulfide, and their antimicrobial properties were assessed against a wide range of bacterial and fungal pathogens.
Materials and Methods
Chemicals
All chemicals were purchased from Merck Company (Germany). The progress of the reaction was checked by aluminum thin-layer chromatography plates (20 × 20 cm) with silica gel 60 coated with the fluorescent indicator F254. Additionally, the Kruss type KSP1N melting point meter (Germany) and UV-2100 RAY Leigh UV-Vis spectrophotometer (China) were used to determine uncorrected melting points and the absorption spectra, respectively. The Fourier-transform infrared (FT-IR) spectra of all synthesized dithiocarbamates were recorded on a Bruker Tensor-27 FT-IR spectrometer (Germany) available in the instrumental analysis laboratory of University of Zabol. Their 1H- and 13C-NMR (400 & 100 MHz) spectra were collected via a Bruker FT-NMR Ultra Shield-400 spectrometer (Germany).
General Process for the Preparation of Dithiocarbamates 3a-f
A mixture containing 10 mmol of hydrazines 1a-f, 10 mmol of potassium hydroxide (0.56 g), and 25 mL of diethyl ether in a 50-mL round-bottom flask was stirred in an ice-bath. Next, 10 mmol of carbon disulfide (0.76 g) was added dropwise to it for 1 hour under these conditions. The reaction continued for another 3 hours at room temperature. Eventually, the solids were collected, washed with cold ethanol (5 mL) and diethyl ether (5 mL), respectively, and then dried over P2O5 in the vacuum desiccator to give potassium salts of dithiocarbamate 3a-f.
Potassium 2-Phenylhydrazine-1-carbodithioate (3a)
IR ν: 3413, 1623, 618, 475 cm-1; 1H NMR (400 MHz, DMSO-d6) 𝛿: 7.39 (m, 2H, H-3,5 Ar), 7.09 (m, 2H, H-2,6 Ar), 6.71 (m, 1H, H-4 Ar), 5.29 (s, 2H, 2×NH) ppm; 13C NMR (100 MHz, DMSO-d6) 𝛿: 228.7 (C=S), 147.6 (C-1 Ar), 129.3 (C-3,5 Ar), 123.0 (C-4 Ar), 113.7 (C-2,6 Ar) ppm.
Potassium 2-(p- Tolyl)hydrazine -1-carbodithioate (3b)
IR ν: 3413, 1624, 619, 475 cm-1; 1H NMR (400 MHz, DMSO-d6) 𝛿: 7.51 (d, J = 8.2 Hz, 2H, H-3,5 Ar), 7.22 (d, J = 8.2 Hz, 2H, H-2,6 Ar), 5.12 (s, 2H, 2×NH), 2.31 (s, 3H, CH3) ppm; 13C NMR (100 MHz, DMSO-d6) 𝛿: 230.3 (C=S), 144.8 (C-1 Ar), 137.6 (C-4 Ar), 129.2 (C-3,5 Ar), 125.9 (C-2,6 Ar), 21.1 (CH3) ppm.
Potassium 2-(4- Fluorophenyl)hydrazine -1-carbodithioate (3c) (New Compound)
IR ν: 3413, 1624, 618, 475 cm-1; 1H NMR (400 MHz, DMSO-d6) 𝛿: 7.25 (d, J = 8.0 Hz, 2H, H-2,6 Ar), 6.94 (d, J = 8.0 Hz, 2H, H-3,5 Ar), 4.90 (s, 2H, 2×NH) ppm; 13C NMR (100 MHz, DMSO-d6) 𝛿: 229.8 (C=S), 144.4 (C-4 Ar), 136.0 (C-1 Ar), 116.3 (C-3,5 Ar), 115.1 (C-2,6 Ar) ppm.
Potassium 2-(4- Chlorophenyl)hydrazine -1-carbodithioate (3d) (New Compound)
IR ν: 3413, 1624, 619, 475 cm-1; 1H NMR (400 MHz, DMSO-d6) 𝛿: 7.74 (d, J = 8.4 Hz, 2H, H-3,5 Ar), 7.50 (d, J = 8.4 Hz, 2H, H-2,6 Ar), 4.17 (s, 2H, 2×NH) ppm; 13C NMR (100 MHz, DMSO-d6) 𝛿: 225.4 (C=S), 132.2 (C-1 Ar), 128.8 (C-3,5 Ar), 127.8 (C-2,6 Ar), 122.1 (C-4 Ar) ppm.
Potassium 2-(2,4- Dinitrophenyl)hydrazine -1-carbodithioate (3e)
IR ν: 3413, 1625, 1324, 620 cm-1; 1H NMR (400 MHz, DMSO-d6) 𝛿: 8.75 (1H, s, H-3 Ar), 8.16 (d, J = 10.2 Hz, 1H, H-5 Ar), 7.60 (d, J = 9.2 Hz, 1H, H-6 Ar), 5.03 (s, 2H, 2×NH) ppm; 13C NMR (100 MHz, DMSO-d6) 𝛿: 230.3 (C=S), 149.3 (C-1 Ar), 134.5 (C-4 Ar), 129.8 (C-5 Ar), 128.1 (C-2 Ar), 123.9 (C-3 Ar), 116.0 (C-6 Ar), 55.6 (CH3) ppm.
Potassium 2-(4- Bromophenyl)hydrazine -1-carbodithioate (3f) (New Compound)
IR ν: 3413, 1623, 1112, 619, 477 cm-1; 1H NMR (400 MHz, DMSO-d6) 𝛿: 7.72-7.62 (m, 4H, Ar-H), 4.20 (s, 2H, 2×NH) ppm; 13C NMR (100 MHz, DMSO-d6) 𝛿: 230.3 (C=S), 138.9 (C-1 Ar), 131.8 (C-3,5 Ar), 128.1 (C-2,6 Ar), 120.7 (C-4 Ar) ppm.
Antimicrobial Assay
Microorganisms
Roswell Park Memorial Institute (RPMI) 1640 media (Roswell Park Memorial Institute 1640), Mueller-Hinton broth (MHB), and Mueller-Hinton agar (MHA) were purchased from HiMedia Company (India). Gram-negative and -positive bacterial strains and fungal strains were prepared from the Persian Type Culture Collection. The first group included Salmonella enterica subsp. enterica (PTCC 1709), Klebsiella pneumoniae (PTCC 1290), Escherichia coli (PTCC 1399), Shigella dysenteriae (PTCC 1188), Acinetobacter baumannii (PTCC 1855), and Pseudomonas aeruginosa (PTCC 1310). In addition, the second group of strains encompassed Streptococcus pyogenes (PTCC 1447), Listeria monocytogenes (PTCC 1297), Staphylococcus epidermidis (PTCC 1435), and Bacillus cereus (PTCC 1665). Finally, fungal strains included yeast Candida albicans (PTCC 5027), molds Fusarium oxysporum (PTCC 5115), and Aspergillus fumigatus (PTCC 5009). Broth microdilution and streak plate methods were applied to evaluate antibacterial and antifungal susceptibility tests (11). In addition, the initial suspensions of bacteria, yeast, and molds were prepared in appropriate broth media with concentrations of 5 × 105,0.5-2.5 × 103, and 0.4-5 × 104 CFU.mL-1, respectively. The results were reported as the mean of three independent experiments.
Minimum Inhibitory Concentration Experiment
To this end, 20 μL of each dithiocarbamate was added to twelve wells in a row of a 96-well microliter plate at concentrations of 20480, 10240, 5120, 2560, 1280, 640, 320, 160, 80, 40, 20, and 10 μg.mL-1 in distilled water, respectively. Then, 80 μL of RPMI 1640 or MHB, as well as 100 μL of microbial suspensions were added to all wells. Next, the plates were incubated at 37 and 35°C for 20 and 48 hours for bacteria and fungi on an orbital shaker (100 rpm), respectively. Eventually, the minimum inhibitory concentration(MIC) value was the lowest concentration of salts which stopped microbial growth (11).
MBC and Minimum Fungicidal Concentration Experiments
The samples of all non-turbid wells in the MIC experiment were re-cultured in RPMI 1640 or MHA agar media plates. Then, the plates were incubated for 24 hours at appropriate temperatures except that 45%-55% relative humidity was supplied for fungi. Finally, the Minimum Fungicidal Concentration (MFC)or MBC value was determined as the lowest concentration of compounds that killed microorganisms (11).
Results and Discussion
Potassium dithiocarbamates 3a-f were generated via the reaction of benzhydrazines 1a-f and carbon disulfide (2). In addition, potassium hydroxide and diethyl ether were used as base and solvent, respectively (Scheme 1). The synthetic data are reported in Table 1. The chemical structure of synthesized dithiocarbamates was characterized by spectral data (Figure 2).
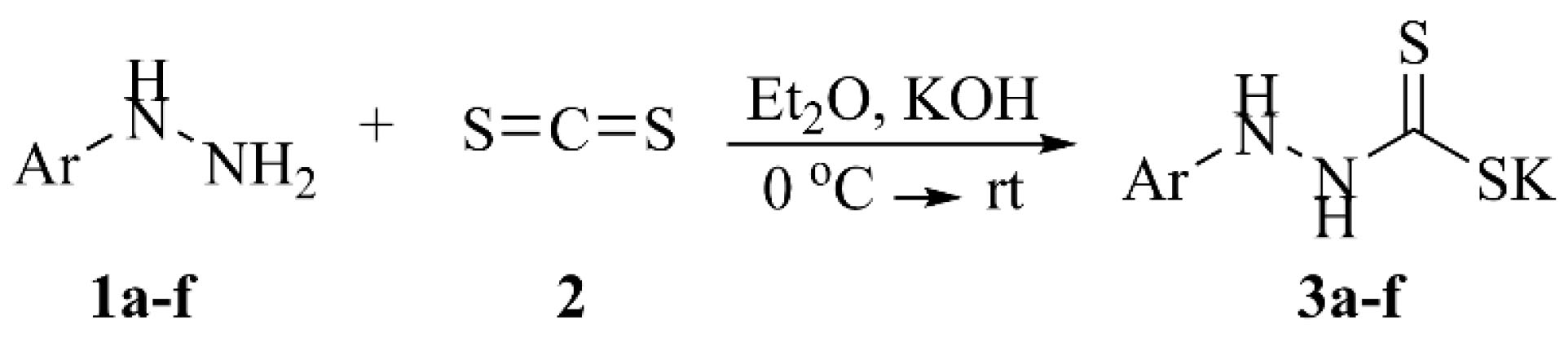
Scheme 1.
Representative Strategy for the Synthesis of Potassium Dithiocarbamates 3a-f.
.
Representative Strategy for the Synthesis of Potassium Dithiocarbamates 3a-f.
Table 1.
The Chemical Structure of Dithiocarbamic Acid Potassium Salts 3a-f
|
Entry
|
Product
|
|
R
|
Yield (%)
|
M. P. (
°
C)
|
|
Found
|
Lit. (Ref.)
|
| 1 |
|
3a
|
C6H5 |
91 |
131-132 (decomp.) |
144-145 (decomp.) (12) |
| 2 |
|
3b
|
4-H3C-C6H4 |
86 |
210-212 |
- (13) |
| 3 |
|
3c
|
4-F-C6H4 |
80 |
289-291 |
- |
| 4 |
|
3d
|
4-Cl-C6H4 |
83 |
295-296 |
- |
| 5 |
|
3e
|
2,4-(O2N)2-C6H3 |
93 |
190-191 |
- (14) |
| 6 |
|
3f
|
4-Br-C6H4 |
89 |
200-202 |
- |
Note. Melting points of salts 3b and 3e were not reported in the literature.
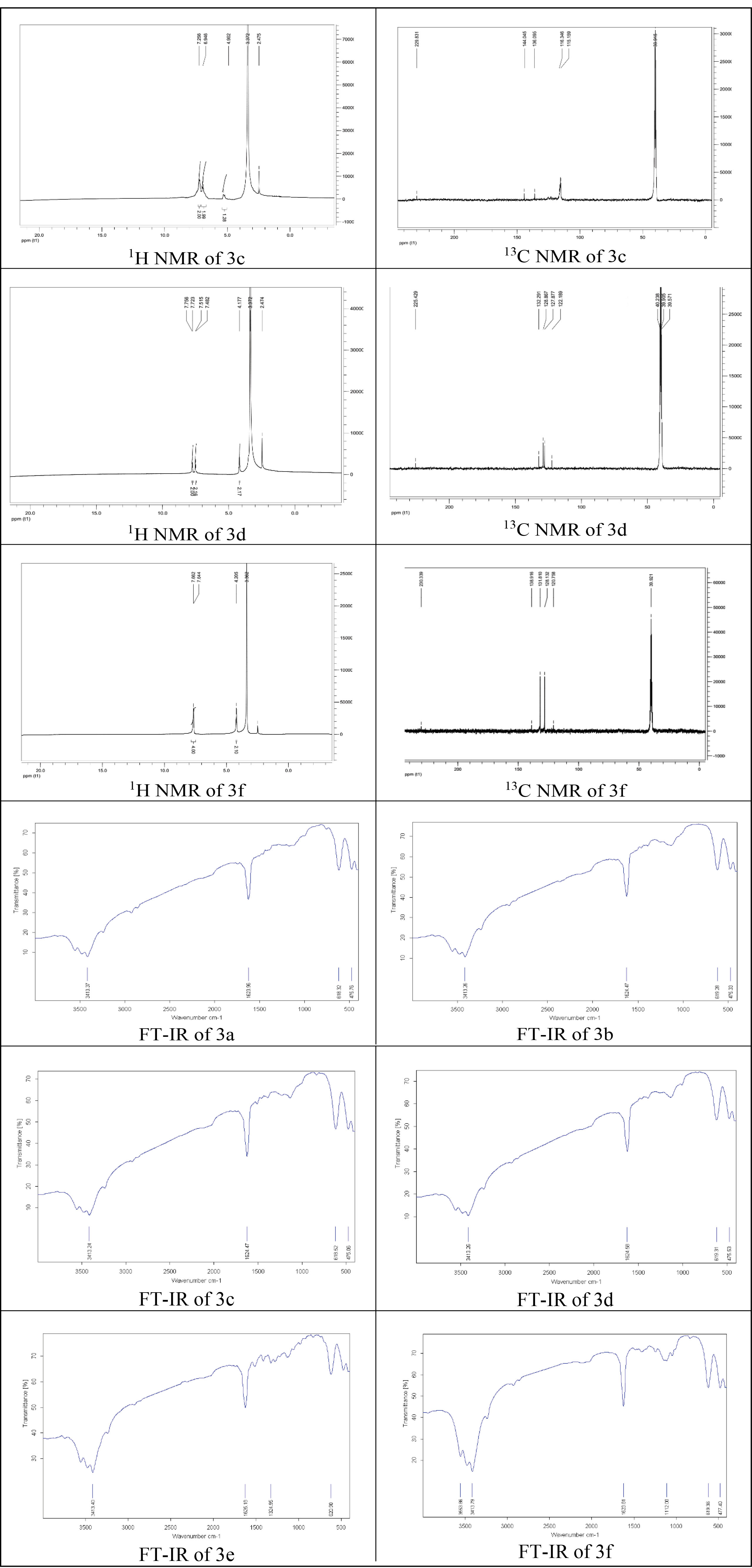
Figure 2.
Selected Spectral Graphs of Dithiocarbamic Acid Potassium Salts 3a-f.
.
Selected Spectral Graphs of Dithiocarbamic Acid Potassium Salts 3a-f.
In vitro blocking potential of all synthesized dithiocarbamates was assessed on some important pathogenic bacteria and fungi including four gram-positive and 6 Gram-negative bacterial strains, 2 molds and one yeast. Further, ampicillin and clotrimazole were used as control drugs. As shown in Table 2, a wide variety of activities was obtained with dithiocarbamates that had substituted or unsubstituted phenyl. All dithiocarbamates except for salts 3c and 3d inhibited the tested bacteria and no blocking effects on Escherichia coli and Klebsiella pneumoniae strains were observed with derivatives 3c and 3d. On the other hand, good inhibitory effects against gram-negative Shigella flexneri and Listeria monocytogenes, as well as gram-positive Bacillus cereus and Staphylococcus aureus strains were observed with the potassium salts of morpholine dithiocarbamate, along with its Cu(II) and Ni(II) complexes. More precisely, complexation decreased antibacterial activities (15). Furthermore, the disk diffusion method was applied to determine the blocking properties of potassium 3-dithiocarboxy-3-aza-5- aminopentanoate on nine hospital bacteria (16). Pyrrolidine dithiocarbamates are potent chelating agents and ionophores that easily enter into the cell (17). Moreover, the antibacterial effects of their Zn(II) complexes are usually higher compared to Cu(II) equivalents.
Table 2.
Antibacterial Properties of Dithiocarbamates 3a-f
|
Bacterial Strains
|
|
Salts
|
Antibiotic
Ampicillin
|
|
3a
|
3b
|
3c
|
3d
|
3e
|
3f
|
| 1855 |
MIC |
1024 |
512 |
256 |
512 |
64 |
512 |
64 |
|
|
MBC |
2048 |
1024 |
256 |
512 |
128 |
1024 |
128 |
| 1399 |
MIC |
1024 |
32 |
˃2048 |
˃2048 |
2048 |
1024 |
32 |
|
|
MBC |
2048 |
64 |
˃2048 |
˃2048 |
2048 |
2048 |
64 |
| 1709 |
MIC |
64 |
128 |
32 |
64 |
256 |
32 |
8 |
|
|
MBC |
64 |
128 |
32 |
64 |
512 |
32 |
16 |
| 1310 |
MIC |
1 |
2 |
32 |
1024 |
2048 |
64 |
1024 |
|
|
MBC |
2 |
2 |
32 |
1024 |
2048 |
64 |
2048 |
| 1290 |
MIC |
128 |
64 |
˃2048 |
˃2048 |
2 |
64 |
32 |
|
|
MBC |
256 |
128 |
˃2048 |
˃2048 |
4 |
128 |
64 |
| 1188 |
MIC |
512 |
256 |
1 |
1 |
1 |
128 |
256 |
|
|
MBC |
512 |
256 |
2 |
1 |
2 |
128 |
256 |
| 1435 |
MIC |
2 |
2 |
1 |
1024 |
2 |
2 |
0.25 |
|
|
MBC |
4 |
2 |
1 |
1024 |
4 |
2 |
2 |
| 1447 |
MIC |
128 |
32 |
512 |
512 |
32 |
128 |
4 |
|
|
MBC |
128 |
32 |
1024 |
512 |
32 |
128 |
8 |
| 1665 |
MIC |
2048 |
1024 |
16 |
256 |
2048 |
2048 |
32 |
|
|
MBC |
2048 |
1024 |
32 |
256 |
2048 |
2048 |
64 |
| 1297 |
MIC |
2 |
64 |
2 |
32 |
256 |
256 |
8 |
|
|
MBC |
2 |
128 |
4 |
32 |
256 |
256 |
16 |
Note. MIC (μg.mL-1): Minimum inhibitory concentration; MBC (μg.mL-1): Minimum bactericidal concentration.
The in vitro blocking effects of synthetic salts were investigated on three pathogenic fungi (Table 3). Based on the results, all synthesized dithiocarbamates affected Candida albicans and Fusarium oxysporum strains. No inhibitory activity against Aspergillus fumigatus was observed with dithiocarbamates 3b and 3d. Additionally, the antifungal activity of some synthesized Pt(II), Pd(II), and Ni(II) dithiocarbamate complexes was studied against Penicillium citrinum, Aspergillus flavus, Aspergillus niger, and Aspergillus parasiticus. They were more efficient at blocking fungi than nystatin (18). Some nontoxic dirutheniumpentadithiocarbamate complexes were synthesized to treat invasive fungal infections. In other words, their MIC values were very similar to those of fluconazole (19). It was proposed that dithiocarbamates act as nonspecific and multisite antifungal agents (20). They further inactivate cellular thiol enzymes and disrupt the energy supply process of fungal cells.
Table 3.
Antifungal Properties of Dithiocarbamates 3a-f
|
Fungal Strains
|
|
Salts
|
Antifungal
|
|
3a
|
3b
|
3c
|
3d
|
3e
|
3f
|
Clotrimazole
|
| 5027 |
MIC |
512 |
1 |
512 |
128 |
512 |
128 |
256 |
|
|
MFC |
512 |
2 |
512 |
128 |
512 |
256 |
512 |
| 5009 |
MIC |
512 |
˃2048 |
128 |
˃2048 |
1024 |
512 |
32 |
|
|
MFC |
512 |
˃2048 |
128 |
˃2048 |
2048 |
512 |
32 |
| 5115 |
MIC |
64 |
2 |
256 |
16 |
64 |
2048 |
256 |
|
|
MFC |
64 |
4 |
256 |
32 |
128 |
2048 |
512 |
Note. MIC (μg.mL-1): Minimum inhibitory concentration; MFC (μg.mL-1); Minimum Fungicidal Concentration.
Conclusions
In general, dithiocarbamates are multi-functionalized compounds with a variety of biological properties and thus their synthesis is important. This study evaluated the antibacterial and antifungal effects of 6 synthesized potassium dithiocarbamates (three new salts) on pathogenic bacteria and fungi. These dithiocarbamates showed relatively acceptable antibacterial effects against Gram-negative S. enterica and S. dysenteriae, as well as Gram-positive S. epidermidis and L. monocytogenes. In addition, good to excellent antifungal activities were observed with dithiocarbamates. More precisely, they are potent chelating ligands and complexation may improve their antimicrobial properties. Eventually, these water-soluble salts can be applied as pesticides and plant growth-promoting agents although their toxic effects must be studied in future studies.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgement
This work was financially supported by the University of Zabol under grant numbers UOZ-GR-9618-10 and UOZ-GR-9618-20.
Ethical Statement
The disclaimer implies that ethical principles have been considered in relation to the proposed work and no ethical issues have been found to be applied to this research proposal.
Authors’ Contribution
Hamid Beyzaei: Supervision, writing original draft, writing- reviewing, and editing; Sedigheh Esmaeilzadeh Bahabadi: Data analysis; Shahla Najafi: Investigation; Fahime Heidari Sadegh: Methodology.
Funding/Support
University of Zabol provided financial support.
References
- Bloomfield SF, Rook GA, Scott EA, Shanahan F, Stanwell-Smith R, Turner P. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health 2016; 136(4):213-24. doi: 10.1177/1757913916650225 [Crossref] [ Google Scholar]
- Smieszek T, Pouwels KB, Dolk FCK, Smith DRM, Hopkins S, Sharland M. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother 2018; 73(suppl_2):ii36-ii43. doi: 10.1093/jac/dkx500 [Crossref] [ Google Scholar]
- Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 2014; 6:25-64. doi: 10.4137/pmc.s14459 [Crossref] [ Google Scholar]
- Oliveira JWF, Rocha HAO, de Medeiros W, Silva MS. Application of dithiocarbamates as potential new antitrypanosomatids-drugs: approach chemistry, functional and biological. Molecules 2019; 24(15). doi: 10.3390/molecules24152806 [Crossref]
- Odularu AT, Ajibade PA. Dithiocarbamates: challenges, control, and approaches to excellent yield, characterization, and their biological applications. Bioinorg Chem Appl 2019; 2019:8260496. doi: 10.1155/2019/8260496 [Crossref] [ Google Scholar]
- Kurpios-Piec D, Grosicka-Maciąg E, Woźniak K, Kowalewski C, Kiernozek E, Szumiło M. Thiram activates NF-kappaB and enhances ICAM-1 expression in human microvascular endothelial HMEC-1 cells. Pestic Biochem Physiol 2015; 118:82-9. doi: 10.1016/j.pestbp.2014.12.003 [Crossref] [ Google Scholar]
- Ohashi Y, Yamada K, Takemoto I, Mizutani T, Saeki K. Inhibition of human cytochrome P450 2E1 by halogenated anilines, phenols, and thiophenols. Biol Pharm Bull 2005; 28(7):1221-3. doi: 10.1248/bpb.28.1221 [Crossref] [ Google Scholar]
- Chripkova M, Drutovic D, Pilatova M, Mikes J, Budovska M, Vaskova J. Brassinin and its derivatives as potential anticancer agents. Toxicol In Vitro 2014; 28(5):909-15. doi: 10.1016/j.tiv.2014.04.002 [Crossref] [ Google Scholar]
- Kartina D, Wahab AW, Ahmad A, Irfandi R, Raya I. In vitro antibacterial and anticancer activity of Zn(II)Valinedithiocarbamate complexes. J Phys Conf Ser 2019; 1341:032042. doi: 10.1088/1742-6596/1341/3/032042 [Crossref] [ Google Scholar]
- Maresca A, Carta F, Vullo D, Supuran CT. Dithiocarbamates strongly inhibit the beta-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 2013; 28(2):407-11. doi: 10.3109/14756366.2011.641015 [Crossref] [ Google Scholar]
- Beyzaei H, Khosravi Z, Aryan R, Ghasemi B. A green one-pot synthesis of 3(5)-substituted 1,2,4-triazol-5(3)-amines as potential antimicrobial agents. J Iran Chem Soc 2019; 16(12):2565-73. doi: 10.1007/s13738-019-01714-2 [Crossref] [ Google Scholar]
- Katritzky AR, Bayyuk S. Novel rearrangement of a 3-amino-1,3-thiazole-2-thione. Heterocycles 1985; 23(12):3099-106. doi: 10.3987/R-1985-12-3099 [Crossref] [ Google Scholar]
- Hanley RN, Ollis WD, Ramsden CA. Synthesis of three new meso-ionic heterocyclic systems. J Chem Soc Chem Commun 1976(9):306-7. doi: 10.1039/C39760000306 [Crossref]
- Bhatia S, Kaushik NK, Sodhi GS. Organomercury(II) dithiocarbazates: synthesis, characterisation and biological studies. Inorganica Chim Acta 1987; 127(2):141-6. doi: 10.1016/S0020-1693(00)82112-X [Crossref] [ Google Scholar]
- Balakrishnan S, Duraisamy S, Kasi M, Kandasamy S, Sarkar R, Kumarasamy A. Syntheses, physicochemical characterization, antibacterial studies on potassium morpholine dithiocarbamate nickel (II), copper (II) metal complexes and their ligands. Heliyon 2019; 5(5):e01687. doi: 10.1016/j.heliyon.2019.e01687 [Crossref] [ Google Scholar]
- Vuksanovic V, Leka Z, Terzic N. Antibacterial effect of synthesized dithiocarbamate K-DAAP. Fresenius Environ Bull 2013; 22(12):3803-7. [ Google Scholar]
- Kang MS, Choi EK, Choi DH, Ryu SY, Lee HH, Kang HC. Antibacterial activity of pyrrolidine dithiocarbamate. FEMS Microbiol Lett 2008; 280(2):250-4. doi: 10.1111/j.1574-6968.2008.01069.x [Crossref] [ Google Scholar]
- Ferreira IP, de Lima GM, Paniago EB, Takahashi JA, Pinheiro CB. Synthesis, characterization and antifungal activity of new dithiocarbamate-based complexes of Ni(II), Pd(II) and Pt(II). Inorganica Chim Acta 2014; 423(Pa A):443-9. doi: 10.1016/j.ica.2014.09.002 [Crossref] [ Google Scholar]
- Donnici CL, Nogueira LJ, Araujo MH, Oliveira SR, Magalhães TF, Lopes MT. In vitro studies of the activity of dithiocarbamate organoruthenium complexes against clinically relevant fungal pathogens. Molecules 2014; 19(4):5402-20. doi: 10.3390/molecules19045402 [Crossref] [ Google Scholar]
- Veignie E, Ceballos C, Len C, Rafin C. Design of new antifungal dithiocarbamic esters having bio-based acrylate moiety. ACS Omega 2019; 4(3):4779-84. doi: 10.1021/acsomega.8b03685 [Crossref] [ Google Scholar]