Avicenna Journal of Clinical Microbiology and Infection. 7(1):22-26.
doi: 10.34172/ajcmi.2020.04
Original Article
Prevalence and Risk Factors Associated to Neospora caninum (Apicomplexa: Toxoplasmatidae) in Pet Dogs From Hamadan, West of Iran, 2016
Jamal Gharekhani 1, 2, *  , Mohammad Yakhchali 2, Reza Khaltabadi-Farahani 3, 4
, Mohammad Yakhchali 2, Reza Khaltabadi-Farahani 3, 4
Author information:
1Department of Laboratory Sciences, Central Veterinary Laboratory, Iranian Veterinary Organization, Hamadan, Iran.
2Department of Pathobiology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
3Department of Molecular Biology, Pasteur Institute of Iran, Tehran, Iran.
4Department of Molecular Biology, National Veterinary Reference Laboratory, Iranian Veterinary Organization, Tehran, Iran.
*
Corresponding author: Jamal Gharekhani, Department of Laboratory Sciences, Central Veterinary Laboratory, Iranian Veterinary Organization, Hamadan, Iran, Postal Code: 6519611156, Tel: + (98)81 32651801, Fax: + (98)81 32644474, Email:
Gharekhani_76@yahoo.com
Abstract
Background: Neosporosis is considered as a ubiquitous disease in Iran and other countries. This research was expected to determine the prevalence and related risk factors of Neospora caninum in household dogs in Hamadan Municipality, Iran.
Methods: A total of 184 whole blood was evaluated for the presence of antibodies to N. caninum by the enzyme-linked immunosorbent assay (ELISA). All seropositive animals were affirmed by molecular techniques.
Results: Based on serology and molecular methods, N. caninum infection was detected in 4.9% (95% CI = 4.9 ± 3.1%) of animals. In addition, the highest infection rate was significantly recognized in female dogs (57.1%) with under 6 months old (54.4%). Additionally, the clinical signs of neosporosis were observed in 2 out of 4 positive dogs (P<0.0001, odds ratio [OR] = 24.71). Finally, the infection had no significant connection (P>0.05) with breeding, food regime, housing, and direct contact with infected animals.
Conclusions: In general, the serological and molecular outcomes were parallel together. It was concluded that this is a universal assessment of risk factors related to N. caninum in Iranian house dogs for the first time.
Keywords: Neospora caninum, PetS, Dogs, Iran, ELISA, PCR
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Neospora caninum as a parasite (Apicomplexa: Toxoplasmatidae) with global prevalence was first reported in puppies from Norway (1). Canine and a wide-range of herbivore animals are the definitive and intermediate hosts for N. caninum (2).
The infected dogs may represent neuromuscular neurologic disorders such as progressive flaccid paralysis of hind limbs and jaw, gradual atrophy of muscles, dysphagia, and heart failure (3). Although puppies are generally born without clinical symptoms, they ordinarily present progressing toward ascending paralysis of the rear limbs in 3 weeks after birth (3,4). Diagnosis is impossible by using only clinical signs. Accordingly, serological tools, namely, enzyme-linked immunosorbent assay (ELISA) are proposed to distinguish N. caninum in epidemiologic works. So far, molecular techniques have been used for affirming the serological assessment (2,5).
N. caninum infectionwas detected among 2.1% and 54.6% in various types of dogs, samples, and diagnostic methods in different regions of Iran (6-21). Considering the above-mentioned explanations (Table 1), the present study aimed to assess the infection rate and risk factors related to N. caninum in the Iranian household dogs of Hamadan municipality, located in the west of Iran. Furthermore, a historical mini-review on N. caninum infection in the dogs of Iran is presented in the Discussion Section.
Table 1.
Prevalence ofNeospora caninum Infection in Dogs in Different Regions of Iran
|
Region
|
Type of Animals
|
Type of Samples
|
Infection Rate (%)
|
Technique
|
Reference
|
|
Location
|
Municipalities
|
| Western Iran |
Hamadan |
Stray |
Blood |
52.8 |
ELISA* |
(6) |
| Shepherd |
Blood |
18 |
ELISA |
| Stray |
Blood |
5 |
ELISA |
(7) |
| Farm |
Blood |
8.6 |
ELISA |
(21) |
| Lorestan |
Farm |
Stool |
2.1 |
PCR |
(8) |
| Northwestern Iran |
Tabriz |
Stray |
Blood |
31 |
IFAT |
(9) |
| Urmia |
Stray |
Blood |
27 |
IFAT |
(10) |
| Sarab, Tabriz |
Shepherd |
Blood |
10.6 |
IFAT |
(11) |
| Meshkin-Shahr, Ardabil |
Rural |
Blood |
30.4 |
ELISA |
(12) |
| Northeastern Iran |
Mashhad |
Farm |
Stool |
2.2 |
PCR |
(13) |
| Central of Iran |
Tehran |
Farm |
Blood |
46 |
IFAT |
(14) |
| Farm |
Blood |
28 |
IFAT |
(15) |
| Urban |
Blood |
20 |
IFAT |
(14) |
| Urban |
Blood |
11.3 |
IFAT |
(15) |
| Stray |
Blood |
2.2 |
ELISA |
(16) |
| Stray |
Brain and other tissues |
35.7 |
Nested-PCR |
(16) |
| Isfahan |
Stray |
Blood |
17.7 |
IFAT |
(17) |
| Clinic animals, mix |
Blood |
10.3 |
IFAT |
(18) |
| Southwestern Iran |
Shahrekord |
Stray |
Blood |
22 |
IFAT |
(18) |
| Shahrekord and Ahvaz |
Stray |
Blood |
43.3 |
ELISA |
(19) |
| Southern Iran |
Shiraz |
Farm |
Blood |
44.4 |
MAT |
(20) |
| Farm |
Blood |
54.6 |
Dot-ELIZA |
(20) |
Note. ELISA: Enzyme-linked immunosorbent assay; PCR: Polymerase chain reaction; IFAT: Indirect fluorescent-antibody test; MAT: Modified agglutination test; *All of ELISA methods were used a commercially Kit, ID-Vet® Company, France.
Methods
Sampling Location, Animals, and Serology
Hamadan Municipality is situated in the west part of Iran (34.77° N and 48.58° E) which is surrounded by mountains and has normal yearly temperature of 11.3°C. During March-September 2016, a total of 184 whole blood was randomly sampled from household dogs that were referred to Veterinary Clinics in Hamedan. Data relating to animals was recorded and sera samples were evaluated after their preparation in order to identify antibodiesto N. caninum using the ELISA (ID-Vet Company, France).
Molecular Examination
The genomic DNA extraction of N. caninum in the whole blood samples of seropositive animals was performed by using the Dyna-BioTM blood kit (Takapouzist Company, Iran). According to the protocol of Müller et al (22), a pair of Np21 plus and Np6 plus primers (Forward: 5’CCCAGTGCGTCCAATCCTGTAAC3’ and Reverse: 5’CTCGCCAGTCAACCTACGTCTTCT3’) was used to amplify a 330 bp-fragment-length of NC5 gene. Additionally, the nested polymerase chain reaction (PCR) was performed to amplify a 100 bp-fragment of the PCR product by using 1 µL of each PCR product and a pair of primers (Forward: 5’GTGTTGCTCTGCTGACGTGT-3’ and Reverse: 5’-TACCAACTCCCTCGGTTCAC-3’) (23).
Data Analysis
The chi-square test (χ2) was used to evaluate the relation among the infection rate and different variables (SPSS 16.0, Chicago, IL, USA) and the probability of ≤ 0.05 was considered significant.
Results
The antibodiesto N. caninum were detected in 4 (4.9%, CI 95% = 1.8-8%) out of 184 examined dogs (Table 2). All seropositive animals were confirmed by nested PCR (Figure 1). The infection rate in female animals (7.6%) was 6.4-fold higher compared to male animals (P = 0.047). The highest infection rate was observed in dogs with 1-2 years old (6.7%) but no significant association was found between infection rate and age groups (P > 0.05). In addition, 5.4% and 3.7% infection rate were found in pure and mixed breed dogs, respectively (P = 0.630). The clinical signs of neosporosis were recorded in 2 seropositive dogs (50%, P < 0.0001, OR = 24.71) while the infection had no connection with a food regime (P = 0.914), housing (P = 0.366), and direct contact with another animal (P = 0.610).
Table 2.
The Infection Rate of Neospora caninum in Household Dogs in Hamadan in Iran
|
Risk Factors
|
No. of Sample (%)
|
No. of Positive (%)
|
Statistical Analysis
|
| Sex |
Male |
79 (42.9) |
1 (1.3) |
χ2= 3.911, P= 0.047,
OR= 6.43 |
| Female |
105 (57.1) |
8 (7.6) |
| Breed |
Pure |
130 (70.7) |
7 (5.4) |
χ2= 0.231, P= 0.630 |
| Mixed |
54 (29.3) |
2 (3.7) |
| Age (month) |
< 6 |
119 (64.7) |
7 (5.9) |
χ2= 0.317, P= 0.853 |
| 6-12 |
50 (27.2) |
1 (2) |
| 12-24 |
15 (8.1) |
1 (6.7) |
| Food regime |
Home made |
46 (25) |
1 (2.2) |
χ2= 0.178, P= 0.914 |
| Commercial food |
21(11.4) |
0 (0) |
| Mixed |
117 (63.6) |
8 (6.8) |
| Housing |
Indoors |
51 (27.7) |
1 (1.9) |
χ2= 2.009, P= 0.366 |
| In- and outdoors |
122 (66.3) |
3 (2.5) |
| In outdoors |
11 (6) |
5 (45.5) |
| Contact with other animals |
Yes |
152 (82.6) |
8 (5.3) |
χ2= 0.259, P= 0.610 |
| No |
32 (17.4) |
1 (3.1) |
| Clinical signs |
Yes |
4 (2.2) |
2 (50) |
χ2= 17.885, P< 0.0001,
OR= 24.71 |
| No |
180 (97.8) |
7 (3.9) |
| Total |
|
184 (100) |
9 (4.9) |
CI 95%= 4.9%± 3.1 |
Note. OR: Odds ratio; CI: Confidence interval.
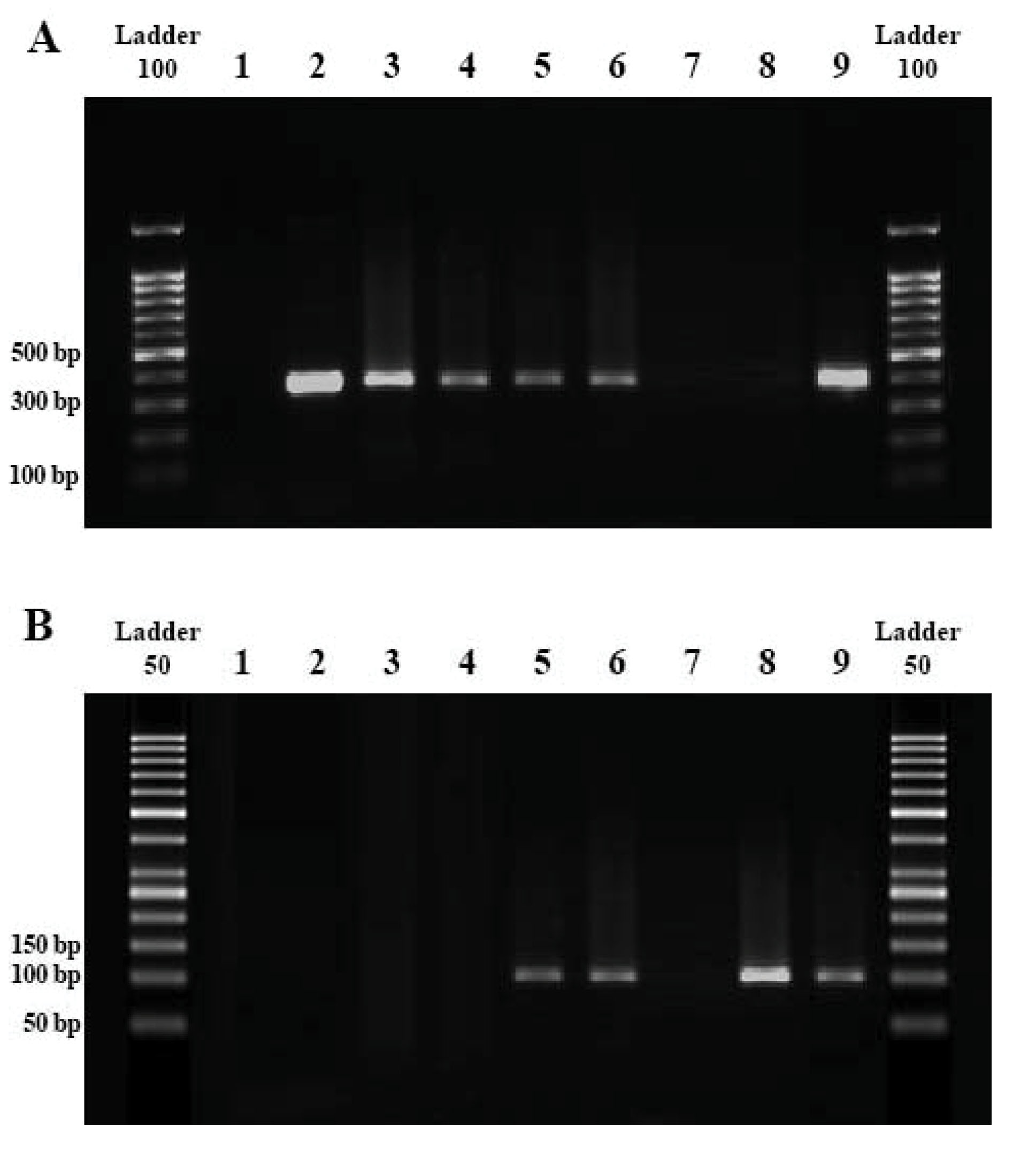
Figure 1.
Gel Electrophoresis Results of Neospora caninum Detection
A (PCR gel): Negative control (L1); Positive control (L2,9); Positive samples (L3-6; bands of 330 bp); Negative samples (L7,8).
B (nested-PCR gel): Negative control (L1); Positive control (L8); Positive samples (L5,6,9; bands of 100 bp); Negative samples (L2-4,7).
.
Gel Electrophoresis Results of Neospora caninum Detection
A (PCR gel): Negative control (L1); Positive control (L2,9); Positive samples (L3-6; bands of 330 bp); Negative samples (L7,8).
B (nested-PCR gel): Negative control (L1); Positive control (L8); Positive samples (L5,6,9; bands of 100 bp); Negative samples (L2-4,7).
Discussion
The interest of keeping pet animals is progressively in spotlights, and therefore, their health status is crucial in the transmission of infection agents to humans. A concern to N. caninum infection in dogs might play a zoonotic role because of its close biologic relationship with Toxoplasma gondii and the occurrence of accidental oocyst ingestion and/or the consumption of raw meat containing tissue cysts. In addition, antibodies to N. caninum were reported in humans with a deficiency in the immune system (24). According to Yakhchali et al (10), assessing seroprevalence and hence the exposure of dogs to N. caninum are considered as the significant parts of investigating the possible transmission routes of the parasite and identifying dogs in which there is the possibility of neosporosis. Additionally, it should be noted that the negative results observed during the examinations of animals do not necessarily confirm the absence of infection (2).
On the global scale, the seroprevalence rate of N. caninum was estimated in 17.14% of the dog’ population (25) while 4.9% of our examined animals were seropositive. This finding contradicts that of the other studies (5,26,27) conducted in Turkey (10%), Switzerland (7.3%), Brazil (10-26.5%), the United Kingdom (5.8%-16.6%), and the United States (2-7%). These discrepancies might be ascribed to apply various laboratory methods, climatic variations, and dog population types (6). Low prevalence in the examined dogs may be due to their living conditions, limited direct exposure to potential sources of infection, feeding habits, and the owners’ attention for eliminating raw meat from the dogs’ diet, along with impossible climates such as low humidity and temperature for a pathogen, which is effective in sporulation time in the region (13,14).
The infection rate of N. caninum was significantly higher in female than male dogs (Table 2), which is in agreement with the findings of Goździk et al (28) while in contrast with those of other studies in Turkey (27) and Iran (6,10,12,14). Alexander and Stinson (29) noted that female dogs were progressively susceptible to N. caninum. The hormonal contrasts may have a significant role in host susceptibility to N. caninum. Since estrogen excretion enhances antibody production and androgen suppresses T and B cell immune responses, in females, the immunity to infection can be broken down as a result of various factors such as nutrition, age, pregnancy, and environmental changes (7).
In the current study, the highest infection rate was recognized in dogs of 12-24 months old (Table 2). In a similar investigation, Basso et al (30) revealed the highest antibodiesto N. caninum in dogs of more than one year old (47.7%). Interestingly, the seroprevalence rate increased in dogs with postnatal exposure to N. caninum through horizontal transmission in prior reports in Iran (10,14,15). The high infection in older animals is due to their chance of exposure to infection over time (17).
N. caninum infection in the pure breed (5.4%) was higher than mixed breed (3.7%) with no significant difference. Nearly all reports confirmed that there is no correlation between breed susceptibility to N. caninum with seroprevalence (31). Further, Nazir et al (32) reported that there were no significant differences between seroprevalence and breeds belonging to Alsatian (12.6%), Bully (20.6%), Pug (20.4%), Bullterrier (18.9%), German Shepherd (31.4%), Labrador Retriever (19.6%), Crossbreds (28.7%), and Mongrel breeds (35.4%). In contrast, Robbe et al (33) reported higher seropositivity to N. caninum in pure breed compared to the other type. Supplementary universal investigations are suggested subsequently because of less information about the impact of breeds in the epidemiology of canine neosporosis.
The clinical signs of neosporosis in dogs, usually neurological disorders, are not pathognomonic because they are very helpful in the exact diagnosis of diseases parallel to laboratory tools (4). In our study, musculoskeletal and neurological signs were recorded in 2 infected animals. Additionally, the infection rate had no association with food regime, housing, and direct contact with another animal (Table 2). The infection with Neospora is associated with animal food materials. Dogs with access to prey or raw food materials outside their home derived from intermediate hosts can increase the risk for seropositivity (5).
In conclusion, the outcomes affirmed the presence and exposure of examined dogs to N. caninum in Hamadan. This investigation, to the best of our knowledge, was the primary presentation of risk factors related to N. caninum infection in Iranian pet dogs. Further investigations on household animal populations are necessary for determining the prevalence and possible routes of infection in dogs from other parts of the country. Finally, using diagnostic screening tools and anti-Neospora compounds in pet dog clinics is recommended for controlling the disease.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgments
The authors wish to thank all clinicians for helping in the sample collection process. This work was not upheld by any foundation.
Ethical Statement
The samples were taken from the dogs with official permission and under the supervision of the Institutional Animal Ethics and Research Committee of Iranian Veterinary Organization, Hamadan office, Iran (Certificate No. 32/1396.4.1).
Authors’ Contribution
JG designed the study, sampling, laboratory techniques, data analysis and writing the paper. MY helped for designing the study and revised the final version of manuscript. RK helped in the design of molecular protocol. All authors reviewed the manuscript.
References
- Bjerkås I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd 1984; 70(2):271-4. doi: 10.1007/bf00942230 [Crossref] [ Google Scholar]
- Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol 2003; 41(1):1-16. doi: 10.3347/kjp.2003.41.1.1 [Crossref] [ Google Scholar]
- Dubey JP, Knickman E, Greene CE. Neonatal N caninum infections in dogs. Acta Parasitol 2005; 50(2):176-9. [ Google Scholar]
- Barber JS, Trees AJ. Clinical aspects of 27 cases of neosporosis in dogs. Vet Rec 1996; 139(18):439-43. doi: 10.1136/vr.139.18.439 [Crossref] [ Google Scholar]
- Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 2007; 20(2):323-67. doi: 10.1128/cmr.00031-06 [Crossref] [ Google Scholar]
- Gharekhani J, Tavoosidana GR, Akbarein H. Serological study of Neospora caninum infection in dogs and cattle from west of Iran. Comp Clin Path 2014; 23(5):1203-7. doi: 10.1007/s00580-013-1763-z [Crossref] [ Google Scholar]
- Gharekhani J, Yakhchali M, Abbasi-Doulatshahi E, Barati E. Seroprevalence of Neospora caninum and Toxoplasma gondii infections in stray dogs of Hamadan suburb, west of Iran, 2018. Avicenna J Clin Microbiol Infect 2019; 6(2):57-60. doi: 10.34172/ajcmi.2019.11 [Crossref] [ Google Scholar]
- Ghafarifar F, Sabevarinejad G, Dalimi A, Forouzandeh-Moghadam M. Molecular detection of Neospora caninum from naturally infected dogs in Lorestan province, west of Iran. Arch Razi Inst 2014; 69(2):185-90. doi: 10.7508/ari.2014.02.010 [Crossref] [ Google Scholar]
- Gharedaghi Y. Seroprevalence of Neospora caninum in stray dogs of Tabriz, Iran. J Anim Vet Adv 2012; 11(6):723-6. doi: 10.3923/javaa.2012.723.726 [Crossref] [ Google Scholar]
- Yakhchali M, Javadi S, Morshedi A. Prevalence of antibodies to Neospora caninum in stray dogs of Urmia, Iran. Parasitol Res 2010; 106(6):1455-8. doi: 10.1007/s00436-010-1824-z [Crossref] [ Google Scholar]
- Khanmohammadi M, Fallah E. Prevalence of Neospora caninum antibodies in shepherd dogs in Sarab district, East Azerbaijan province, Iran. Afr J Microbiol Res 2011; 5(28):5062-6. doi: 10.5897/AJMR11.796 [Crossref] [ Google Scholar]
- Sharifdini M, Mohebali M, Keshavarz H, Hosseininejad M, Hajjaran H, Akhoundi B. Neospora caninum and Leishmania infantum co-infection in domestic dogs (Canis familiaris) in Meshkin-Shahr district, Northwestern Iran. Iran J Arthropod Borne Dis 2011; 5(2):60-8. [ Google Scholar]
- Razmi G. Fecal and molecular survey of Neospora caninum in farm and household dogs in Mashhad area, Khorasan province, Iran. Korean J Parasitol 2009; 47(4):417-20. doi: 10.3347/kjp.2009.47.4.417 [Crossref] [ Google Scholar]
- Malmasi A, Hosseininejad M, Haddadzadeh H, Badii A, Bahonar A. Serologic study of anti-Neospora caninum antibodies in household dogs and dogs living in dairy and beef cattle farms in Tehran, Iran. Parasitol Res 2007; 100(5):1143-5. doi: 10.1007/s00436-006-0385-7 [Crossref] [ Google Scholar]
- Haddadzadeh HR, Sadrebazzaz A, Malmasi A, Talei Ardakani H, Khazraii Nia P, Sadreshirazi N. Seroprevalence of Neospora caninum infection in dogs from rural and urban environments in Tehran, Iran. Parasitol Res 2007; 101(6):1563-5. doi: 10.1007/s00436-007-0678-5 [Crossref] [ Google Scholar]
- Pouramini A, Jamshidi S, Shayan P, Ebrahimzadeh E, Namavari M, Shirian S. Molecular and serological detection of Neospora caninum in multiple tissues and CSF in asymptomatic infected stray dogs. Iran J Vet Med 2017; 11(2):105-12. doi: 10.22059/ijvm.2017.61602 [Crossref] [ Google Scholar]
- Hosseininejad M, Mahzounieh M, Shams Esfandabadi N. Neospora caninum suspects as one of the most important causes of abortion in large dairy farms in Isfahan, Iran. Iran J Parasitol 2017; 12(3):408-12. [ Google Scholar]
- Hosseininejad M, Hosseini F, Mahzounieh M, Raisi-Nafchi A, Moshrraf M. Seroprevalence of Neospora caninum infection in dogs in Chaharmahal-va-Bakhtiari province, Iran. Comp Clin Path 2010; 19(3):269-70. doi: 10.1007/s00580-009-0864-1 [Crossref] [ Google Scholar]
- Hosseininejad M, Hosseini F. Seroprevalence of Neospora caninum and Toxoplasma gondii infection in dogs from west and central parts of Iran using two indirect ELISA tests and assessment of associate risk factors. Iran J Vet Res 2011; 12(1):46-51. doi: 10.22099/ijvr.2011.40 [Crossref] [ Google Scholar]
- Khordadmehr M, Hosseini S, Mohsenifar E, Namavari M, Khordadmehr S. Seroprevalence of Neospora caninum in farm and household dogs determined by ELISA. Online J Vet Res 2012; 16(4):172-81. [ Google Scholar]
- Gharekhani J, Yakhchali M. Neospora caninum infection in dairy farms with history of abortion in west of Iran. Vet Anim Sci 2019; 8:100071. doi: 10.1016/j.vas.2019.100071 [Crossref] [ Google Scholar]
- Müller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol 1996; 34(11):2850-2. [ Google Scholar]
- Macedo CA, Macedo MF, Cardim ST, Paiva MC, Taroda A, Barros LD. Neospora caninum: evaluation of vertical transmission in slaughtered dairy cows (Bos taurus). Rev Bras Parasitol Vet 2013; 22(1):13-7. doi: 10.1590/s1984-29612013000100004 [Crossref] [ Google Scholar]
- McCann CM, Vyse AJ, Salmon RL, Thomas D, Williams DJ, McGarry JW. Lack of serologic evidence of Neospora caninum in humans, England. Emerg Infect Dis 2008; 14(6):978-80. doi: 10.3201/eid1406.071128 [Crossref] [ Google Scholar]
- Anvari D, Saberi R, Sharif M, Sarvi S, Hosseini SA, Moosazadeh M, et al. Seroprevalence of Neospora caninum infection in dog population worldwide: a systematic review and meta-analysis. Acta Parasitol. 2020. 10.2478/s11686-019-00163-4
- Sager H, Moret CS, Müller N, Staubli D, Esposito M, Schares G. Incidence of Neospora caninum and other intestinal protozoan parasites in populations of Swiss dogs. Vet Parasitol 2006; 139(1-3):84-92. doi: 10.1016/j.vetpar.2006.02.021 [Crossref] [ Google Scholar]
- Coskun SZ, Aydýn L, Bauer C. Seroprevalence of Neospora caninum infection in domestic dogs in Turkey. Vet Rec 2000; 146(22):649. doi: 10.1136/vr.146.22.649 [Crossref] [ Google Scholar]
- Goździk K, Wrzesień R, Wielgosz-Ostolska A, Bień J, Kozak-Ljunggren M, Cabaj W. Prevalence of antibodies against Neospora caninum in dogs from urban areas in Central Poland. Parasitol Res 2011; 108(4):991-6. doi: 10.1007/s00436-010-2143-0 [Crossref] [ Google Scholar]
- Alexander J, Stimson WH. Sex hormones and the course of parasitic infection. Parasitol Today 1988; 4(7):189-93. doi: 10.1016/0169-4758(88)90077-4 [Crossref] [ Google Scholar]
- Basso W, Venturini L, Venturini MC, Moore P, Rambeau M, Unzaga JM. Prevalence of Neospora caninum infection in dogs from beef-cattle farms, dairy farms, and from urban areas of Argentina. J Parasitol 2001; 87(4):906-7. doi: 10.1645/0022-3395(2001)087[0906:poncii]2.0.co;2 [Crossref] [ Google Scholar]
- Fernandes BC, Gennari SM, Souza SL, Carvalho JM, Oliveira WG, Cury MC. Prevalence of anti-Neospora caninum antibodies in dogs from urban, periurban and rural areas of the city of Uberlândia, Minas Gerais--Brazil. Vet Parasitol 2004; 123(1-2):33-40. doi: 10.1016/j.vetpar.2004.05.016 [Crossref] [ Google Scholar]
- Nazir MM, Maqbool A, Akhtar M, Ayaz M, Ahmad AN, Ashraf K. Neospora caninum prevalence in dogs raised under different living conditions. Vet Parasitol 2014; 204(3-4):364-8. doi: 10.1016/j.vetpar.2014.05.041 [Crossref] [ Google Scholar]
- Robbe D, Passarelli A, Gloria A, Di Cesare A, Capelli G, Iorio R. Neospora caninum seropositivity and reproductive risk factors in dogs. Exp Parasitol 2016; 164:31-5. doi: 10.1016/j.exppara.2016.02.003 [Crossref] [ Google Scholar]