Avicenna Journal of Clinical Microbiology and Infection. 11(4):147-155.
doi: 10.34172/ajcmi.3570
Original Article
Identification of a Novel Extracellular Alkaline Protease-Producing Bacillus cereus G2 Isolated From Petroleum Sludge and Optimization of Enzyme Production
Bassam Al Balaa 1, *  , Samah Qasem 1, Nermeen Haj Mahmoud 1
, Samah Qasem 1, Nermeen Haj Mahmoud 1
Author information:
1Department of Molecular Biology and Biotechnology, Atomic Energy Commission of Syria (AECS), Damascus, Syria
Abstract
Background: Alkaline proteases are of significant utility in the field of biotechnology, with extensive applications in spanning numerous sectors, including those in the medical and health, pharmaceuticals, food, detergents, and leather industries.
Methods: The bacterial strain G2, isolated from petroleum sludge at the Homs Refinery in Syria, was evaluated for its ability to produce alkaline protease using a skim milk agar medium. Protease production was confirmed through a protease assay. The molecular identification of the selected isolate was conducted through sequencing of the 16S ribosomal deoxyribonucleic acid (rDNA). Culture conditions, including variables such as inoculum size, incubation duration, temperature, pH, and various carbon and nitrogen sources, were optimized to achieve protease production.
Results: Strain G2 exhibited alkaline proteolytic activity, thereby establishing its status as an effective protease producer. A 16S rDNA analysis of the intended strain revealed that the organism was identified as Bacillus cereus. The highest level of enzyme production was observed when the isolate, at an inoculum size of 3%, was cultured in the production medium for 48 hours at a pH rate of 10 and 45° C, using 1% glucose and 1% yeast extract as carbon and nitrogen sources, respectively. The alkaline protease activity was ultimately found to be 4.44-fold higher (533.3 U/mL) compared to the initial optimization of enzyme production (120 U/mL).
Conclusion: This constitutes the inaugural account of the alkaliphilic B. cereus G2 strain, which was isolated from sludge petroleum and is capable of secreting alkaline protease. The findings regarding the optimal cultural conditions for the production of thermophilic alkaline protease provide a crucial foundation for future research into the potential biotechnological applications of this strain in alkaline protease production.
Keywords: Bacillus cereus, Alkaline protease, Temperature, pH, Biotechnology, Sludge
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Al Balaa B, Qasem S, Haj Mahmoud N. Identification of a novel extracellular alkaline protease-producing Bacillus cereus G2 isolated from petroleum sludge and optimization of enzyme production. Avicenna J Clin Microbiol Infect. 2024; 11(4):147-155. doi:10.34172/ajcmi.3570
Introduction
Proteases represent a significant class of industrial enzymes, comprising a substantial portion of the global enzyme market. These hydrolytic enzymes play an integral role in the global enzyme market, contributing significantly to overall enzyme production and sales (1,2). The commercial value of these enzymes is underscored by their diverse sources, including bacteria, fungi, plants, and animals (3). Among these, microbial proteases are particularly noteworthy due to their extracellular secretion, facilitating easier downstream processing than their plant and animal counterparts (4). This quality renders microbial proteases highly advantageous for industrial applications, facilitating the production process and enhancing efficiency.
Alkaline proteases demonstrate a fascinating class of enzymes with significant biotechnological importance. These enzymes are classified as proteases and catalyze the hydrolysis of proteins into smaller peptide fragments. The defining characteristic of alkaline proteases is their ability to function optimally at alkaline pH levels, typically ranging from 7.5 to 12. This distinguishes them from other proteases, which are most effective at neutral or acidic pH ranges (4).
The production of alkaline proteases is primarily attributed to various microorganisms, with bacteria representing a particularly significant source (5). These bacteria have evolved to flourish in alkaline environments, including alkaline-rich water bodies, industrial waste sites, and alkaline soils. Bacteria are vital sources of enzymes due to their rapid growth, minimal space requirements for cultivation, and the ability to be genetically modified to produce new enzymes with modified properties (6,7). It has been reported that approximately 35% of the microbial enzymes utilized in the detergent industry are bacterial proteases (8). These alkaliphilic bacteria have evolved sophisticated cellular mechanisms that enable their survival in extreme alkaline conditions, including the secretion of alkaline proteases. A number of studies have identified alkaline protease-producing bacteria from a variety of sources, thereby underscoring the diversity of their enzymatic capabilities and the potential for their use in industrial applications (9-11).
The selection of an appropriate organism is of paramount importance for the achievement of high yields of the desired enzymes. The continued isolation and characterization of novel, promising strains utilizing cost-effective carbon and nitrogen sources are essential for advancements in industrial enzyme production (12). Notable among bacterial species are those of the genus Bacillus, which are known for their production of extracellular proteases. These bacteria are commonly found in soil and water, with certain strains demonstrating the capacity to withstand extreme environmental conditions, including high alkalinity (13). Consequently, they can be cultivated under extreme pH and temperature conditions, resulting in the production of enzymes that remain stable in a variety of harsh environments (14). These proteases are employed in a multitude of industrial sectors, including pharmaceuticals, food production, laundry, leather manufacturing, and waste management (15).
The components of the medium, including carbon and nitrogen sources, exert a profound influence on the organism’s growth and enzyme production. In addition to nutritional factors, cultural parameters, including pH, temperature, and incubation time, have been demonstrated to significantly affect enzyme production (16). It is, therefore, imperative to optimize both the components of the medium and the cultural parameters in any biological process.
The applications of alkaline proteases are numerous and diverse, encompassing a multitude of industries, including the medical and health sector, pharmaceuticals, detergent manufacturing, food processing, the leather industry, and waste treatment. Their capacity to perform effectively under harsh alkaline conditions renders them indispensable in these industrial processes. For example, alkaline proteases are extensively employed in the detergent industry to enhance the efficacy of stain removal, particularly for protein-based stains (17). In the leather industry, alkaline proteases facilitate dehairing processes, offering an environmentally preferable alternative to traditional chemical methods (18). Alkaline proteases have been the subject of investigation as a potential avenue for pharmaceutical therapeutics due to their proteolytic activity and compatibility with physiological conditions (19). They have a variety of applications in the medical field, including the development of medicines, drugs, and vaccines to combat a range of diseases, such as genetic disorders. Alkaline-fibrinolysis protease has been identified as a promising agent for selectively breaking down fibrin, suggesting its potential utility in thrombolytic therapy and anticancer treatments. Alkaline proteases are extensively utilized in medical applications, particularly in the form of collagenases. Furthermore, Bacillus species, which are renowned for their protease production abilities, are regarded as safe for human consumption.
In light of the aforementioned factors, the present investigation has been undertaken to identify an alkaliphilic bacterial strain capable of producing alkaline proteases and being isolated from petroleum sludge. Subsequently, the optimal conditions for alkaline protease production by this alkaliphilic strain are evaluated qualitatively under various chemical and physical conditions.
Materials and Methods
Bacterial Strain
This research focused on the bacterial strain G2, recognized for its protease production. This strain was isolated and purified in our laboratory from petroleum sludge at Homs Refinery in Syria.
Protease Production Determination
The ability of the bacterium to break down protein was tested by inoculating a bacterial culture on nutrient agar plates enriched with 1% skim milk. The plates were incubated at 37°C for 48 hours. Protease activity was confirmed by the formation of a clearance zone around colonies. Additionally, protease activity was quantified using a protease assay with casein as the substrate.
Molecular Identification of Selected Isolate
The molecular method was employed to identify the isolate. This isolate was confirmed through 16S ribosomal deoxyribonucleic acid (rDNA) sequencing and the Basic Local Alignment Search Tool (BLAST) analysis. The bacterial isolate was cultured overnight in nutrient broth, and then the genomic DNA was extracted from the bacterial cells. The amplification of 16S rDNA was performed using universal primers fD1 (5′-CCGAATTCGTCGACAACA GAGTTTGATCCTGGCTCAG-3′) and rP1 (5′-CCCGG GATCCAAGCTTACGGTTACCTTGTTACGACTT-3′) (20). Polymerase chain reaction (PCR)-amplified product was analyzed using agarose gel electrophoresis, and the molecular size of the fragment was estimated by referencing the DNA ladder (GeneRuler DNA Ladder Mix, Thermo Scientific). A sequence similarity search was performed using BLAST to identify the most closely related sequences in GenBank. The resulting sequences were aligned and analyzed to determine the closest microbial homologies.
Inoculum Preparation
A loopful of the culture was transferred into 10 mL of nutrient broth and incubated at 37 °C for 24 hours. Next, an inoculum size of 107 CFU from the bacterial culture was added to 50 mL of the production medium (Horikoshi I, pH: 9) containing 1% glucose, 0.5% peptone, 0.5% yeast extract, 1% Na2CO3, 0.1% K2HPO4, and 0.02% MgSO4.7H2O. The medium was then incubated at 160 rpm for 48 hours at 37 °C. After fermentation, the broth was centrifuged at 6000 rpm for 20 minutes at 4 °C, and the protease activity in the supernatant was assessed through a protease assay.
Proteolytic Activity Assay
To evaluate proteolytic activity, 0.1 mL of the supernatant was mixed with 1.1 mL of a reaction mixture containing 1% casein in 100 mM Tris-HCl buffer at a pH rate of 8. This mixture was incubated at 37 °C for 15 minutes. The reaction was halted by adding 1.8 mL of 5% (w/v) trichloroacetic acid, followed by centrifugation at 10 000 rpm for 15 minutes. One unit of enzyme activity was defined as a change in absorbance at 280 nm by 0.001 per minute under the specified conditions (21). All measurements were conducted in triplicate.
Effects of Culture Conditions on Protease Production of the Bacillus cereus G2 Strain
The influence of various culture conditions on the production of protease by B. cereus G2 underwent investigation. The physical and chemical parameters, including inoculum size, incubation time, temperature, initial pH, and sources of carbon and nitrogen, were evaluated as well. After centrifugation at 6000 rpm for 20 minutes at 4 °C, the protease production assay was performed as described in the previous paragraph. All experiments were conducted in triplicate, and this methodology was consistently applied across all parameters.
Inoculum Size
To investigate the impact of inoculum size on protease production, the production medium was inoculated with varying inoculum percentages of 0.5%, 1%, 2%, 3%, 4%, 5%, and 6% (v/v). The medium was then incubated at 37 °C for 48 hours.
Incubation Time
The incubation period was assessed by inoculating the production medium with the previously determined optimal inoculum size of 3%. The medium was then incubated at 37 °C for varying durations of 24 hours, 48 hours, 72 hours, and 96 hours.
Temperature
The impact of temperature on protease production was examined by inoculating the production medium with an inoculum size of 3% and incubating at various temperatures of 30 °C, 37 °C, 40 °C, 45 °C, 50 °C, and 55 °C for 48 hours.
Initial pH
The effect of initial pH was evaluated by inoculating the production medium with an inoculum size of 3% and incubating at 45°C for 48 hours at different initial pH rates of 9, 10, 11, and 12.
Carbon and Nitrogen Sources
To investigate the effect of different chemical parameters on protease production, the production medium was supplemented with a 1% carbon source (starch, sucrose, galactose, and glucose) or 1% nitrogen source (sodium nitrate, casein, urea, beef extract, tryptone, a mixture of peptone and yeast extract, peptone, ammonium chloride, and yeast extract). The media were then inoculated with an inoculum size of 3% and incubated at a pH rate of 10 for 48 hours at 45 °C.
Results
Protease Production Determination
Bacillus G2, isolated from petroleum sludge, was investigated for its ability to produce protease by the point inoculation technique on an agar medium supplemented with skim milk. The presence of protease production was indicated by the formation of clear hydrolytic areas around colonies. The productivity of protease was quantified through the protease assay using casein as the substrate. The findings revealed a protease activity level of 146.67 U/mL.
Molecular Identification of Selected Isolate
The PCR amplification of the 16S rDNA gene revealed a single band of the amplified DNA product of approximately 1500 bp (Figure 1). Based on the sequence analysis of the 16S rDNA gene, the selected strain G2 demonstrated high sequence similarity to the members of the genus Bacillus. The BLAST analysis conducted against the 16S rDNA sequence database (Bacteria and Archaea) confirmed that the G2 strain exhibited a 100% similarity to the Bacillus cereus strain MRY14-0060 (NCBI: AP022877.1).
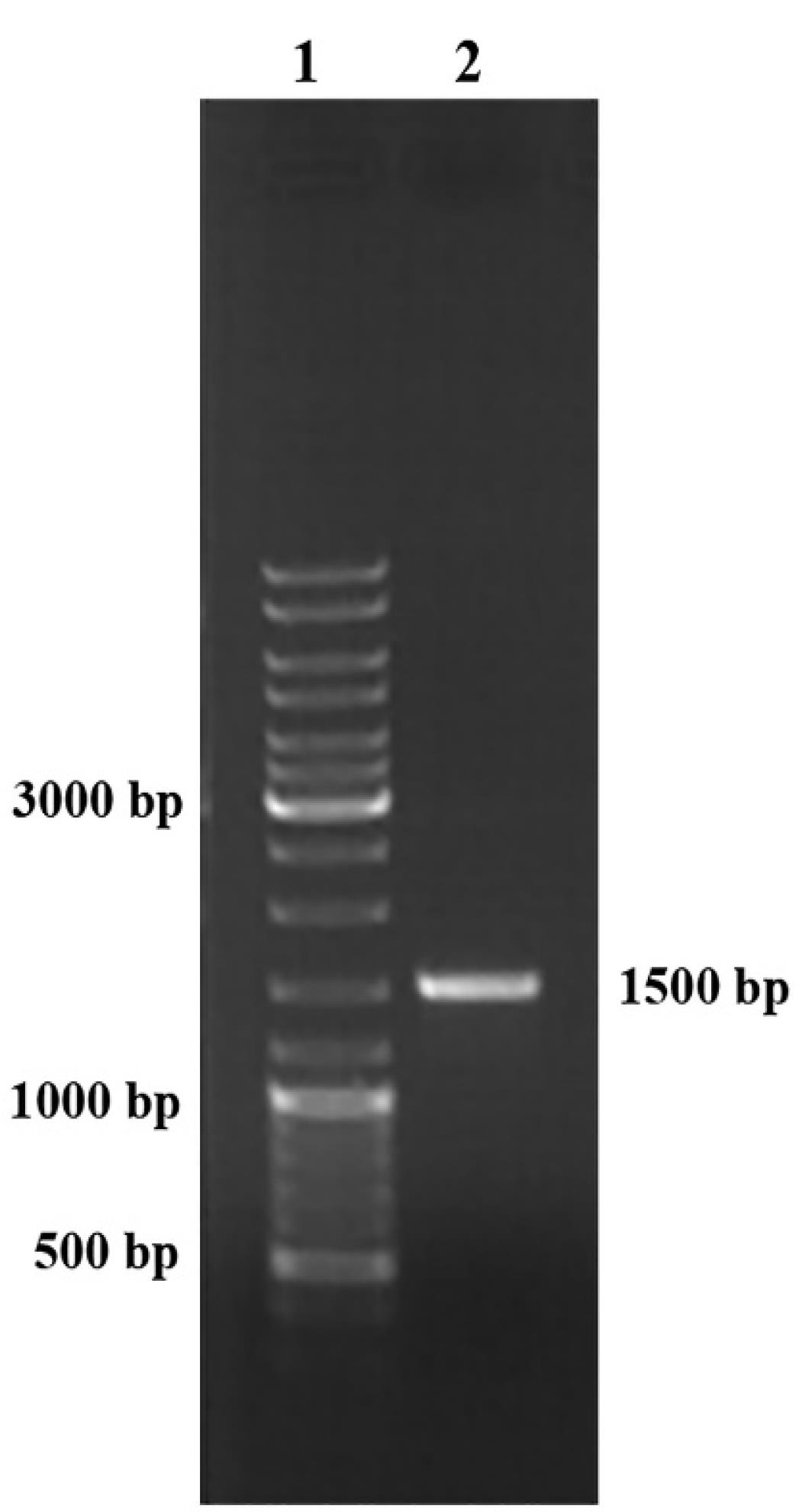
Figure 1.
16S rDNA Gene-Based Molecular Identification of Bacillus cereus G2. Note. rDNA: Ribosomal deoxyribonucleic acid; PCR: Polymerase chain reaction. Representative electrophoretic image of amplified PCR product. Lane 1: DNA ladder (GeneRuler DNA Ladder Mix, Thermo Scientific); Lane 2: PCR amplified the 16S rDNA gene of the respective bacterial isolate
.
16S rDNA Gene-Based Molecular Identification of Bacillus cereus G2. Note. rDNA: Ribosomal deoxyribonucleic acid; PCR: Polymerase chain reaction. Representative electrophoretic image of amplified PCR product. Lane 1: DNA ladder (GeneRuler DNA Ladder Mix, Thermo Scientific); Lane 2: PCR amplified the 16S rDNA gene of the respective bacterial isolate
Optimization of Culture Conditions for Protease Production by Bacillus cereus G2
The influence of various parameters on the production of protease by B. cereus G2 was investigated in this phase. The physical and chemical parameters, including inoculum size, incubation time, temperature, initial pH, and sources of carbon and nitrogen, were analyzed as well.
Effect of Inoculum Size
The optimum inoculum size for producing protease was examined by testing different inoculum percentages, ranging from 0.5% (120 U/mL) to 6% (162 U/mL), in the production medium incubated at 37°C for 48 hours. The findings showed that protease activity increased as the inoculum size peaked at 3% (194.3 U/mL). Beyond this point, further increases in the inoculum size resulted in a decrease in enzyme activity. As a result, the inoculum size of 3% was determined to be the optimal condition (Figure 2).
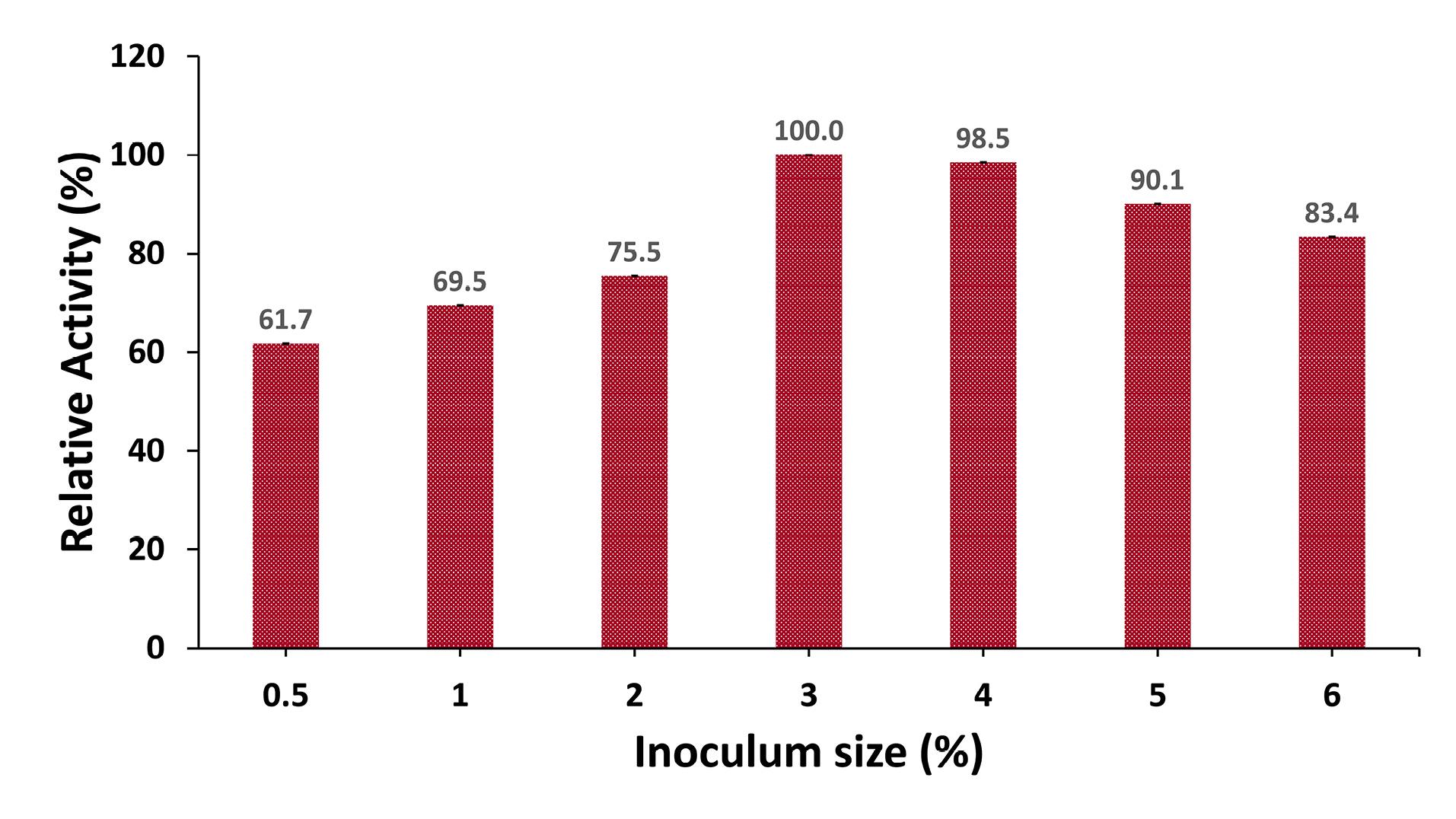
Figure 2.
Effect of Inoculum Size on Protease Production. Note. Culture media were inoculated with different inoculum sizes of 0.5%, 1%, 2%, 3%, 4%, 5%, and 6% (v/v), and incubated at 37 °C for 48 hours. Protease activity with an inoculum size of 3% (v/v) was considered 100%
.
Effect of Inoculum Size on Protease Production. Note. Culture media were inoculated with different inoculum sizes of 0.5%, 1%, 2%, 3%, 4%, 5%, and 6% (v/v), and incubated at 37 °C for 48 hours. Protease activity with an inoculum size of 3% (v/v) was considered 100%
Effect of Incubation Time
The effect of the incubation period on protease production was assessed by inoculating the production medium with an inoculum size of 3% and incubating at 37°C for durations ranging from 24 hours to 96 hours. The findings revealed that the maximum protease activity (194.3 U/mL) for B. cereus G2 occurred at 48 hours (Figure 3). The activity was negligible during the first 24 hours (127 U/mL) and declined to 142 U/mL by 96 hours.
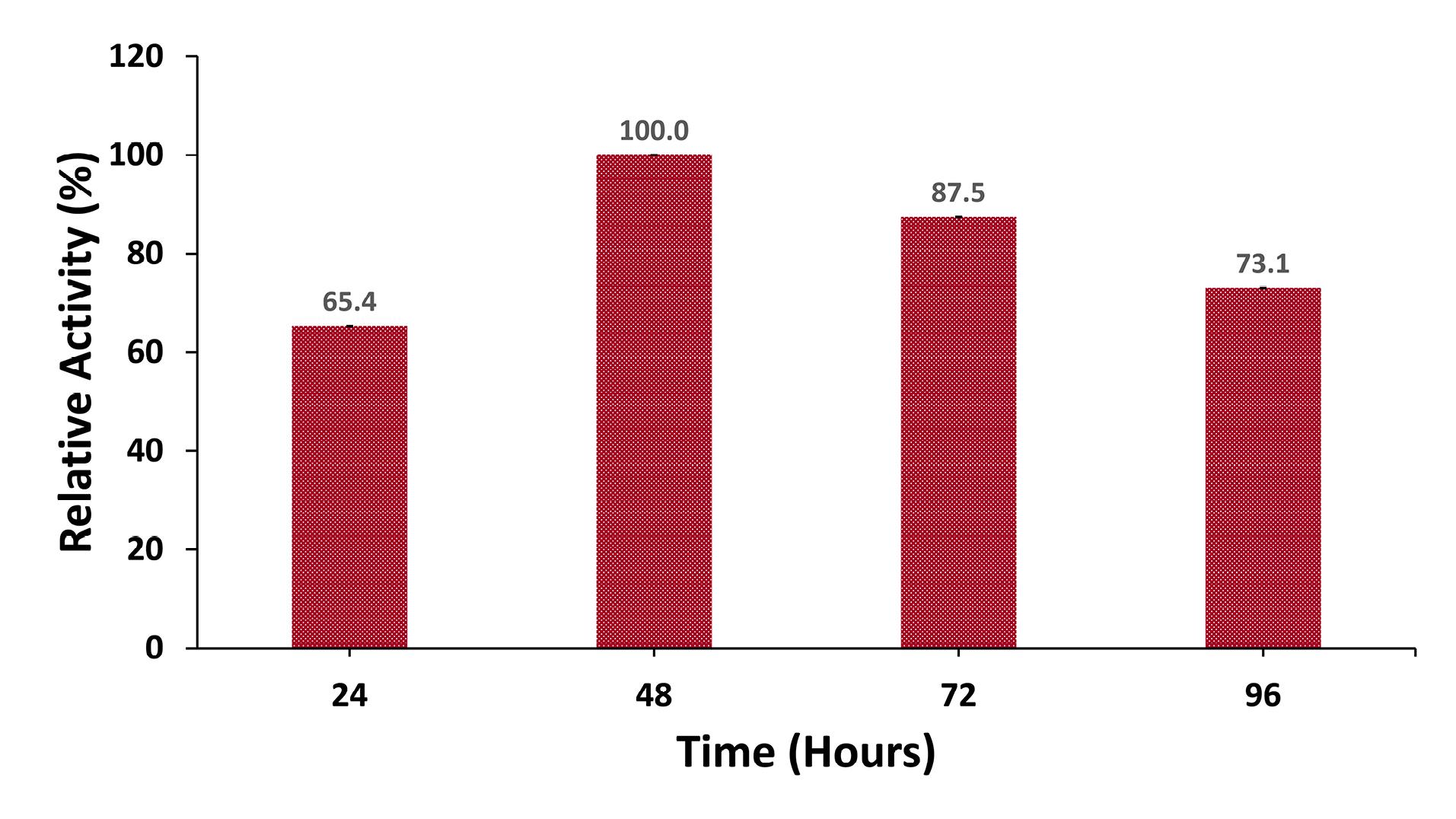
Figure 3.
Effect of Incubation Time on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated for 24 hours, 48 hours, 72 hours, 45 hours, and 96 hours at 37 °C. Protease activity for 48 hours of incubation was considered 100%
.
Effect of Incubation Time on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated for 24 hours, 48 hours, 72 hours, 45 hours, and 96 hours at 37 °C. Protease activity for 48 hours of incubation was considered 100%
Effect of Temperature
The optimal temperature for protease production was examined within the range of 30 °C to 55 °C. B. cereus G2 exhibited a steady increase in protease production, reaching its peak at 45 °C with an activity of 253.3 U/mL. Beyond this temperature, a gradual decline was observed in enzyme activity (Figure 4).
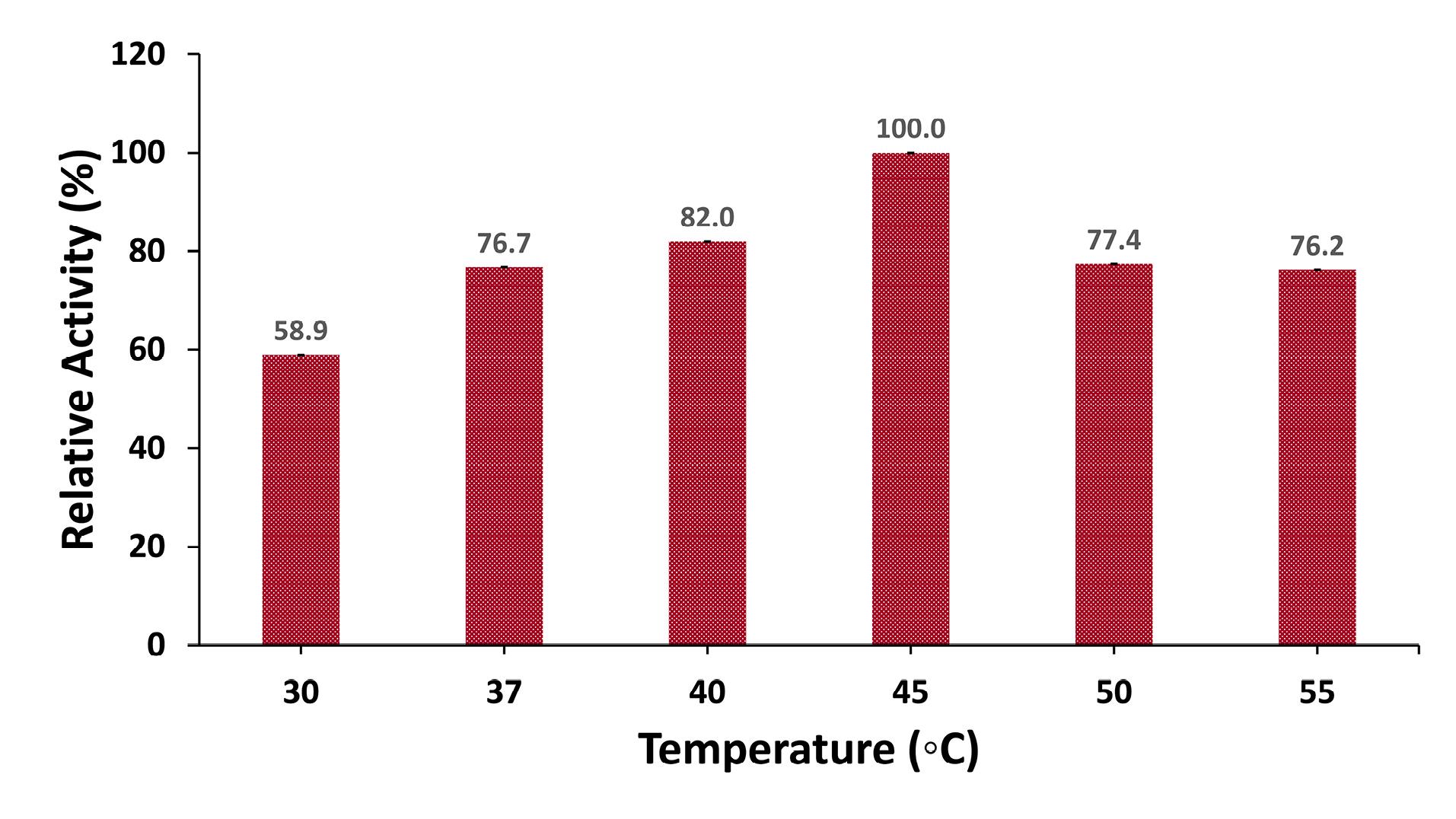
Figure 4.
Effect of Temperature on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated at 30 °C, 37 °C, 40 °C, 45 °C, 50 °C, and 55 °C for 48 hours. Protease activity at 45 °C was considered 100%
.
Effect of Temperature on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated at 30 °C, 37 °C, 40 °C, 45 °C, 50 °C, and 55 °C for 48 hours. Protease activity at 45 °C was considered 100%
Effect of Initial pH
The effect of the initial pH of the medium, ranging from 9 to 12, on protease production was evaluated by inoculating the medium with an inoculum size of 3% and incubating at 45°C for 48 hours. The results showed that increasing the initial medium pH from 9 to 10 significantly enhanced protease production, which then slightly decreased at pH rates of 11 and 12. The maximum protease activity was observed at a pH rate of 10, with an activity of 367.3 U/mL (Figure 5).
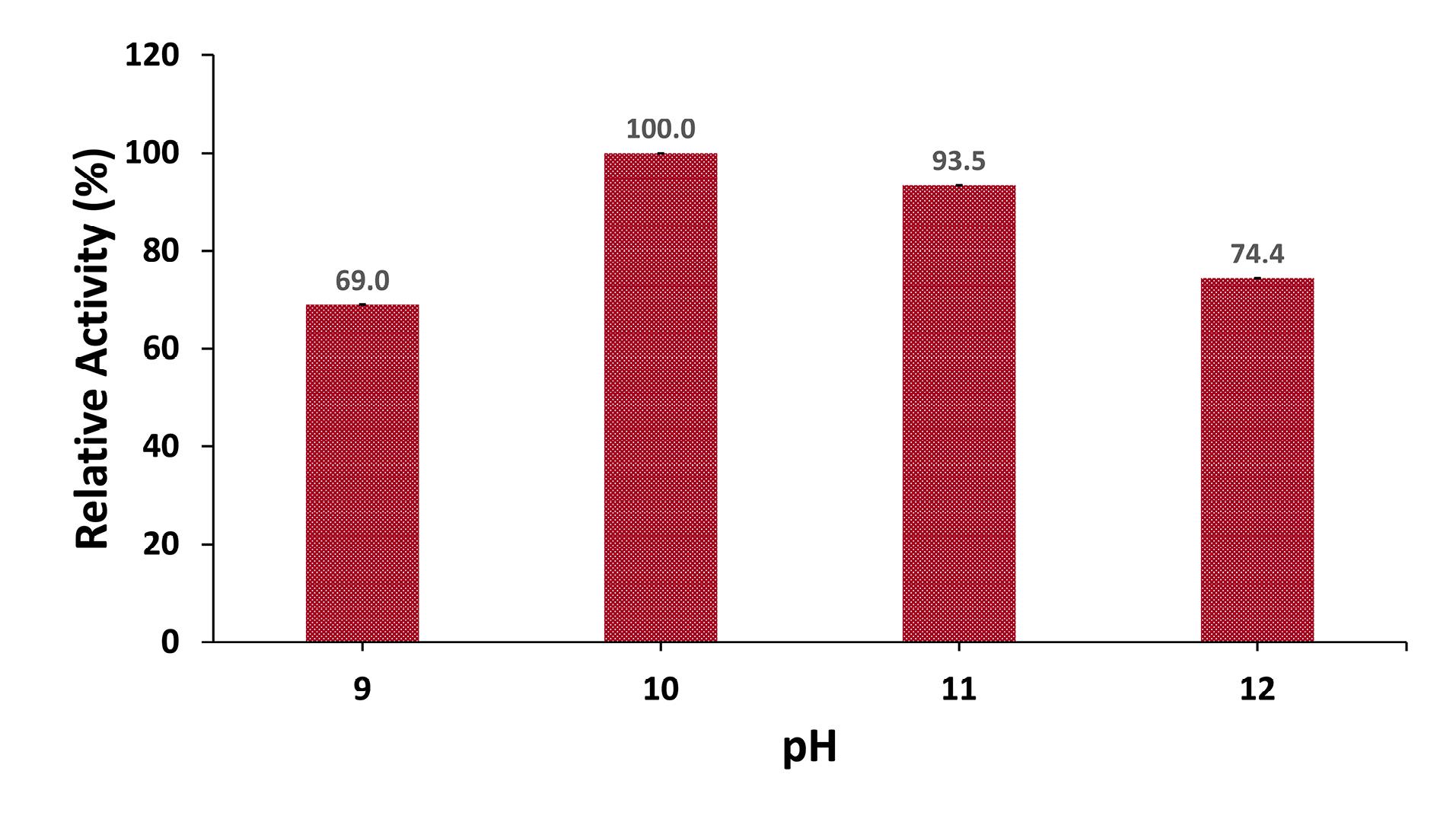
Figure 5.
Effect of pH on Protease Production. Note.Bacterial cultures were adjusted to pH rates of 9, 10, 11, and 12, inoculated with an inoculum size of 3%, and incubated at 45 °C for 48 hours. Protease activity at a pH rate of 10 was considered 100%
.
Effect of pH on Protease Production. Note.Bacterial cultures were adjusted to pH rates of 9, 10, 11, and 12, inoculated with an inoculum size of 3%, and incubated at 45 °C for 48 hours. Protease activity at a pH rate of 10 was considered 100%
Effect of Carbon Source
To evaluate different carbon sources, 1% of each carbon source was incorporated into the production medium, which was then inoculated with an inoculum size of 3% and incubated at a pH rate of 10 for 48 hours at 45 °C. Among the tested carbon sources, glucose significantly enhanced enzyme production, achieving a protease activity of 367.3 U/mL. In contrast, other sources, such as starch, sucrose, and galactose, yielded only 37.2%, 46.6%, and 96%, respectively, of the enzyme activity compared to glucose (Figure 6).
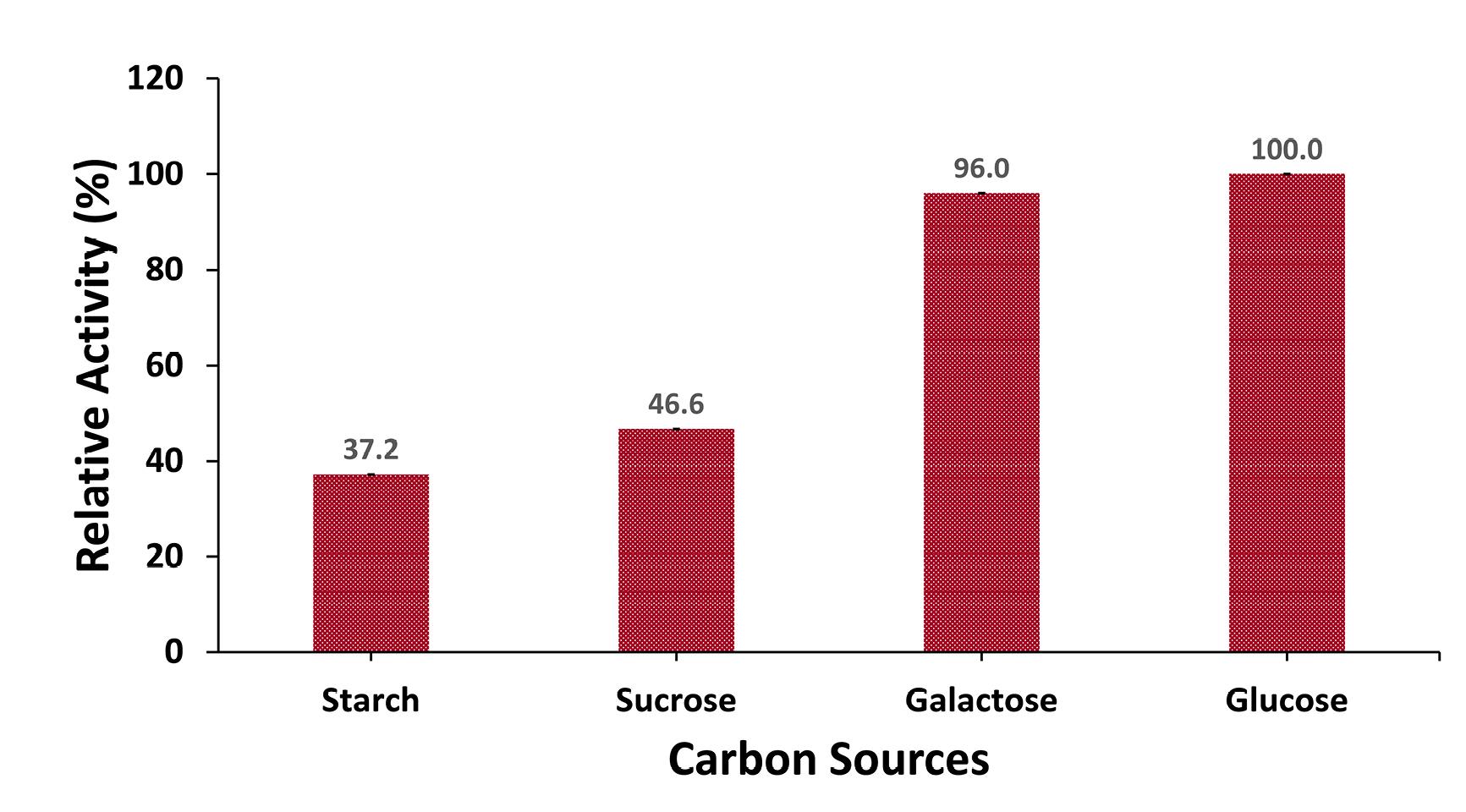
Figure 6.
Effect of Carbon Source on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated with 1% carbon source (starch, sucrose, galactose, and glucose) at 45°C for 48 hours. Protease activity using glucose as a carbon source was considered 100%
.
Effect of Carbon Source on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated with 1% carbon source (starch, sucrose, galactose, and glucose) at 45°C for 48 hours. Protease activity using glucose as a carbon source was considered 100%
Effect of Nitrogen Source
In this study, each nitrogen source was incorporated into the production medium at a concentration of 1%. The medium was then inoculated with an inoculum size of 3% and incubated at a pH rate of 10 for 48 hours at 45°C. Yeast extract emerged as the most effective nitrogen source, yielding the highest protease activity of 533.3 U/mL, compared to other tested inorganic and organic nitrogen sources (Figure 7).
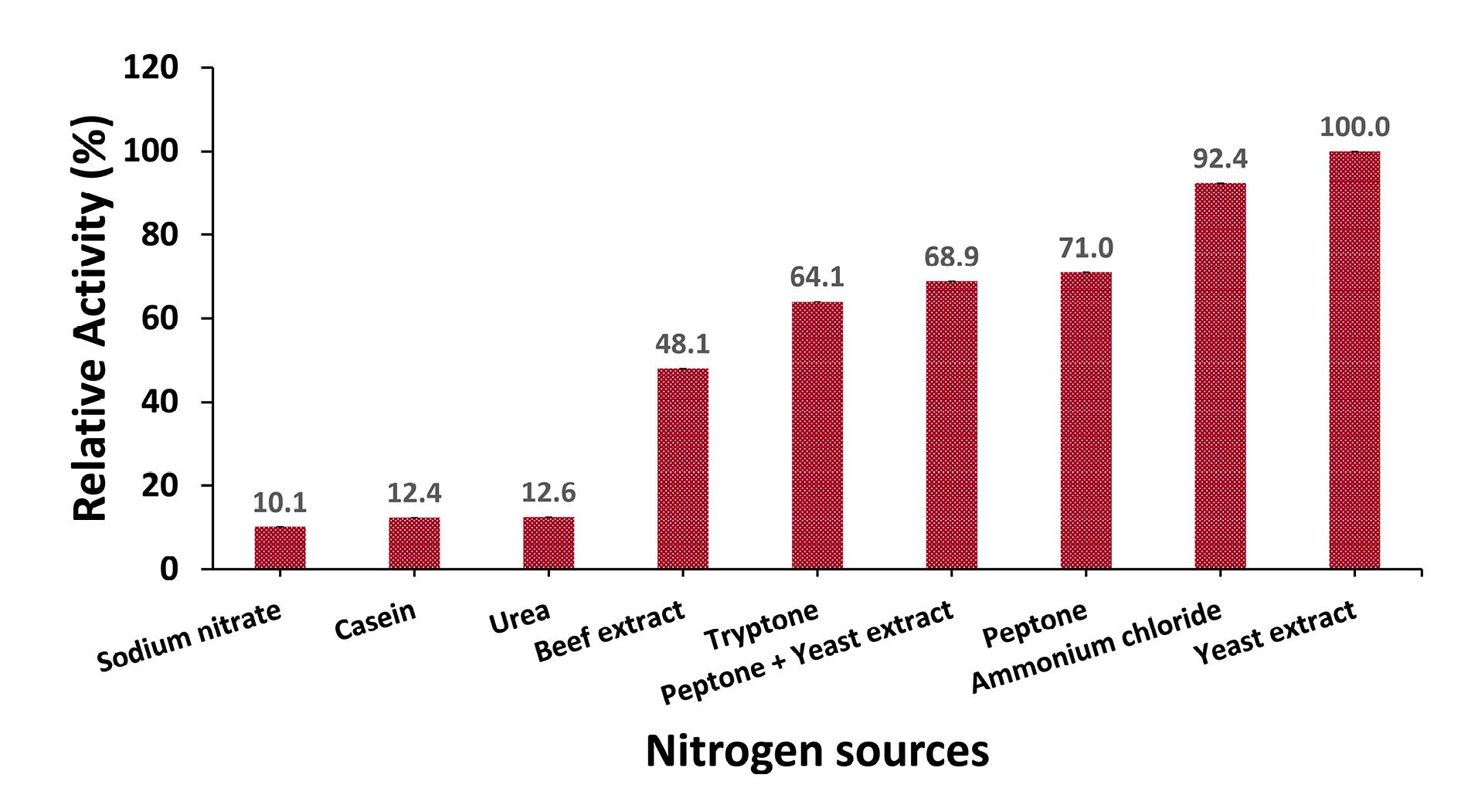
Figure 7.
Effect of Nitrogen Source on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated with a 1% nitrogen source (sodium nitrate, casein, urea, beef extract, tryptone, a mixture of peptone and yeast extract, peptone, ammonium chloride, and yeast extract) at a pH rate of 10 for 48 hours at 45°C. Protease activity using yeast extract as a nitrogen source was considered 100%
.
Effect of Nitrogen Source on Protease Production. Note. Culture media were inoculated with an inoculum size of 3% and incubated with a 1% nitrogen source (sodium nitrate, casein, urea, beef extract, tryptone, a mixture of peptone and yeast extract, peptone, ammonium chloride, and yeast extract) at a pH rate of 10 for 48 hours at 45°C. Protease activity using yeast extract as a nitrogen source was considered 100%
Discussion
Identification of the G2 Strain
The bacterial strain G2 demonstrated the ability to degrade skimmed milk. In addition, the bacterial strain G2 represented the capacity to generate a discernible zone of clearance on agar plates containing skimmed milk. The formation of a clear zone on the plate indicated that the strain had secreted proteolytic enzymes into the medium, effectively degrading the skimmed milk. Accordingly, this strain was identified as a protease producer, and its activity was quantified using a protease assay in the presence of casein as a substrate.
The taxonomic identification of strain G2 was conducted through 16S rDNA sequencing, which has demonstrated the potential for distinguishing strains at the subspecies level (22). The 16S rDNA gene from strain G2 was amplified, and the results confirmed the presence of a single band of amplified DNA, indicative of efficient amplification. A BLAST (NCBI) search of the 16S rDNA gene sequence comparison revealed that the strain is classified under the Bacillaceae family, specifically within the genus Bacillus, and displays 100% sequence similarity with B cereus strain MRY14-0060 (NCBI: AP022877.1).
Effect of Inoculum Size
The determination of the optimal inoculum size is of paramount importance in the context of the fermentation process. To achieve the greatest possible enzyme production within a limited medium volume, it is essential to control the bacterial inoculum size. The greatest protease production was achieved at an inoculum size of 3%. Reddy et al (23) observed comparable outcomes in protease production by B. subtilis SVR-07, with the highest yield attained at an inoculum size of 3%. Other researchers have reported varying optimal inoculum sizes. In their studies, Ahmed et al (24) and Lakshmi et al (25) determined that 2% was the optimal inoculum size for B. cereus, while Shine et al (26) found that 4% was optimal for B. cereus RS3. In a separate study, Sharma et al (27) identified 5% as the optimal inoculum size for Bacillus aryabhattai K3. The relationship between enzyme production and inoculum size was elucidated through an examination of the competition between bacterial cells for nutrients. Venkata Mohan and Venkateswar Reddy (28) concluded that increasing the concentration of the microorganism inoculum enhances growth up to a certain threshold. Once this threshold is exceeded, microbial activity declines due to the depletion of nutrients essential for bacterial growth and enzyme production. Conversely, a reduction in inoculum size results in a lower cell concentration, thus leading to a reduction in the yield of the target product. Furthermore, lower cell concentrations necessitate a longer period to achieve the optimal growth levels required for substrate consumption and enzyme production.
Effect of Incubation Time
The incubation period was found to have a significant impact on enzyme activity, with B. cereus G2 achieving its maximum protease production at 48 hours. Similarly, Ahmed et al (24) reported findings for B. cereus ASM1 that are consistent with the aforementioned results, noting that the peak protease production occurred at the same incubation duration. Similarly, other studies have observed the attainment of maximum protease yields for Bacillus isolates at 48 hours (22,23,27,29,30). However, different optimal incubation periods have been reported in the literature. The optimal incubation period for Bacillus sp. was found to be 60 hours (31), while B. cereus S8, B. licheniformis NCIM-2042, and Bacillus subtilis RSKK96 required 72 hours (24), 96 hours (32), and 120 hours (33), respectively. In contrast with the aforementioned studies, strain G2 requires a shorter incubation period for protease production. The decline in enzyme activity beyond 48 hours is attributed to the depletion of nutrients in the medium and changes in conditions that are unfavorable for microbial growth (34).
Effect of Temperature
Temperature is a critical parameter that exhibits variability between different organisms and must be regulated meticulously (35). B. cereus G2 demonstrated the highest protease production at 45°C. Similar findings were observed by Shine et al (26) for B. cereus RS3, with the highest protease production occurring at 45 °C. Other researchers have determined that the optimal temperature for protease production in Bacillus isolates is 45 °C (22,36). However, there is a discrepancy in the literature regarding the optimal temperature for alkaline protease production. The optimal temperature for protease production in B. cereus S8 (25), B. licheniformis NCIM-2042 (32), B. subtilis RSKK96 (33), and B. licheniformis N-2 (37) has been reported to be 37 °C. In contrast, B. subtilis NS represented optimal protease production at 40 °C (29), while in B. subtilis SVR-07, the optimal temperature was 55 °C (23). The decline in enzymatic activity at elevated temperatures is attributed to the inhibition of microbial growth, which in turn restricts enzyme production (38). It is widely acknowledged that elevated temperatures can result in alterations to protein conformation and subsequent degradation, ultimately leading to a reduction in protease activity (39).
Effect of Initial pH
The pH level of the culture medium exerts a profound influence on a multitude of enzymatic activities and the transport of diverse substances across the cell membrane (40). The maximum production of protease for B. cereus G2 was observed at the pH rate of 10, indicating that this strain exhibits alkaliphilic characteristics. Similar findings were found by Nadeem et al (37) and Pant et al (41), demonstrating that B. licheniformis N-2 and B. subtilis also exhibited the greatest protease production at the pH rate of 10. In contrast, other studies indicated that the optimal pH for alkaline protease production is 9 for B. cereus ASM1 (24), B. cereus RS3 (26), B. subtilis SVR-07 (23), and B. subtilis NS (29). Lakshmi et al (25) observed that B. cereus S8 had peak protease production at the pH rate of 12. It has been established that enzyme production declines at pH levels outside the optimal range due to a reduction in the metabolic activity of the microorganisms, which is significantly impacted by pH changes in the medium (28).
Effect of Carbon Source
Carbon is a vital component in culture media, providing essential energy for microorganisms and playing a significant role in their growth and production of both primary and secondary metabolites. The type of applied carbon source can exert a profound influence on protease production, with different bacterial strains exhibiting a proclivity for disparate sources in their metabolic processes. Glucose has been identified as the most effective carbon source for the maximum production of proteases by B. cereus G2, exhibiting superior performance compared to galactose, sucrose, and starch. This finding corroborates the results of studies on B. cereus ASM1 (24), B. licheniformis N-2 (37), and B. subtilis MTCC7312 (2), demonstrating optimal protease production with glucose. Furthermore, fructose has been identified as a notable carbon source. In a study conducted by Joshi et al (42), the highest levels of protease were produced by B. cereus MTCC 6840 when fructose was employed as the carbon source in comparison to galactose, glucose, sucrose, or starch. Similarly, Shine et al (26) reported that B. cereus RS3 achieved the greatest protease production when fructose was used as the carbon source in comparison to other sources. Moreover, Lakshmi et al (25) observed that B. cereus S8 exhibited a preference for molasses over glucose for optimal protease production.
Effect of Nitrogen Source
The maintenance of the physiological and biochemical activities of microorganisms is contingent upon the availability of suitable nitrogen sources, exerting a significant influence on enzyme production. A variety of nitrogen substrates have been confirmed to be effective in enhancing protease production in different bacterial strains (43). In the case of B. cereus G2, yeast extract was identified as the most effective nitrogen source, exhibiting superior performance compared to other organic and inorganic sources. Similar findings have been reported for B. cereus RS3 (26), B. pumilus D3 (44), and B. sp. NPST-AK15 (45), where yeast extract was also identified as the preferred nitrogen source for maximum alkaline protease production. In contrast, other studies have demonstrated peptone as the optimal nitrogen source for B. subtilis RMK (22). Moreover, Lakshmi et al (25) demonstrated that potassium nitrate significantly enhanced protease production in B. cereus S8. Conversely, Joshi et al (42) indicated that a combination of peptone and yeast extract was the most efficacious for B. cereus MTCC 6840.
Refineries produce a considerable quantity of sludge, which is classified as a hazardous pollutant (46). Microorganisms that flourish in extreme environments are adept at utilizing hydrocarbons as carbon and energy sources, thereby producing a plethora of extracellular enzymes with a range of catabolic functions. These enzymes are of considerable value for a multitude of practical and industrial applications. The results of this study revealed that petroleum sludge provides an optimal environment for the isolation of bacteria that produce proteases.
Alkaline proteases are widely used in various industries due to their ability to function effectively in high pH environments. The ability of B. cereus G2 to produce these enzymes under thermophilic conditions suggests that it could be a valuable resource for industries requiring robust and efficient proteases. This could lead to more sustainable and cost-effective production processes, enhancing the overall efficiency and environmental friendliness of industrial operations. Furthermore, the unique properties of the B. cereus G2 strain could inspire the development of new biotechnological applications, such as in waste management and bioremediation, where the degradation of complex organic materials is essential. The strain’s resilience and enzymatic capabilities could be harnessed to improve the degradation of pollutants in harsh environmental conditions, contributing to cleaner and more sustainable industrial practices.
The study has successfully demonstrated enzyme production at the laboratory scale; however, scaling up to industrial production may introduce unforeseen challenges to the commercial viability of this process. Variations in environmental conditions, equipment differences, and the need for optimization at larger scales can significantly influence enzyme production.
Conclusion
Alkaline proteases are employed in a multitude of industries, including those related to medicine and environmental remediation. The primary objective in selecting an organism for commercial enzyme production is to achieve the greatest possible enzyme yield, given the constraints of the production process. This research investigated and produced an alkaline protease from a locally isolated Bacillus strain found in petroleum sludge. The producing bacterium was evaluated for protease production using agar plates containing skim milk. The presence of clear zones around colonies indicated the presence of the enzyme, which was subsequently confirmed through a protease assay. The bacterial isolate was identified as B. cereus G2 through 16S rDNA sequence analysis. The optimization of protease production from B. cereus G2 entailed the adjustment of multiple parameters, including inoculum size, incubation time, carbon and nitrogen sources, temperature, and pH. The highest level of alkaline protease productivity was observed when the inoculum size was 3%, the incubation period was 48 hours, the pH was 10, and the temperature was 45°C. In addition, 1% glucose and 1% yeast extract were employed as the carbon and nitrogen sources, respectively. Subsequently, the alkaline protease activity was found to be 4.44-fold higher (533.3 U/mL) in comparison to the initial stage of enzyme production optimization (120 U/mL).
The results of this study confirmed that bacteria indigenous to refinery sludge with petroleum hydrocarbons may serve as a valuable source of microbial enzymes. The isolate, designated B. cereus G2, was obtained from petroleum sludge in Syria and has been identified as a promising candidate for the production of alkaline protease. Further research is required to purify and characterize this enzyme, as well as to investigate potential genetic modifications that could enhance its activity. Future directions also include industrial application testing and enzyme stability studies to expand the practical applications of this enzyme.
Acknowledgements
The authors express their gratitude to the Director General of AECS for the great support provided throughout this research.
Authors’ Contribution
Conceptualization: Bassam Al Balaa.
Data curation: Bassam Al Balaa and Samah Qasem.
Formal analysis: Bassam Al Balaa and Samah Qasem.
Investigation: Bassam Al Balaa and Samah Qasem.
Methodology: Bassam Al Balaa.
Project administration: Bassam Al Balaa.
Resources: Bassam Al Balaa.
Software: Bassam Al Balaa, Samah Qasem, and Nermeen Haj Mahmoud.
Supervision: Bassam Al Balaa.
Validation: Bassam Al Balaa.
Visualization: Bassam Al Balaa and Nermeen Haj Mahmoud.
Writing–original draft: Bassam Al Balaa and Nermeen Haj Mahmoud.
Writing–review & editing: Bassam Al Balaa, Samah, Qsem, and Nermeen Haj Mahmoud.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
This study was conducted in vitro. There is no experiment conducted on humans or live animals.
Funding
This study was funded by Atomic Energy Commission of Syria.
References
- Chellapandi P. Production and preliminary characterization of alkaline protease from Aspergillus flavus and Aspergillus terreus. J Chem 2010; 7(2):502583. doi: 10.1155/2010/502583 [Crossref] [ Google Scholar]
- Verma OP, Kumari P, Shukla S, Singh A. Production of alkaline protease by Bacillus subtilis (MTCC7312) using submerged fermentation and optimization of process parameters. Eur J Exp Biol 2011; 1(3):124-9. [ Google Scholar]
- Aftab S, Ahmed S, Saeed S, Rasool SA. Screening, isolation and characterization of alkaline protease producing bacteria from soil. Pak J Biol Sci 2006; 9(11):2122-6. doi: 10.3923/pjbs.2006.2122.2126 [Crossref] [ Google Scholar]
- Gupta R, Beg QK, Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 2002; 59(1):15-32. doi: 10.1007/s00253-002-0975-y [Crossref] [ Google Scholar]
- Asker MM, Mahmoud MG, El Shebwy K, Abd el Aziz MS. Purification and characterization of two thermostable protease fractions from Bacillus megaterium. J Genet Eng Biotechnol 2013; 11(2):103-9. doi: 10.1016/j.jgeb.2013.08.001 [Crossref] [ Google Scholar]
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 1998; 62(3):597-635. doi: 10.1128/mmbr.62.3.597-635.1998 [Crossref] [ Google Scholar]
- Kumar AG, Nagesh N, Prabhakar TG, Sekaran G. Purification of extracellular acid protease and analysis of fermentation metabolites by Synergistes sp utilizing proteinaceous solid waste from tanneries. Bioresour Technol 2008; 99(7):2364-72. doi: 10.1016/j.biortech.2007.05.001 [Crossref] [ Google Scholar]
- Ferrero MA, Castro GR, Abate CM, Baigorí MD, Siñeriz F. Thermostable alkaline proteases of Bacillus licheniformis MIR 29: isolation, production and characterization. Appl Microbiol Biotechnol 1996; 45(3):327-32. doi: 10.1007/s002530050691 [Crossref] [ Google Scholar]
- Gemechu G, Masi C, Tafesse M, Bekele GK. A review on the bacterial alkaline proteases. J Xidian Univ 2020; 14(11):264-74. doi: 10.37896/jxu14.11/022 [Crossref] [ Google Scholar]
- Song P, Zhang X, Wang S, Xu W, Wang F, Fu R. Microbial proteases and their applications. Front Microbiol 2023; 14:1236368. doi: 10.3389/fmicb.2023.1236368 [Crossref] [ Google Scholar]
- Adetunji AI, Olaitan MO, Erasmus M, Olaniran AO. Microbial proteases: a next generation green catalyst for industrial, environmental and biomedical sustainability. Food Mater Res 2023; 3:12. doi: 10.48130/fmr-2023-0012 [Crossref] [ Google Scholar]
- Parekh S, Vinci VA, Strobel RJ. Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 2000; 54(3):287-301. doi: 10.1007/s002530000403 [Crossref] [ Google Scholar]
- Khan MA, Ahmad N, Zafar AU, Nasir IA, Abdul Qadir M. Isolation and screening of alkaline protease producing bacteria and physio-chemical characterization of the enzyme. Afr J Biotechnol 2011; 10(33):6203-12. doi: 10.5897/ajb11.413 [Crossref] [ Google Scholar]
- Han XQ, Damodaran S. Isolation, identification, and fermentation of a Bacillus species producing a detergent-stable endopeptidase. J Agric Food Chem 1997; 45(11):4191-5. doi: 10.1021/jf970727f [Crossref] [ Google Scholar]
- Pastor MD, Lorda GS, Balatti A. Protease obtention using Bacillus subtilis 3411 and amaranth seed meal medium at different aeration rates. Braz J Microbiol 2001; 32(1):6-9. doi: 10.1590/s1517-83822001000100002 [Crossref] [ Google Scholar]
- Jameel A, Khan MM. Production and characterization of alkaline protease from locally isolated alkaliphilic Bacillus species. Int J Eng Sci Res Technol 2011; 3(6):4596-603. [ Google Scholar]
- Tanwar M, Debnath M, Debnath S, Sharma P, Mukhopadhay A, Kakar N. Exploring the utility of nanoprotease as environmentally friendly benign laundry detergent fabric cleaner. J Clean Prod 2022; 334:130243. doi: 10.1016/j.jclepro.2021.130243 [Crossref] [ Google Scholar]
- Hasan MJ, Haque P, Rahman MM. Protease enzyme based cleaner leather processing: a review. J Clean Prod 2022; 365:132826. doi: 10.1016/j.jclepro.2022.132826 [Crossref] [ Google Scholar]
- Nounou MI, Zaghloul TI, Ahmed NA, Eid AA, El-Khordagui LK. Skin permeability enhancement by Bacillus subtilis alkaline protease: Application to transdermal drug delivery. Int J Pharm 2017; 529(1-2):423-32. doi: 10.1016/j.ijpharm.2017.06.057 [Crossref] [ Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 1991; 173(2):697-703. doi: 10.1128/jb.173.2.697-703.1991 [Crossref] [ Google Scholar]
- David Troncoso F, Alberto Sánchez D, Luján Ferreira M. Production of plant proteases and new biotechnological applications: an updated review. ChemistryOpen 2022; 11(3):e202200017. doi: 10.1002/open.202200017 [Crossref] [ Google Scholar]
- Krishnaveni K, Mukesh Kumar DJ, Balakumaran MD, Ramesh S, Kalaichelvan PT. Production and optimization of extracellular alkaline protease from Bacillus subtilis isolated from dairy effluent. Pharm Lett 2012; 4(1):98-109. [ Google Scholar]
- Reddy MN, Kumar CG, Swathi K, Nagamani B, Venkateshwar S, Rao LV. Extracellular alkaline protease production from isolated Bacillus subtilis SVR-07 by using submerged fermentation. Int J Pharm Res Dev 2011; 3(1):216-23. [ Google Scholar]
- Ahmed M, Rehman R, Siddique A, Hasan F, Ali N, Hameed A. Production, purification and characterization of detergent-stable, halotolerant alkaline protease for eco-friendly application in detergents’ industry. Int J Biosci 2016; 8(2):47-65. doi: 10.12692/ijb/8.2.47-65 [Crossref] [ Google Scholar]
- Lakshmi BK, Ratna Sri PV, Ambika Devi K, Hemalatha KP. Screening, optimization of production and partial characterization of alkaline protease from haloalkaliphilic Bacillus sp. Int J Res Eng Technol 2014; 3(2):435-45. doi: 10.15623/ijret.2014.0302077 [Crossref] [ Google Scholar]
- Shine K, Kanimozhi K, Panneerselvam A, Muthukumar C, Thajuddin N. Production and optimization of alkaline protease by Bacillus cereus RS3 isolated from desert soil. Int J Adv Res Biol Sci 2016; 3(7):193-202. [ Google Scholar]
- Sharma KM, Kumar R, Vats S, Gupta A. Production, partial purification and characterization of alkaline protease from Bacillus aryabhattai K3. Int J Adv Pharm Biol Chem 2014; 3(2):290-8. [ Google Scholar]
- Venkata Mohan S, Venkateswar Reddy M. Optimization of critical factors to enhance polyhydroxyalkanoates (PHA) synthesis by mixed culture using Taguchi design of experimental methodology. Bioresour Technol 2013; 128:409-16. doi: 10.1016/j.biortech.2012.10.037 [Crossref] [ Google Scholar]
- Nisha NS, Divakaran J. Optimization of alkaline protease production from Bacillus subtilis NS isolated from sea water. Afr J Biotechnol 2014; 13(16):1707-13. doi: 10.5897/ajb2014.13652 [Crossref] [ Google Scholar]
- Sankareswaran M, Anbalaga S, Prabhavathi P. Optimization of production of an extracellular alkaline protease by soil isolated Bacillus species using submerged and solid-state fermentation with agricultural wastes. Afr J Microbiol Res 2014; 8(9):872-7. doi: 10.5897/ajmr11.1273 [Crossref] [ Google Scholar]
- Prakasham RS, Rao Ch S, Sarma PN. Green gram husk--an inexpensive substrate for alkaline protease production by Bacillus sp in solid-state fermentation. Bioresour Technol 2006; 97(13):1449-54. doi: 10.1016/j.biortech.2005.07.015 [Crossref] [ Google Scholar]
- Bhunia B, Dutta D, Chaudhuri S. Selection of suitable carbon, nitrogen and sulphate source for the production of alkaline protease by Bacillus licheniformis NCIM-2042. Not Sci Biol 2010; 2(2):56-9. doi: 10.15835/nsb224630 [Crossref] [ Google Scholar]
- Akcan N, Uyar F. Production of extracellular alkaline protease from Bacillus subtilis RSKK96 with solid state fermentation. Eurasia J Biosci 2011; 5(8):64-72. doi: 10.5053/ejobios.2011.5.0.8 [Crossref] [ Google Scholar]
- Palaniyappan M, Vijayagopal V, Viswanathan R, Viruthagiri T. Screening of natural substrates and optimization of operating variables on the production of pectinase by submerged fermentation using Aspergillus niger MTCC 281. Afr J Biotechnol 2009; 8(4):682-6. [ Google Scholar]
- Kumar CG, Takagi H. Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv 1999; 17(7):561-94. doi: 10.1016/s0734-9750(99)00027-0 [Crossref] [ Google Scholar]
- Akhavan Sepahy A, Jabalameli L. Effect of culture conditions on the production of an extracellular protease by Bacillus sp isolated from soil sample of Lavizan jungle park. Enzyme Res 2011; 2011:219628. doi: 10.4061/2011/219628 [Crossref] [ Google Scholar]
- Nadeem M, Qazi JI, Baig S, Syed Q. Effect of medium composition on commercially important alkaline protease production by Bacillus licheniformis N-2. Food Technol Biotechnol 2008; 46(4):388-94. [ Google Scholar]
- Haq IH, Ashraf H, Ali S, Qadeer MA. Submerged fermentation of alpha amylase by Bacillus licheniformis GCB-36. Biologia (Bratislava) 1997; 43:39-45. [ Google Scholar]
- Johnvesly B, Naik GR. Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp JB-99 in a chemically defined medium. Process Biochem 2001; 37(2):139-44. doi: 10.1016/s0032-9592(01)00191-1 [Crossref] [ Google Scholar]
- Moon SH, Parulekar SJ. A parametric study ot protease production in batch and fed-batch cultures of Bacillus firmus. Biotechnol Bioeng 1991; 37(5):467-83. doi: 10.1002/bit.260370509 [Crossref] [ Google Scholar]
- Pant G, Prakash A, Pavani JV, Bera S, Deviram GV, Kumar A. Production, optimization and partial purification of protease from Bacillus subtilis. Taibah Univ Sci 2015; 9(1):50-5. doi: 10.1016/j.jtusci.2014.04.010 [Crossref] [ Google Scholar]
- Joshi GK, Kumar S, Sharma V. Production of moderately halotolerant, SDS stable alkaline protease from Bacillus cereus MTCC 6840 isolated from lake Nainital, Uttaranchal state, India. Braz J Microbiol 2007; 38(4):773-9. doi: 10.1590/s1517-83822007000400034 [Crossref] [ Google Scholar]
- Saurabh S, Jasmine I, Pritesh G, Kumar SR. Enhanced productivity of serine alkaline protease by Bacillus sp using soybean as substrate. Malays J Microbiol 2007; 3(1):1-6. doi: 10.21161/mjm.00107 [Crossref] [ Google Scholar]
- Özçelik B, Aytar P, Gedikli S, Yardımcı E, Çalışkan F, Çabuk A. Production of an alkaline protease using Bacillus pumilus D3 without inactivation by SDS, its characterization and purification. J Enzyme Inhib Med Chem 2014; 29(3):388-96. doi: 10.3109/14756366.2013.788503 [Crossref] [ Google Scholar]
- Ibrahim AS, Al-Salamah AA, Elbadawi YB, El-Tayeb MA, Ibrahim SS. Production of extracellular alkaline protease by new halotolerant alkaliphilic Bacillus sp NPST-AK15 isolated from hyper saline soda lakes. Electron J Biotechnol 2015; 18(3):236-43. doi: 10.1016/j.ejbt.2015.04.001 [Crossref] [ Google Scholar]
- Patel R, Dodia M, Singh SP. Extracellular alkaline protease from a newly isolated haloalkaliphilic Bacillus sp: production and optimization. Process Biochem 2005; 40(11):3569-75. doi: 10.1016/j.procbio.2005.03.049 [Crossref] [ Google Scholar]