Avicenna Journal of Clinical Microbiology and Infection. 11(4):163-170.
doi: 10.34172/ajcmi.3567
Original Article
Investigating the Antibacterial Effects of Cuminum cyminum and Foeniculum vulgare Essential Oils on Escherichia coli and Listeria monocytogenes
Farnaz Zakikhani 1  , Saman Afshar 2
, Saman Afshar 2  , Faezeh Mirzaei 3
, Faezeh Mirzaei 3  , Maryam Kazemian 4
, Maryam Kazemian 4  , Fatemeh Ataei Masjedlou 5
, Fatemeh Ataei Masjedlou 5  , Javid Taghinejad 6, *
, Javid Taghinejad 6, * 
Author information:
1Researcher and manager of Rasa Gene, Shiraz, Iran
2Department of Animal Biology, Faculty of Natural Science, University of Tabriz, Tabriz, Iran
3Department of Microbiology, Institute of Higher Education Nabi Akram University, Tabriz, Iran
4Department of Chemical Engineering, Faculty of Chemical Engineering, Babol Noshirvani University of Technology, Mazandaran, Iran
5Department of Biology, Ardabil Branch, Islamic Azad University, Ardabil, Iran
6Department of Microbiology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
Abstract
Background: Due to the increasing demand for new antibiotics, extensive research is being conducted on various compounds, particularly plant-based compounds. Among them, plant essential oils (EOs) have been investigated for their antibacterial properties in numerous studies. Accordingly, the inhibitory and bactericidal effects of Cuminum cyminum and Foeniculum vulgare EOs on two bacterial species, Escherichia coli and Listeria monocytogenes, were examined in this study.
Methods: The compounds present in these extracts were evaluated using gas chromatography-mass spectrometry analysis. Subsequently, molecular docking methods were employed to depict the interactions of these compounds with the active site of the beta-lactamase enzyme of these bacteria in three-dimensional illustrations.
Results: Laboratory methods such as disk diffusion agar, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) revealed that although the bactericidal properties of these extracts were weak, they exhibited strong growth inhibitory properties, particularly the effect of F. vulgare EO on L. monocytogenes (MIC=1/104). Additionally, with the presence of p-cumin aldehyde at 33.7% in the cumin extract and trans-anethole at 46.5% in the fennel extract, molecular docking analyses showed that the binding ability and antibacterial properties of the cumin extract against E. coli by two hydrogen bonds and fennel extract against L. monocytogenes by two hydrogen bonds were more pronounced considering the level of binding energies.
Conclusion: Although the extracts represented weak bactericidal properties, their strong inhibitory effects on bacterial growth, especially with F. vulgare EO on L. monocytogenes, are notable. Moreover, the active compounds, such as p-cumin aldehyde and trans-anethole, demonstrated significant potential as alternative antibacterial agents.
Keywords: Antibiotic resistance, Essential oils, Antibiotics, Docking
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Zakikhani F, Afshar S, Mirzaei F, Kazemian M, Ataei Masjedlou F, Taghinejad J. Investigating the antibacterial effects of Cuminum cyminum and Foeniculum vulgare essential oils on Escherichia coli and Listeria monocytogenes. Avicenna J Clin Microbiol Infect. 2024; 11(4):163-170. doi:10.34172/ajcmi.3567
Introduction
Antibiotic resistance is a process by which bacteria become resistant to different antibiotics, rendering the intended antibiotic unable to treat the infection caused by the resistant bacteria. This phenomenon can lead to many medical and economic problems. Therefore, antibiotic resistance can create many complications for people, the medical system, and governments (1).
Bacteria can develop resistance to antibiotics through various mechanisms. One method involves bacterial cells preventing the entry of antibiotics, thereby stopping these drugs from reaching their target within the bacteria. Another strategy uses efflux pumps to expel antibiotic molecules out of the cells, thus reducing the intracellular concentration of the drug. Additionally, some cells modify the chemical structure of antibiotics to disrupt their activity. Finally, bacteria can alter the molecular structure of the antibiotic’s target molecules, such as ribosomes, to prevent the antibiotic from binding and functioning effectively (2,3).
Nowadays, the level of antibiotic resistance is considerably increasing due to the incorrect and excessive usage of antibiotics, causing concerns within the medical community. Thus, to combat resistant bacteria, there is a need to discover or synthesize new antibiotics. This requires various research efforts to identify these compounds. They can be extracted from a multitude of living organisms, including animals, microorganisms, and plants (4).
Several studies have been conducted on the antibacterial effects of compounds extracted from different plant species, resulting in the discovery of many effective substances (5-7). Among them, research has also been performed on green cumin (Cuminum cyminum) and fennel (Foeniculum vulgare), examining their health-related impacts, which make them suitable candidates for research on the antibacterial compounds in their essential oils (EOs) (8).
C. cyminum, commonly referred to as cumin or ‘zeera’ in Persian, is a member of the Apiaceae family. This herb is noted for its aromatic qualities and is utilized both as a spice and a natural preservative in foods (9). Cumin is a staple for adding flavor to various dishes, salads, and dairy products across numerous cultures (10). The EO derived from C. cyminum and its primary constituents are recognized as safe, natural alternatives to artificial preservatives and additives in the food sector (11). Furthermore, studies have highlighted the plant’s diverse pharmacological benefits, including potent antioxidant (12,13), antibacterial (14), antifungal (15), and pain-relieving properties (7,12).
Fennel, scientifically named F. vulgare, is highly regarded as a therapeutic herb in Morocco and is a member of the Apiaceae family. This plant thrives in various global regions and serves multiple purposes (16). The fruits and extracted EOs from mature fennel are incorporated into cosmetic and medicinal products. Further, they enhance the taste of food items, including liqueurs, bread, and cheese (17,18). The leaves and seeds are integral to diverse cooking practices (19). Notably, fennel is used to address health issues such as cardiovascular problems, allergies, kidney stones, menopausal symptoms, nausea, and obesity. Moreover, it is known to boost appetite and aid in digestion (20-22). In this study, the antibacterial effects of green cumin and fennel EOs on the bacterial species Escherichia coli and Listeria monocytogenes were examined using various antibacterial susceptibility laboratory techniques. Subsequently, the active substances of these extracts were investigated using gas chromatography-mass spectrometry (GC-MS) test.
Materials and Methods
In Vitro Studies
Essential Oil Extraction Method
After preparing the desired plants in dried form, the plant samples were powdered using a milling device and prepared for EO extraction. A Clevenger apparatus was utilized for the extraction process. In this method, 40 g of the dried plant powder was mixed in 1 L of distilled water within the flask and set on an electric heater for 4 hours to boil. Subsequently, the essence was separated from the plant distillate using the discharge valve.
Bacteria Species
In this study, the bacterial strains L. monocytogenes IBRC-M 10671 and E. coli ATCC 25922 were utilized, and these strains were purchased from the Rasa Gene Company, Shiraz, Iran.
Investigation of Growth Inhibition by the Disc Diffusion Method
A 2000-ppm concentration of EOs was used in all the following tests. For the disc diffusion method, a cotton swab was utilized to apply the bacterial suspension onto a Petri dish containing Mueller-Hinton agar (MHA) medium. The dish was then incubated at 37 °C for 24 hours. Next, 50 µL of each essence was applied onto 6-mm paper discs using a sampler. Moreover, a disc containing dimethyl sulfoxide (DMSO) was considered the control group. The 0.5 McFarland concentration equal to 1 × 108 CFU/mL of the microbial samples, which were in the logarithmic growth phase, was prepared and cultured on the culture media. Then, the paper discs containing the EO were placed at specified distances, and after 24 hours of incubation at 35-37 degrees Celsius, the diameter of the inhibition zone was measured using calipers based on the Kirby Bauer method (23). Finally, the results were analyzed using Tukey’s analysis of variance statistical test with IBM SPSS software, version 25.
Determination of Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) of the EO was identified as the concentration at which bacterial growth is inhibited. The broth microdilution method was used for this purpose. In 96-well plates, serial dilutions of EOs ranging from 1/101 to 1/110 v/v were prepared using Mueller-Hinton broth (MHB). In addition, 2% of the DMSO was added to each well. After being diluted in the MHB, bacterial suspensions with a turbidity of 0.5 McFarland were made and added to each well. Furthermore, a mixture of MHB and DMSO devoid of EOs was employed as growth controls. The plates were incubated under aerobic conditions for 18–24 hours at 37 °C. Following the incubation period, the bacterial growth was evaluated visually.
Determination of Minimum Bactericidal Concentration
The MBC test is recognized as the lowest concentration of the antimicrobial agent that kills 99.9% of the bacterial cells. To determine the MBC, 10 µL of the samples were taken using a pipette from all the wells that had been evaluated in the MIC test. Subsequently, each sample was cultured on a sterile MHA medium. The culture plates were incubated for 24 hours at 37 °C. The lowest dilution of the essences that had resulted in bacterial death was identified by the absence of bacterial colonies on the plate and considered the MBC of that essence.
Chemical Composition Analysis of Essential Oils Using Gas Chromatography–Mass Spectrometry
The chromatographic analysis of EOs was performed using a Perkin Elmer gas chromatograph. A column with a length of 30 meters, an internal diameter of 0.25 mm, and a film thickness of 0.25 µm was used in this method. Furthermore, the fragmentation process was conducted by ionization energy at 70 eV. The temperature program was initially set at 50 °C for 2 minutes, then increased by 8 °C per minute for 18 minutes until reaching 290 °C. Helium was utilized as the carrier gas at a flow rate of 1 mL/min. The sample size was 1 µL of the EO, which was diluted in hexane. The mass spectra (GC-MS) of the EOs were analyzed by comparing them with the mass spectra in the Wiley NBS75K.L and NIST/EPA/NIH libraries (2002 edition, National Institute of Standards and Technology, USA). This method is especially employed for identifying various compounds in EOs. The mass spectra obtained from this analysis were compared with those in the libraries, and the compounds were identified accordingly (20).
In Silico Studies
Three-Dimensional Structure and Molecular Docking
In this study, the H Dock server was utilized for molecular docking; the ligand and protein files were uploaded on the site, and the docking results were observed. The research focused on compounds with medicinal properties. The structures of the compounds were obtained from the PubChem database (http://pubchem.ncbi.nlm.nih.gov). Moreover, the appropriate crystal structure of the beta-lactamase enzyme containing the central catalytic domain was selected and downloaded from the RCSB Protein Data Bank (PDB; https://www.rcsb.org/pdb). The enzyme codes for E. coli and L. monocytogenes were 1JWZ and 5ZQB, respectively.
The enzyme structure downloaded from the PDB site was opened with Discovery software. The panel was opened, and HETATM structures were removed to exclude non-protein parts. Additionally, except for the main chain, other chains were removed, leaving only the main chain and protein parts. The file was then saved in the PDB format with the extension pro1.pdb.
Enzyme File Preparation
The file containing the desired sample was created on the desktop, containing the enzyme downloaded from the PDB site, the enzyme file prepared with Discovery, and the compounds prepared with HyperChem.
Enzyme Preparation With Chimera
The option File → Open was selected, and the file containing the enzyme downloaded from the PDB site, the enzyme file prepared with Discovery, and the compounds prepared with HyperChem was opened. The enzyme file prepared with Discovery, named pro.pdb, was selected. Several options were used, including the Select → Residue standard and without it, and Tool → Structure Editing → Dock Prep. In this section, all hypotheses were accepted, and default settings were chosen throughout. Next, the options Tool → Structure Editing → Minimize Structure → Minimize were utilized, and the process was allowed to run until fully optimized. Finally, the file was saved with the pdb format using File → Save.
Analysis of Docking Results
The ten models with the most negative energy from the docking results were downloaded. The first model, with the most negative energy, was selected and analyzed using Discovery software. The panel was opened from the top left corner, and the interactions of the compounds with the active site of the beta-lactamase enzyme underwent examination. Several steps were performed, including Ligand → Structure → H-bond Monitor, where all hydrogen bonds formed in the enzyme’s active site were shown. The possible outcomes include the formation of no bonds or the formation of several bonds. The compound’s effect on the enzyme under study can be determined based on the number of formed bonds.
The results, including the most favored regions, disallowed regions, ligand binding energies, and types of ligand-protein interactions (e.g., hydrogen bonds, hydrophobic interactions, π interactions, interactions with copper ions in the enzyme’s active site, and other interactions), were observed and analyzed after performing the docking procedure. The Discovery software and two servers, HDOCK and PDBsum Generate, were employed to obtain this information.
Results
In Vitro Studies Results
The Inhibition Zone Size in the Disk Diffusion Method
Based on the examination of the inhibition zone diameter, cumin EO exhibited better inhibitory performance against the bacterial species of interest, L. monocytogenes and E. coli. In contrast, the inhibitory property of fennel EO was slightly lower than that of the cumin EO. Table 1 provides data on the extent of the inhibition zone, showing results from three repetitions for each EO.
Table 1.
The Inhibition Zone of Discs Containing Plant Essential Oils
|
Bacteria Species
|
Cuminum cyminum
EO Inhibition Zone
|
Foeniculum
vulgare
EO Inhibition Zone
|
|
Escherichia coli (ATCC 25922) |
15 mm |
16 mm |
15 mm |
14 mm |
14 mm |
13 mm |
|
Listeria monocytogenes (IBRC-M 10671) |
20 mm |
21 mm |
21 mm |
19 mm |
18 mm |
19 mm |
| Control group |
0 mm |
0 mm |
0 mm |
0 mm |
0 mm |
0 mm |
Note. The results of Tukey’s analysis of variance test showed that all four groups (cumin-Escherichia coli, cumin-Listeria monocytogenes, fennel-Escherichia coli, and fennel-Listeria monocytogenes) had significant differences with each other (P < 0.05). The highest inhibition zones belonged to the essential oil of cumin against Listeria monocytogenes, fennel against Listeria monocytogenes, cumin against Escherichia coli, and fennel against Escherichia coli, respectively.
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
The MIC and MBC test results indicated that the cumin EO had greater antibacterial effects on the studied bacteria, where cumin EO inhibited L. monocytogenes at a dilution of 1/400 and fennel at 1/100, with the MBC observed at a dilution of 10 for both EOs, as evidenced by the absence of bacterial colonies on MHA. Additionally, it showed inhibitory effects on E. coli at a dilution of 1/10 and fennel at a dilution of 1/200. Table 2 and Table 3 present the MIC and MBC results of EOs, respectively.
Table 2.
The Minimum Inhibitory Concentration of Essential Oils on Escherichia coli and Listeria monocytogenes
|
Species
|
Essential Oils
|
1/10 (v/v)
|
1/100 (v/v)
|
1/200 (v/v)
|
1/400 (v/v)
|
1/600 (v/v)
|
1/800 (v/v)
|
1/1000 (v/v)
|
1/1200 (v/v)
|
1/1500 (v/v)
|
1/2000 (v/v)
|
Control
|
Escherichia coli
(ATCC 25922) |
Cuminum cyminum
|
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Foeniculum vulgare
|
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Listeria monocytogenes (IBRC-M 10671) |
Cuminum cyminum
|
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Foeniculum vulgare
|
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Note. Inhibition: (-); Growth: (+).
Table 3.
The Minimum Bactericidal Concentration of Essential Oils on Escherichia coli and Listeria monocytogenes
|
Species
|
Essential Oils
|
1/10 (v/v)
|
1/100 (v/v)
|
1/200 (v/v)
|
1/400 (v/v)
|
1/600 (v/v)
|
1/800 (v/v)
|
1/1000 (v/v)
|
1/1200 (v/v)
|
1/1500 (v/v)
|
1/2000 (v/v)
|
Control
|
Escherichia coli
(ATCC 25922) |
Cuminum cyminum
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Foeniculum vulgare
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Listeria monocytogenes (IBRC-M 10671) |
Cuminum cyminum
|
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Foeniculum vulgare
|
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Note. Colony inhibition: (-); Colony Growth: (+).
Chemical Composition of Cuminum cyminum and Foeniculum vulgare Essential Oils
According to the GC-MS analysis results, different compounds were identified in each EO. Almost 92.55% of the chemicals and 99.36% of the compounds were identified in the cumin and fennel EOs, respectively. The main compounds in these EOs were p-cumin aldehyde andtrans-anethole,with 33.7%and 46.5%,respectively. All compounds and values are listed in Tables 4 and 5.
Table 4.
Compounds in the Cuminum cyminum Essential oil Using Gas Chromatography-Mass Spectrometry
|
Compositions
|
%
|
Retention Time/min (RT)
|
| α-Thujene |
0.2 |
9.37 |
| α-Pinene |
0.6 |
9.49 |
| Sabinene |
1.3 |
9.76 |
| β-Pinene |
8.2 |
9.91 |
| Myrcene |
0.5 |
10.33 |
| δ-2-carene |
0.8 |
10.43 |
| α-Terpinene |
0.8 |
10.53 |
| p-Cymene |
15.8 |
10.59 |
| Limonene |
0.6 |
10.63 |
| β-Phellandrene |
0.1 |
10.84 |
| 1,8-Cineole |
0.4 |
12.39 |
| γ-Terpinene |
13.4 |
12.50 |
| Linalool |
0.05 |
12.97 |
| Terpineol |
0.5 |
13.39 |
| p-Cumin aldehyde |
33.7 |
14.73 |
| Perillaldehyde |
0.8 |
14.87 |
| Safranal |
10.6 |
14.92 |
| 9-epi-β-caryophyllene |
3.5 |
15.09 |
| Germacrene D |
0.2 |
15.26 |
| E-α-Farnesene |
0.5 |
15.30 |
| Detected compounds |
92.55 |
- |
Table 5.
Compounds in the Foeniculum vulgare Essential oil Using Gas Chromatography-Mass Spectrometry
|
Compositions
|
%
|
Retention Time/min (RT)
|
| α-Pinene |
0.6 |
7.38 |
| β-Phellandrene |
0.1 |
7.77 |
| β-PINENE |
0.08 |
7.83 |
| α-Phellandrene |
0.1 |
8.08 |
| р-Cymene |
0.2 |
8.56 |
| D-limonene |
9.5 |
8.90 |
| Eucalyptol |
0.06 |
9.35 |
| Bicyclo[3.1.1]hept-2-ene, 3,6,6-trimethyl- |
0.09 |
9.93 |
| L-fenchone |
13.20 |
10.04 |
| Terpinene-4-ol |
0.09 |
11.79 |
| Estragole |
7.2 |
12.60 |
| Bicyclo[2.2.1]heptan-2-ol, 1,3,3-trimethyl-, acetate, (1S-exo)- |
0.07 |
12.87 |
| Trans –anethole |
46.5 |
13.60 |
| 1-Butanone, 2-chloro-3-methyl-1 |
21.20 |
13.70 |
| 4-[(S)-sec-Butyl] anisole |
0.1 |
14.87 |
| 4-Methoxyphenylacetone |
0.07 |
15.03 |
| m-Anisic acid, 4-chlorophenyl ester |
0.1 |
16.30 |
| Myristicin |
0.1 |
17.36 |
| Detected compounds |
99.36 |
- |
Results of in Silico Studies
Molecular Docking of the Active Compounds of Essential Oils
The p-cumin aldehyde, the main compound in the C. cyminum EO based on GC-MS analysis, is shown to dock in the active site of the beta-lactamase enzyme in L. monocytogenes, forming a hydrogen bond with the amino acid TRP79, with a binding energy of -82.72 kcal/mol (Figure 1).
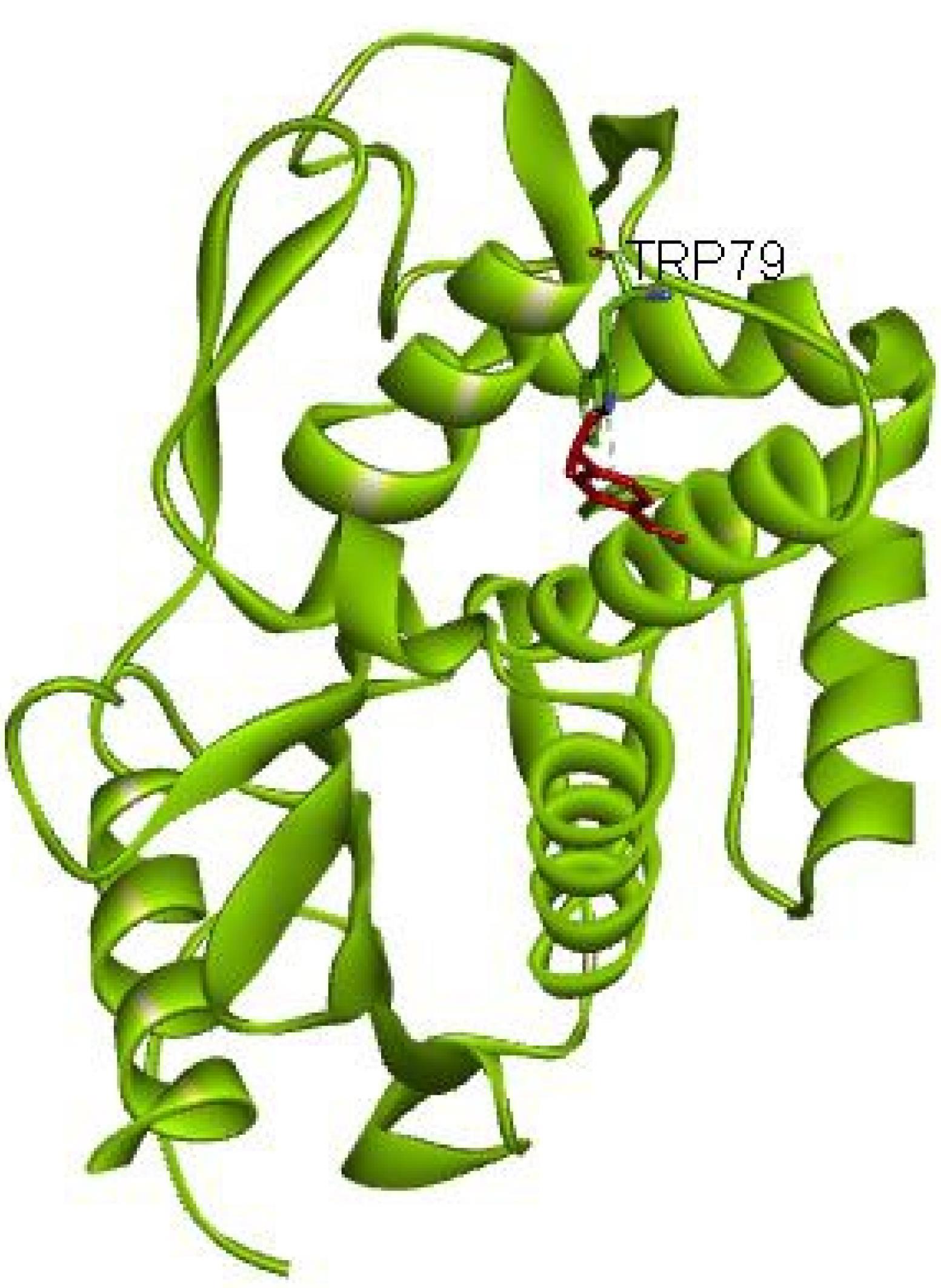
Figure 1.
The Interaction Between P-Cumin Aldehyde and Listeria monocytogenes Beta-Lactamase
.
The Interaction Between P-Cumin Aldehyde and Listeria monocytogenes Beta-Lactamase
Additionally, the interaction of trans-anethole, the main chemical compound in fennel, with the enzyme beta-lactamase of L. monocytogenes underwent evaluation. This molecule, when positioned in the enzyme’s active site, participates in hydrogen bonding with the amino acids GLU190 and ARG108, with a binding energy of -78.71 kcal/mol.
Therefore, the docking results regarding the interactions of molecules and L. monocytogenes beta-lactamase indicated that the compounds trans-anethole and p-cumin aldehyde bind to the active site of the enzyme, possibly inhibiting the enzyme. The compound p-cumin aldehyde forms one hydrogen bond with the beta-lactamase enzyme in the active site, while trans-anethole forms two hydrogen bonds with the beta-lactamase enzyme in the active site. Among these compounds, trans-anethole represents a better inhibitory effect on beta-lactamase (Figure 2).
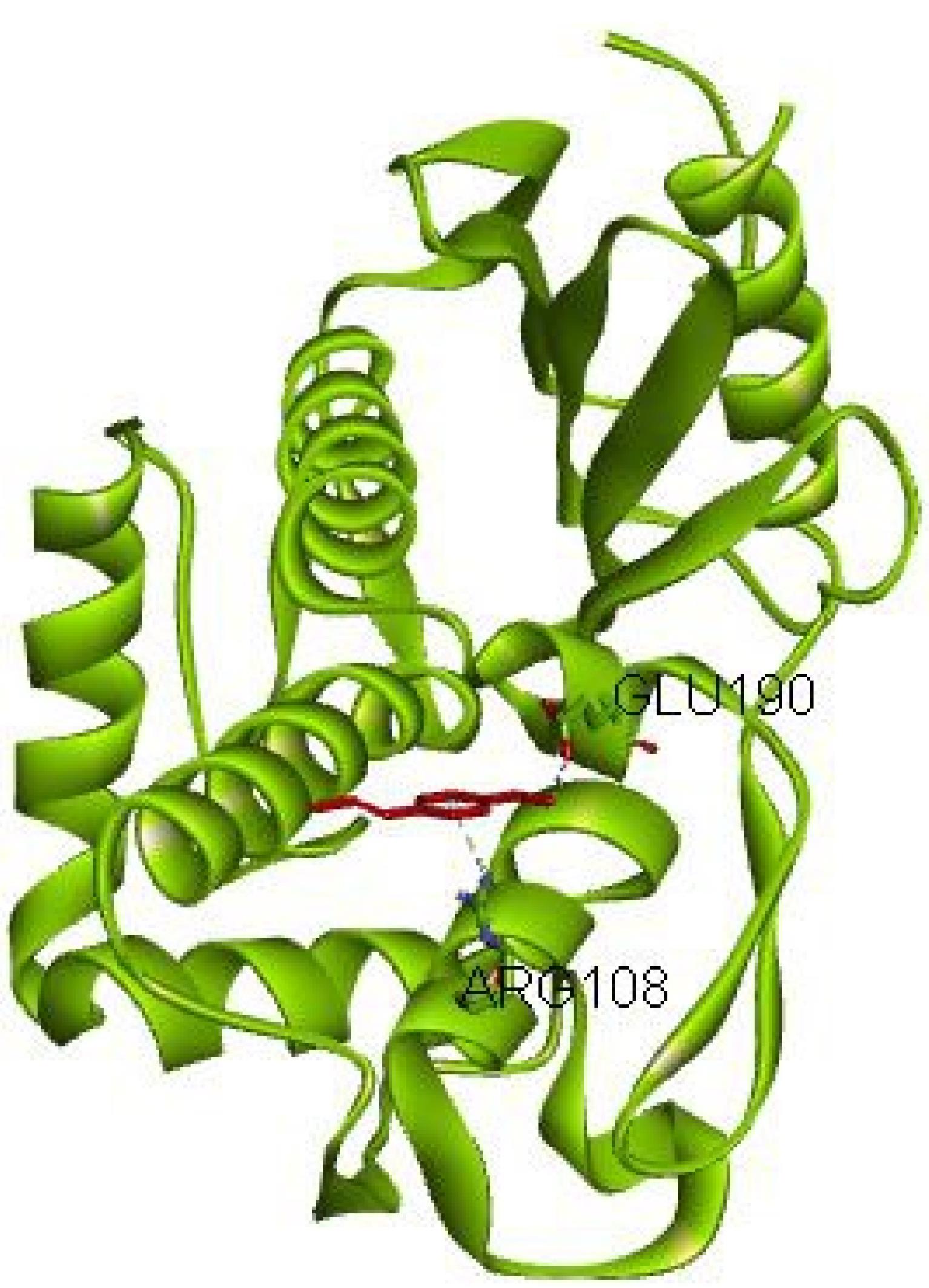
Figure 2.
The Interaction Between Trans-anethole and Listeria monocytogenes Beta-Lactamase
.
The Interaction Between Trans-anethole and Listeria monocytogenes Beta-Lactamase
Considering the structural differences between the beta-lactamase enzymes of E. coli and L. monocytogenes, all of the above-mentioned steps were repeated for E. coli. It was demonstrated that p-cumin aldehyde can bind to amino acids LYS73 and SER70 in the enzyme’s active site by hydrogen bonding, with a binding energy of -79.12 kcal/mol (Figure 3).
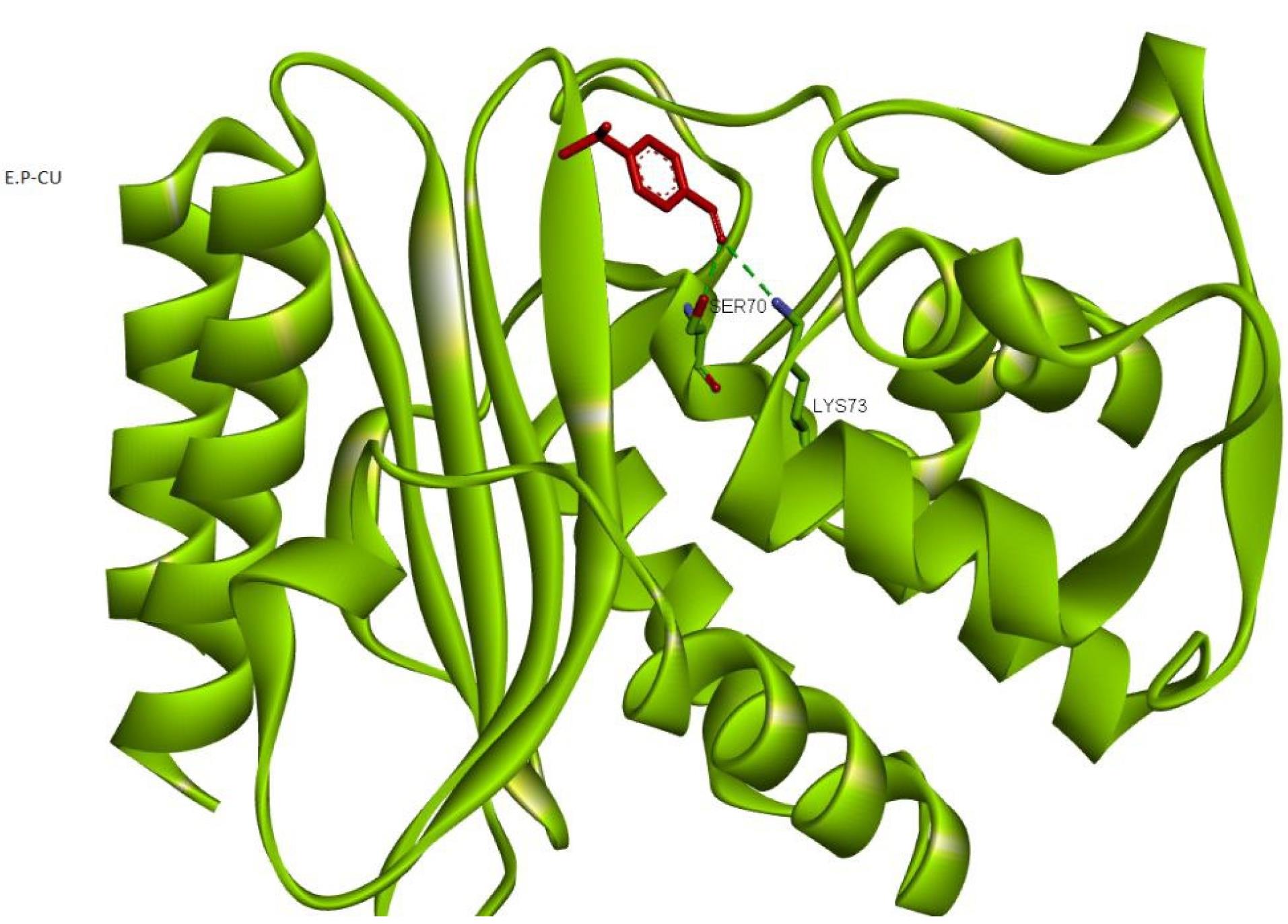
Figure 3.
The Interaction Between P-Cumin Aldehydeand Escherichia coli Beta-Lactamase
.
The Interaction Between P-Cumin Aldehydeand Escherichia coli Beta-Lactamase
The analysis of trans-anethole revealed that this compound is positioned in the enzyme’s active site and participates in hydrogen bonding with the amino acid ALA237, with a binding energy of -74.71 kcal/mol.
Hence, the docking results confirmed that the compounds trans-anethole and p-cumin aldehyde bind to the active site of the beta-lactamase enzyme, leading to its inhibition. The compound p-cumin aldehyde forms two hydrogen bonds with the beta-lactamase enzyme in the active site, while trans-anethole forms one hydrogen bond with the beta-lactamase enzyme in the active site. Among these compounds, p-cumin aldehyde is the most effective inhibitor of the beta-lactamase enzyme (Figure 4).
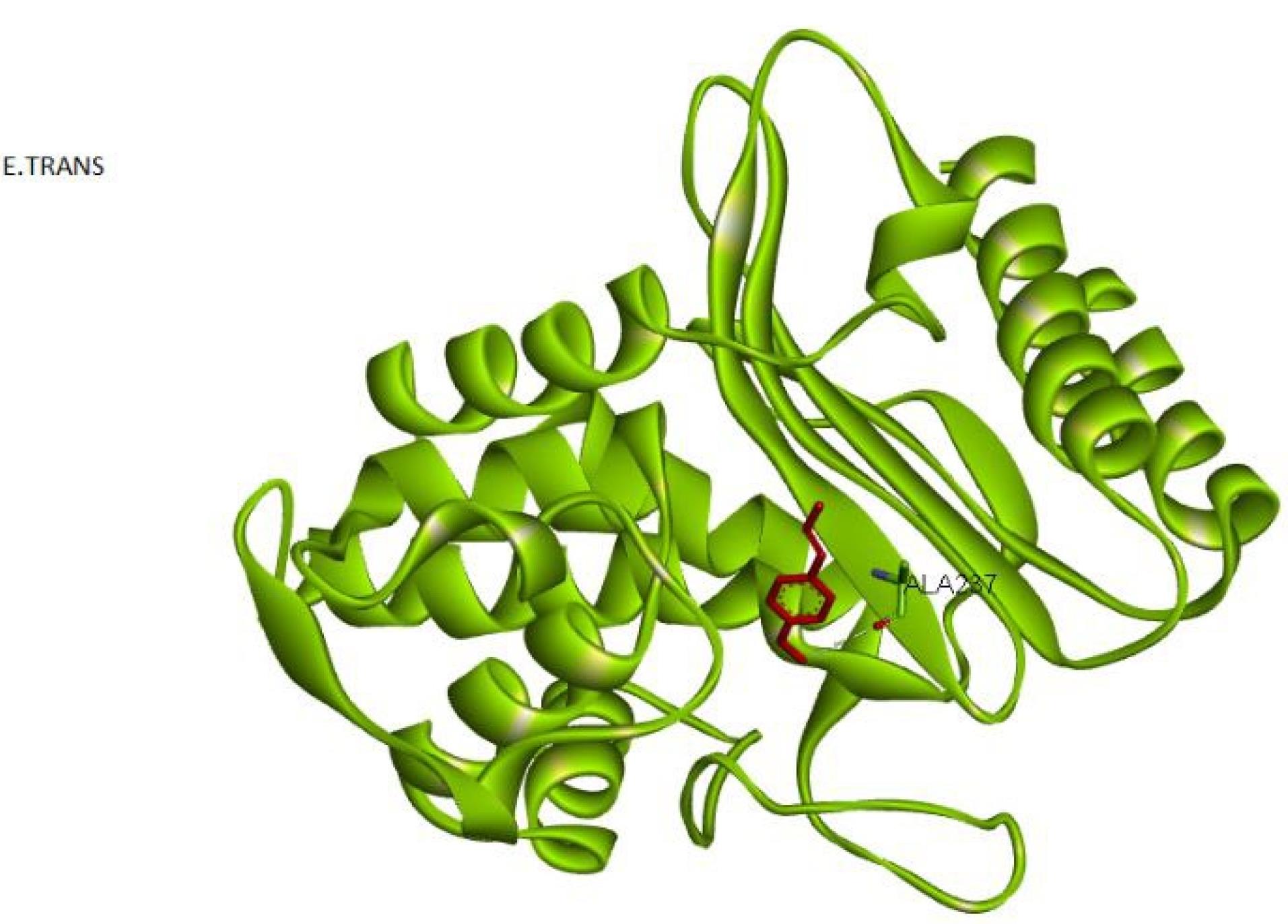
Figure 4.
The Interaction Between Trans-anetholeand Escherichia coli Beta-Lactamase
.
The Interaction Between Trans-anetholeand Escherichia coli Beta-Lactamase
Discussion
Antibiotic resistance is currently one of the major concerns among researchers, with significant risks to human health and the global economy. Consequently, various studies are being conducted to find new antibiotics from different sources. One group of compounds that has shown promising antimicrobial properties in various studies is plants’ EOs. These compounds are particularly important because they can also be used in food products (4,24,25). Given that E. coli and L. monocytogenes are significant causes of food poisoning, extensive research has been performed on the antibiotic resistance of these bacteria. Additional studies are also being implemented to discover new antibiotics, focusing on plant-based compounds that can be utilized in food products to limit the growth of the above-mentioned bacterial species (26-28).
In the present study, the antimicrobial properties of extracts from two plants, C. cyminum and F. vulgare, were examined both in vitro and in silico. The extracts of these two plants demonstrated antimicrobial activity against E. coli and L. monocytogenes. Furthermore, molecular docking studies showed the interactions and binding of the active compounds of these extracts with the beta-lactamase enzymes of the examined bacteria. The compound trans-anethole from fennel exhibited better binding to the active site of the beta-lactamase enzyme of L. monocytogenes, while p-cumin aldehyde from cumin represented better binding to a similar enzyme in E. coli.
Based on the disk diffusion test, it was determined that the diameter of the inhibition zone formed for L. monocytogenes was larger than that for E. coli. Furthermore, cumin exhibited a greater inhibitory effect (larger inhibition zone) against both bacteria compared to fennel. In the study performed by Barrahi et al, the antibacterial properties of the fennel extract were examined on Gram-positive and Gram-negative bacteria, revealing that the inhibition zone diameter for Gram-positive bacteria was larger than that for Gram-negative species. Among Gram-negative bacteria, the largest inhibition zone was observed for Klebsiella pneumoniae at 14 mm, whereas among Gram-positive bacteria, Acinetobacter baumannii showed the largest inhibition zone at 26 mm (20).
Numerous studies have explored the antibiotic properties of cumin and fennel. For instance, Barrahi et al examined the effects of the fennel extract on various Gram-positive and Gram-negative bacterial species. Their findings, which are in line with the present study’s GC/MS results, identified trans-anethole as the main compound in the fennel extract, though its concentration differed by 2.1% (20). Additionally, Lemiasheuski et al investigated the impact of the fennel extract on opportunistic bacteria in human microflora. Despite the lack of the analysis of the ingredients using GC/MS, their findings demonstrated positive antibiotic activity (29).
Based on the GC/MS test, the main compound in cumin was identified as cumin aldehyde, comprising 33.7% of the total compounds. Sharifi et al also used the cumin extract to evaluate its antibiotic properties against methicillin-resistant Staphylococcus aureus and found that the amount of p-cumin aldehyde in their studied cumin was 4.3% higher. Although Sharifi et al did not perform molecular docking tests, the reason for the antibiotic properties remained unidentified. In the present study, the results showed that p-cumin aldehyde and trans-anethole could form hydrogen bonds with the active site of the beta-lactamase enzymes of the studied bacteria (9).
Despite the various studies performed on each plant species, due to differences in the amounts of compounds in each plant under different environmental conditions and genetic variations, further research is still needed in this area (18,30). Zoubiri et al measured the amounts of different compounds in the fennel seed extract and compared these amounts with various studies, finding discrepancies in this regard. For example, the main compound of fennel in the present study was trans-anethole at 46%, while in the studies reviewed by Zoubiri et al, this number ranged from 62% to 88% (18).
To the best of our knowledge, no in silico research has so far been conducted on the docking of active compounds from fennel (trans-anethole) and cumin (p-cumin aldehyde) onto the beta-lactamase enzyme of the studied bacteria. However, some studies indicated that certain active compounds isolated from plants possess the ability to bind to the beta-lactamase enzyme. For example, in a study by Ghodrati and Ataie Kachoie, it was found that compounds from the plant Hypericum perforatum exhibited inhibitory effects on the beta-lactamase enzyme of E. coli. Notably, when comparing the binding energy from their study to the present study, it is evident that the binding energy of p-cumin aldehyde was higher than that of the compounds isolated from H. perforatum (31). The results of the present study demonstrated that the binding energies of p-cumin aldehyde were -82.72 and -78.71 kcal/mol, while those of trans-anethole were -79.12 and -74.71 kcal/mol for the beta-lactamase enzymes of L. monocytogenes and E. coli, respectively. These findings indicate that p-cumin aldehyde exhibits the strongest binding affinity with the beta-lactamase enzyme of L. monocytogenes.
In this research, the researchers examined the antibiotic properties in the laboratory, identified the active ingredient, and then investigated the effects of these compounds on the beta-lactamase enzyme of the studied bacteria through molecular docking analysis. It should be noted that this study has limitations, such as the restriction to certain bacterial species; for instance, gram-positive bacteria ought to be examined more during studies working on antibiotics that affect bacterial species’ cell walls. Additionally, fungal species, particularly foodborne pathogens, could be evaluated as samples. Finally, further molecular docking tests could also be employed to evaluate the effects of these compounds on other parts and activities of bacterial cells.
Conclusion
Our findings demonstrated that the cumin EO exhibits potential antibacterial properties, particularly against L. monocytogenes and E. coli, as evidenced by its larger inhibition zones and lower MIC and MBC values compared to the fennel EO. The GC-MS analysis identified p-cumin aldehyde and trans-anethole as the major active compounds in cumin and fennel EOs, respectively. Moreover, molecular docking studies revealed that these compounds effectively bind to the beta-lactamase enzyme’s active site of both species, with p-cumin aldehyde showing superior inhibitory effects. Overall, these findings suggest that the cumin EO, particularly its active compound p-cumin aldehyde, may hold promise as a potent antibacterial agent.
Authors’ Contribution
Conceptualization: Javid Taghinejad.
Data curation: Farnaz Zakikhani, Javid Taghinejad, Saman Afshar.
Formal analysis: Saman Afshar, Faezeh Mirzaei.
Funding acquisition: Farnaz Zakikhani.
Investigation: Farnaz Zakikhani.
Methodology: Javid Taghinejad, Farnaz Zakikhani.
Project administration: Javid Taghinejad.
Resources: Farnaz Zakikhani.
Software: Saman Afshar, Faezeh Mirzaei.
Supervision: Javid Taghinejad.
Validation: Javid Taghinejad, Saman Afshar.
Visualization: Saman Afshar, Faezeh Mirzaei.
Writing-original draft: Javid Taghinejad, Saman Afshar, Maryam Kazemian, Fatemeh Ataei Masjedlou.
Writing-review & editing: Javid Taghinejad, Saman Afshar, Maryam Kazemian, Fatemeh Ataei Masjedlou.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Funding
This research was conducted with funding provided by the authors.
References
- Hasan TH, Al-Harmoosh RA. Mechanisms of antibiotics resistance in bacteria. Syst Rev Pharm 2020; 11(6):817-23. doi: 10.31838/srp.2020.6.118 [Crossref] [ Google Scholar]
- Urban-Chmiel R, Marek A, Stępień-Pyśniak D, Wieczorek K, Dec M, Nowaczek A. Antibiotic resistance in bacteria-a review. Antibiotics (Basel) 2022; 11(8):1079. doi: 10.3390/antibiotics11081079 [Crossref] [ Google Scholar]
- Chinemerem Nwobodo D, Ugwu MC, Oliseloke Anie C, Al-Ouqaili MT, Chinedu Ikem J, Victor Chigozie U. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal 2022; 36(9):e24655. doi: 10.1002/jcla.24655 [Crossref] [ Google Scholar]
- Chin KW, Michelle Tiong HL, Luang-In V, Ma NL. An overview of antibiotic and antibiotic resistance. Environ Adv 2023; 11:100331. doi: 10.1016/j.envadv.2022.100331 [Crossref] [ Google Scholar]
- Gavanji S, Mohammadi E, Larki B, Bakhtari A. Antimicrobial and cytotoxic evaluation of some herbal essential oils in comparison with common antibiotics in bioassay condition. Integr Med Res 2014; 3(3):142-52. doi: 10.1016/j.imr.2014.07.001 [Crossref] [ Google Scholar]
- Su T, Qiu Y, Hua X, Ye B, Luo H, Liu D. Novel opportunity to reverse antibiotic resistance: to explore traditional Chinese medicine with potential activity against antibiotics-resistance bacteria. Front Microbiol 2020; 11:610070. doi: 10.3389/fmicb.2020.610070 [Crossref] [ Google Scholar]
- Mohammed FS, Sevindik M, Uysal İ, Çesko C, Koraqi H. Chemical composition, biological activities, uses, nutritional and mineral contents of cumin (Cuminum cyminum). Meas Food 2024; 14:100157. doi: 10.1016/j.meafoo.2024.100157 [Crossref] [ Google Scholar]
- Shuja S, Owais A, Fatima N, Shan M, Khan M, Zaman M. The use, phytochemical and antimicrobial activity evaluation of Foeniculum vulgare, Cuminum cyminum, and Trachyspermumammi were reviewed for the treatment of gastric diseases as future compounds. J Pharm Pharmacol 2022; 10:273-82. doi: 10.17265/2328-2150/2022.10.005 [Crossref] [ Google Scholar]
- Sharifi A, Mohammadzadeh A, Salehi TZ, Mahmoodi P, Nourian A. Cuminum cyminum L essential oil: a promising antibacterial and antivirulence agent against multidrug-resistant Staphylococcus aureus. Front Microbiol 2021; 12:667833. doi: 10.3389/fmicb.2021.667833 [Crossref] [ Google Scholar]
- Johri RK. Cuminum cyminum and Carum carvi: an update. Pharmacogn Rev 2011; 5(9):63-72. doi: 10.4103/0973-7847.79101 [Crossref] [ Google Scholar]
- Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L and Carum carvi L essential oils. J Agric Food Chem 2005; 53(1):57-61. doi: 10.1021/jf0487351 [Crossref] [ Google Scholar]
- De M, De AK, Mukhopadhyay R, Banerjee AB, Miro M. Actividad antimicrobiana de Cuminum cyminum L. Ars Pharm 2003; 44(3):257-69. [ Google Scholar]
- Megraj KV, Raju K, Balaraman R, Meenakshisundaram K. Biological activities of some Indian medicinal plants. J Adv Pharm Educ Res 2011; 1:12-22. [ Google Scholar]
- Hajlaoui H, Mighri H, Noumi E, Snoussi M, Trabelsi N, Ksouri R. Chemical composition and biological activities of Tunisian Cuminum cyminum L essential oil: a high effectiveness against Vibrio spp strains. Food Chem Toxicol 2010; 48(8-9):2186-92. doi: 10.1016/j.fct.2010.05.044 [Crossref] [ Google Scholar]
- Pai MB, Prashant GM, Murlikrishna KS, Shivakumar KM, Chandu GN. Antifungal efficacy of Punica granatum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: an in vitro study. Indian J Dent Res 2010; 21(3):334-6. doi: 10.4103/0970-9290.70792 [Crossref] [ Google Scholar]
- Raal A, Orav A, Arak E. Essential oil composition of Foeniculum vulgare Mill fruits from pharmacies in different countries. Nat Prod Res 2012; 26(13):1173-8. doi: 10.1080/14786419.2010.535154 [Crossref] [ Google Scholar]
- Telci I, Demirtas I, Sahin A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill) fruits during stages of maturity. Ind Crops Prod 2009; 30(1):126-30. doi: 10.1016/j.indcrop.2009.02.010 [Crossref] [ Google Scholar]
- Zoubiri S, Baaliouamer A, Seba N, Chamouni N. Chemical composition and larvicidal activity of Algerian Foeniculum vulgare seed essential oil. Arab J Chem 2014; 7(4):480-5. doi: 10.1016/j.arabjc.2010.11.006 [Crossref] [ Google Scholar]
- Ehsanipour A, Razmjoo J, Zeinali H. Effect of nitrogen rates on yield and quality of fennel (Foeniculum vulgare Mill) accessions. Ind Crops Prod 2012; 35(1):121-5. doi: 10.1016/j.indcrop.2011.06.018 [Crossref] [ Google Scholar]
- Barrahi M, Esmail A, Elhartiti H, Chahboun N, Benali A, Amiyare R. Chemical composition and evaluation of antibacterial activity of fennel (Foeniculum vulgare Mill) seed essential oil against some pathogenic bacterial strains. Casp J Environ Sci 2020; 18(4):295-307. doi: 10.22124/cjes.2020.4276 [Crossref] [ Google Scholar]
- Akbari A, Bahmani K, Kafkas NE, Bilgin OF, Hamijo T, Izadi Darbandi A. Evaluation of seed yield, essential oil compositions, and fatty acid profiles in advanced fennel (Foeniculum vulgare Mill) breeding populations. Biocatal Agric Biotechnol 2024; 57:103118. doi: 10.1016/j.bcab.2024.103118 [Crossref] [ Google Scholar]
- Repajić M, Elez Garofulić I, Marčac Duraković N, Balun M, Cegledi K, Cegledi E. Physico-chemical characterization of encapsulated fennel essential oil under the influence of spray-drying conditions Processes. 2024 Ma r 14; 12(3):577. doi: 10.3390/pr12030577 [Crossref] [ Google Scholar]
- Shiri Y, Neyriz Naghadehi M. Co-effects of lactic acid and sodium chloride on the antibacterial activity of cumin and dill essential oils under laboratory conditions. Iran J Infect Dis Trop Med 2022;27(98):1-13. [Persian].
- Khammassi M, Polito F, Caputo L, Abidi A, Mabrouk Y, Nazzaro F. Antibacterial, antibiofilm, and chemical profiles of Ammi visnaga L and Foeniculum vulgare mill essential oils, and ADMET, molecular docking investigation of essential oils major components. Fitoterapia 2024; 177:106047. doi: 10.1016/j.fitote.2024.106047 [Crossref] [ Google Scholar]
- de Sousa Fernandes PA, Pereira RL, Dos Santos AT, Coutinho HD, Morais-Braga MF, da Silva VB. Phytochemical analysis, antibacterial activity and modulating effect of essential oil from Syzygiumcumini (L) skeels. Molecules 2022; 27(10):3281. doi: 10.3390/molecules27103281 [Crossref] [ Google Scholar]
- Nyarko RO, Kumar R, Sharma S, Chourasia A, Roy A, Saha P. Antibacterial activity of herbal plant-Tinospora cordifolia And Catharnthus roseus. World J Pharm Pharm Sci 2022; 11(5):1973-7. doi: 10.20959/wjpps20225-22075 [Crossref] [ Google Scholar]
- Wiktorczyk-Kapischke N, Skowron K, Wałecka-Zacharska E. Genomic and pathogenicity islands of Listeria monocytogenes-overview of selected aspects. Front Mol Biosci 2023; 10:1161486. doi: 10.3389/fmolb.2023.1161486 [Crossref] [ Google Scholar]
- Silva FV. Pasteurization of food and beverages by high pressure processing (HPP) at room temperature: inactivation of Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Salmonella, and other microbial pathogens. Appl Sci 2023; 13(2):1193. doi: 10.3390/app13021193 [Crossref] [ Google Scholar]
- Lemiasheuski V, Ji Y, Buchenkov I, Gritskevitch E, Sysa A. Evaluation of the antibacterial effect of Foeniculum vulgare Mill essential oil on opportunistic microflora: growth and enzymatic activity indicators. Asian J Res Biochem 2024; 14(4):138-48. [ Google Scholar]
- Elbaz M, Abdesslem SB, St-Gelais A, Boulares M, Moussa OB, Timoumi M. Essential oils profile, antioxidant and antibacterial potency of Tunisian fennel (Foeniculum vulgare Mill) leaves grown under conventional and organic conditions. Food Chem Adv 2024; 4:100734. doi: 10.1016/j.focha.2024.100734 [Crossref] [ Google Scholar]
-
Ghodrati L, Ataie Kachoie M. In silico antimicrobial activity of Hypericum perforatum to inhibit some enzymes of three bacterial species and in vitro antimicrobial effects of its extracts. Casp J Environ Sci. 2024:1-11. doi: 10.22124/cjes.2024.7469.