Avicenna Journal of Clinical Microbiology and Infection. 11(4):191-203.
doi: 10.34172/ajcmi.3564
Review Article
Mpox Resurgence: Preventing a Potential Pandemic Through Lessons Learned From COVID-19 Experience and Future Research Directions
Seyi Samson Enitan 1, *  , Richard Yomi Akele 2
, Richard Yomi Akele 2  , Adesuyi Ayodeji Omoare 3
, Adesuyi Ayodeji Omoare 3  , Banenat Bajehson Dogonyaro 4
, Banenat Bajehson Dogonyaro 4  , Michael Unata Iduh 5
, Michael Unata Iduh 5  , Kester Awharentomah Digban 6
, Kester Awharentomah Digban 6  , Seto Saint Tunrayo Aladenika 7
, Seto Saint Tunrayo Aladenika 7  , Grace Eleojo Itodo 8
, Grace Eleojo Itodo 8  , Grace Amarachi John-Ugwuanya 9
, Grace Amarachi John-Ugwuanya 9  , Okeoghene Marcel Edafetanure-Ibeh 10
, Okeoghene Marcel Edafetanure-Ibeh 10  , Ameh Raphael Adole 11
, Ameh Raphael Adole 11  , Agbolahan Adegboyega Adebanjo 12
, Agbolahan Adegboyega Adebanjo 12  , Oluwanifemi Adefiyinfoluwa Ajayi 1
, Oluwanifemi Adefiyinfoluwa Ajayi 1  , Ayomide Oluwatobiloba Okuneye 1
, Ayomide Oluwatobiloba Okuneye 1  , Temitayo Oluwatomisin Ajayi 1
, Temitayo Oluwatomisin Ajayi 1 
Author information:
1Department of Medical Laboratory Science, School of Public and Allied Health, Babcock University, Ilishan-Remo 121109, Ogun State, Nigeria
2Department of Biomedical Science, School of Applied Science, University of Brighton, London, United Kingdom
3Department of Public Health Laboratory Services, Nigeria Centre for Disease Control and Prevention (NCDC). FCT Abuja, Nigeria
4Division of Virology, Parasitology and Bacteriology, National Veterinary Research Institute Vom, Plateau state, Nigeria
5Department of Medical Microbiology, School of Medical Laboratory Science, Usmanu Danfodiyo University, Sokoto, Sokoto State Nigeria
6Department of Medical Laboratory Science, College of Medical & Health Sciences, Novena University, Ogume, Kwale 322107, Delta State, Nigeria
7Department of Medical Laboratory Sciences, University of Nigeria Enugu Campus, Enugu, Enugu State, Nigeria
8Department of Microbiology, Federal Teaching Hospital, Lokoja, Kogi State, Nigeria
9Department of Medical Laboratory Science, Medbury Medical Services, Lagos, Lagos State, Nigeria
10Department of Environmental and Occupational Health, Texas A&M School of Public Health, 212 Adriance Lab Rd, College Station, TX 77843, United States of America
11Department of Infectious Disease, Clinton Health Access Initiative, FCT-Abuja, Nigeria
12Department of Medical Laboratory Science, Grandville Medical Center, Surulere, 101232, Lagos State, Nigeria
Abstract
Mpox, declared a Public Health Emergency of International Concern by the World Health Organization (WHO), poses a growing global threat. The resurgence, driven by the highly transmissible and severe clade Ib strain from the Democratic Republic of the Congo, raises concerns about a potential pandemic. This narrative review aims to identify actionable strategies to mitigate the current Mpox outbreak by leveraging lessons from past health crises, particularly COVID-19, and to highlight future research priorities essential for global preparedness. A comprehensive literature review from 2018 to 2024 was conducted using Google Scholar, Scopus, and PubMed databases, focusing on Mpox transmission, vaccine efficacy, antiviral treatments, and public health responses. Our findings revealed that the resurgence of Mpox is fueled by viral evolution, ecological changes, and inadequate surveillance, exacerbated by inequitable healthcare access and insufficient public health infrastructure. While existing vaccines, such as Jynneos and ACAM2000, offer protection, their long-term efficacy and safety across diverse populations require further study. Antiviral treatments, including tecovirimat and brincidofovir, show promise but lack optimized care protocols and face challenges such as emerging resistance. Lessons from the COVID-19 response underscore the importance of enhancing global surveillance systems, strengthening healthcare infrastructure, ensuring equitable vaccine distribution, and fostering community engagement. Effective Mpox management also requires standardized treatment guidelines and the integration of genomic monitoring to track viral evolution and drug resistance. Urgent international collaboration is needed to avert a potential Mpox pandemic. Priorities include advancing research on long-term vaccine efficacy, optimizing antiviral therapies, and improving care for severe cases. Actionable recommendations include robust policies, sustainable funding, and global collaboration to strengthen pandemic preparedness and mitigate Mpox’s impact.
Keywords: Mpox, Pandemic preparedness, Surveillance, Global cooperation, Healthcare infrastructure, Vaccine distribution
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Enitan SS, Akele RY, Omoare AA, Dogonyaro BB, Iduh MU, Digban KA, et al. Mpox resurgence: preventing a potential pandemic through lessons learned from COVID-19 experience and future research directions. Avicenna J Clin Microbiol Infect. 2024; 11(4):191-203. doi:10.34172/ajcmi.3564
Introduction
In the wake of the COVID-19 pandemic, the global community has become acutely aware of the profound impact viral outbreaks can have on public health (1). The resurgence of Mpox, formerly known as Monkeypox, underscores the ongoing risks posed by infectious diseases. Once confined to endemic regions, Mpox has now spread significantly, highlighting the interconnectedness of global health threats (2). The World Health Organization (WHO) has recently declared Mpox a Public Health Emergency of International Concern (PHEIC) due to its rapid spread across multiple African countries and the rise of a new, more transmissible strain, clade Ib (3). This declaration is a stark reminder of the evolving nature of viral threats and the necessity for immediate and coordinated global action. A PHEIC is defined by the WHO as an “extraordinary event” that “constitutes a public health risk to states through the international spread of disease” (2). In 2022, the WHO declared Mpox a PHEIC for the first time. The outbreak was primarily driven by the Clade IIb variant, which spread mainly through sexual transmission (4). However, the current surge is being caused by the more lethal Clade I strain, which has a mortality rate of 3%, significantly higher than the 0.2% rate observed in 2022 (2,3,5). As Mpox transitions from a regional concern to a global emergency, it is crucial to reflect on the lessons learned from the COVID-19 pandemic. The COVID-19 experience revealed significant vulnerabilities in our global health systems, including gaps in early detection, response capabilities, and preparedness (1,6). Applying these lessons to Mpox preparedness can help mitigate the risk of a full-scale pandemic. This article explores how insights gained from COVID-19 can inform our approach to managing Mpox and preventing future pandemics. By linking past experiences with current strategies, it is possible to enhance our readiness and response, ultimately fostering a more resilient global health system. The aim of this narrative review is to identify actionable strategies to mitigate the current Mpox outbreak by leveraging lessons from past health crises, particularly COVID-19, and to highlight future research priorities essential for global preparedness.
Methods
Search and Selection Strategy
This narrative review employed a systematic search of Google Scholar, Scopus, and PubMed databases to gather peer-reviewed articles and reports related to Mpox transmission, vaccine efficacy, antiviral treatments, and public health responses. The search was restricted to studies published between 2018 and 2024, a timeframe chosen to encompass the impact of COVID-19 on global health strategies and its lessons for Mpox management.
The search strategy included a combination of keywords and medical subject heading (MeSH) terms, such as “mpox”, “global response”, “epidemiology”, “vaccination”, “antiviral treatments”, “pandemic preparedness”, “COVID-19 response”, and “lessons from COVID-19”. Boolean operators (AND, OR) refined the results to ensure relevance and comprehensiveness. The search was conducted in July 2024.
Inclusion and Exclusion Criteria
The following criteria were applied to select articles:
Inclusion Criteria
-
Peer-reviewed studies, systematic reviews, or official reports published between 2018 and 2024.
-
Studies addressing Mpox epidemiology, transmission dynamics, vaccine efficacy, antiviral treatments, and public health interventions.
-
Research providing insights into pandemic preparedness or drawing comparisons to the COVID-19 response.
-
English-language publications for consistency and accessibility.
Exclusion Criteria
-
Studies not directly addressing Mpox or its management strategies.
-
Articles lacking empirical evidence or methodological rigor.
-
Duplicates, abstracts without full texts, and preprints not peer-reviewed.
Data Extraction and Analysis
Relevant data were extracted systematically, focusing on study design, findings, and implications. Thematic categorization identified patterns, trends, and research gaps. This synthesis emphasized parallels between Mpox and COVID-19, highlighting lessons from the latter’s global response to improve Mpox prevention, management, and research strategies.
Ethical Considerations
As publicly available literature was utilized, ethical approval was unnecessary. All cited works were appropriately acknowledged to maintain academic integrity.
Results and Discussion
Understanding Mpox
Mpox, formerly known as monkeypox, is a zoonotic viral disease caused by the monkeypox virus, a member of the Orthopoxvirus genus. First identified in laboratory monkeys in 1958 and humans in 1970 in the Democratic Republic of Congo (DRC), Mpox was historically confined to Central and West Africa. However, recent outbreaks, including the 2022–2023 surge, have spread to non-endemic regions, raising global alarm (7-9).
The Mpox virus (Figure 1) is a double-stranded DNA virus with a complex structure. It features a lipid bilayer outer membrane with proteins and glycoproteins, facilitating cell attachment and immune evasion. The viral core, encased in a palisade layer, contains DNA, nucleocapsid proteins, and enzymes critical for replication. These features enable the virus to infect host cells and evade the immune system effectively (10). The virus has two clades, namely, clade I and clade II. Clade IIb, identified during the 2015 Nigeria outbreak, demonstrated person-to-person transmission via sexual contact-a shift in its epidemiology. This clade has driven recent global outbreaks, highlighting its increased transmissibility (11-13).
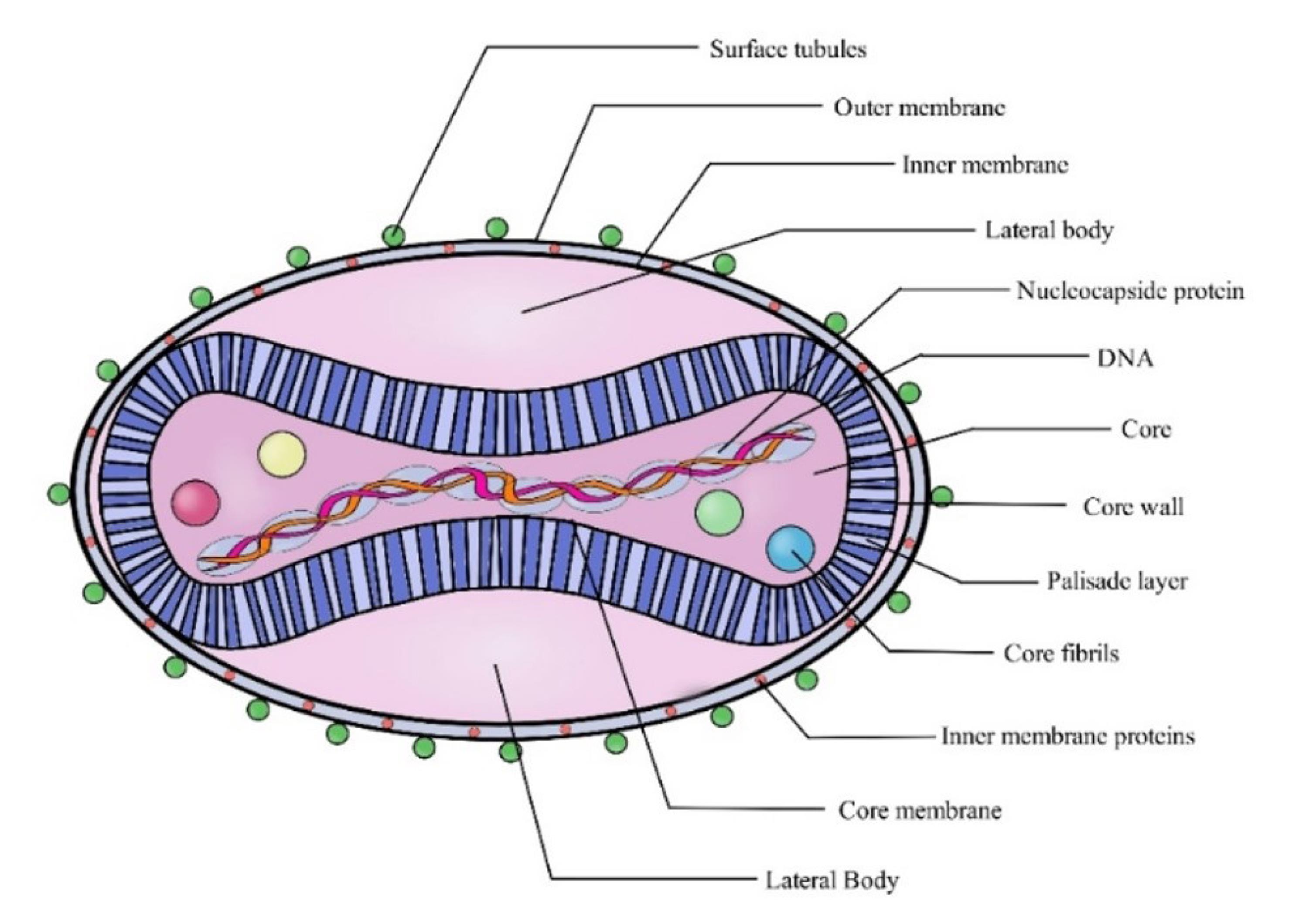
Figure 1.
The Morphology of the Mpox Virus. Source (11)
.
The Morphology of the Mpox Virus. Source (11)
Diagnosis relies on polymerase chain reaction (PCR) testing of lesion samples. Enhanced PCR assays and multiplex testing have improved diagnostic accuracy and outbreak management (14). Preventive strategies include avoiding contact with infected individuals and utilizing smallpox vaccines, which provide cross-protection. Emerging Mpox-specific vaccines and antiviral treatments, though still under research, offer targeted management options. Supportive care remains the primary treatment, emphasizing symptom alleviation and complication management. Effective prevention and management are critical to mitigating Mpox’s global health impact (15-19).
Mpox Vaccines
Vaccines developed for smallpox, such as Jynneos (Imvamune/Imvanex) and ACAM2000, are effective against Mpox due to the genetic similarity between the viruses (20,21). Jynneos, a non-replicating live virus vaccine (MVA-BN strain), is approved for both smallpox and Mpox. Its safety profile makes it suitable for individuals with weakened immune systems, pregnant women, and those with certain skin conditions. ACAM2000, a live replicating virus vaccine, is primarily used for smallpox but is also effective against Mpox. However, it has a higher risk of side effects and is less suitable for individuals with health conditions. The comparison of ACAM2000® and JYNNEOSTM (APSV) vaccines is presented in Table 1.
Table 1.
Comparison of ACAM2000® and JYNNEOSTM (APSV) Vaccines
|
Parameters
|
ACAM2000®
|
JYNNEOSTM (APSV)
|
| Year of approval |
2007 |
2019 |
| Manufacturer |
Sanofi Pasteur/Emergent BioSolutions |
Bavarian Nordic |
| Country of approval |
United States (FDA)/Australia |
United States (FDA) and European Union (EMA) |
| Vaccine type |
Live attenuated smallpox vaccine |
Live attenuated vaccine against smallpox and monkeypox |
| Age of recipients |
Licensed for use in individuals aged 1 year and older |
Licensed for use in individuals aged 18 and older |
| Efficacy |
Effective against smallpox; efficacy against monkeypox not well established |
Demonstrated efficacy against monkeypox; efficacy against smallpox not as well established |
| Safety |
Associated with potentially serious side effects, including myocarditis and pericarditis, |
Generally well-tolerated with a lower side effect profile compared to ACAM2000® |
| Contraindications |
Not recommended for individuals with immunocompromised conditions, pregnancy, or certain skin conditions |
Contraindicated in individuals with severe allergies to components of the vaccine; less restrictive than ACAM2000® |
| Storage |
Requires freezing (-15 °C to -25 °C) |
Requires refrigeration (2 °C to 8 °C) |
| Administration route |
Intradermal |
Subcutaneous |
| dosing schedule |
Single dose |
Single dose |
| Post-vaccination effects |
Possible reactions include fever, rash, and local site reactions |
Typical reactions include mild fever, fatigue, and local site reactions |
| Vaccine development |
Derived from the original smallpox vaccine strain |
Derived from a modified vaccinia virus |
| Coverage |
Provides immunity primarily against smallpox |
Provides immunity against both smallpox and monkeypox |
| Cost |
Generally lower cost |
Typically higher cost due to newer technology |
| Public health use |
Historically used for smallpox outbreaks and in pre-exposure vaccination programs |
Used in current monkeypox outbreaks and pre-exposure vaccination programs |
Vaccination is most effective within 4 days of exposure but can still provide protection if administered within 14 days, particularly before the development of symptoms. Preventive vaccination is recommended for high-risk populations, even without direct exposure (22,23). Challenges in vaccine distribution, including limited healthcare infrastructure and cold storage requirements, necessitate strategies such as mobile vaccination units and local health partnerships to ensure equitable access.
Mpox Medications
Antivirals developed for smallpox, such as tecovirimat (TPOXX), have been repurposed for Mpox treatment. Tecovirimat inhibits the VP37 protein, preventing viral maturation and spread and reducing infection severity (24). Other options include brincidofovir and cidofovir. The first one is an oral prodrug that inhibits viral DNA replication and shows promise in clinical studies for Mpox. The second one, which targets viral DNA polymerase, is used for severe Orthopoxvirus infections and is under evaluation for Mpox treatment. Both are being assessed for safety and efficacy (25). Table 2 compares some common Mpox medications.
Table 2.
Comparison of Some Common Mpox Medications
|
Parameter
|
Tecovirimat
|
Brincidofovir
|
Cidofovir
|
| Brand name |
TPOXX |
CMX001 |
Vistide |
| Manufacturer |
SIGA Technologies |
Chimerix |
Gilead Sciences |
| Approval date |
2018 (for smallpox); 2022 (for Mpox) |
2021 (for smallpox) |
1996 (for cytomegalovirus, used off-label for Mpox) |
| Country of approval |
USA |
USA |
USA |
| Drug class |
Antiviral (Orthopoxvirus-specific) |
Antiviral (Orthopoxvirus-specific) |
Antiviral (nucleotide analog) |
| Mechanism of action |
Inhibits viral envelope protein synthesis |
Inhibits viral DNA polymerase |
Inhibits viral DNA polymerase |
| Administration route |
Oral |
Oral |
Intravenous (IV) |
| Dosage |
600 mg twice daily for 14 days |
200 mg orally once weekly |
5 mg/kg IV once a week for 2 weeks |
| Efficacy |
Effective against smallpox and Mpox (in clinical trials) |
Effective against smallpox (not yet widely used for Mpox) |
Effective against smallpox (limited data for Mpox) |
| Safety |
Generally well-tolerated; common side effects include nausea, diarrhea, and headache |
Generally well-tolerated; side effects include nausea, headache, and abdominal pain |
Potential for nephrotoxicity; a need for monitoring kidney function |
| Contraindications |
Pregnancy and severe renal impairment |
Pregnancy, breastfeeding, severe liver, or kidney disease |
Renal impairment, pregnancy, and lactation |
| Storage requirements |
Stored at room temperature (20-25 °C) |
Stored at room temperature (20-25 °C) |
Stored at room temperature (20-25 °C) |
| Pharmacokinetics |
Well-absorbed orally, metabolized by the liver |
Well-absorbed orally, metabolized by the liver |
Requires dose adjustment in renal impairment |
| Common side effects |
Nausea, diarrhea, headache |
Nausea, headache, and abdominal pain |
Nephrotoxicity, nausea, and headache |
| Use in pediatrics |
Not well-studied in children |
Not well-studied in children |
Used with caution in pediatric patients |
| Current status |
Approved for Mpox in clinical settings |
Approved for smallpox; off-label use for Mpox pending further data |
Limited use in Mpox; primary use for cytomegalovirus |
Global Spread of Mpox
The Mpox outbreak accelerated in May 2022, involving clade IIb in over 116 countries with nearly 100 000 cases reported globally (26,27) (Figure 2 depicts the global maps of Mpox cases in 2022 and 2024). In Africa, the clade I variant, specifically clade Ib, has driven the crisis, causing severe disease. Since early 2023, the DRC reported over 22 000 cases and 1200 deaths, with clade Ib spreading to neighboring countries such as Kenya, Rwanda, and Burundi. This highlights the virus’s adaptability and cross-border transmission.
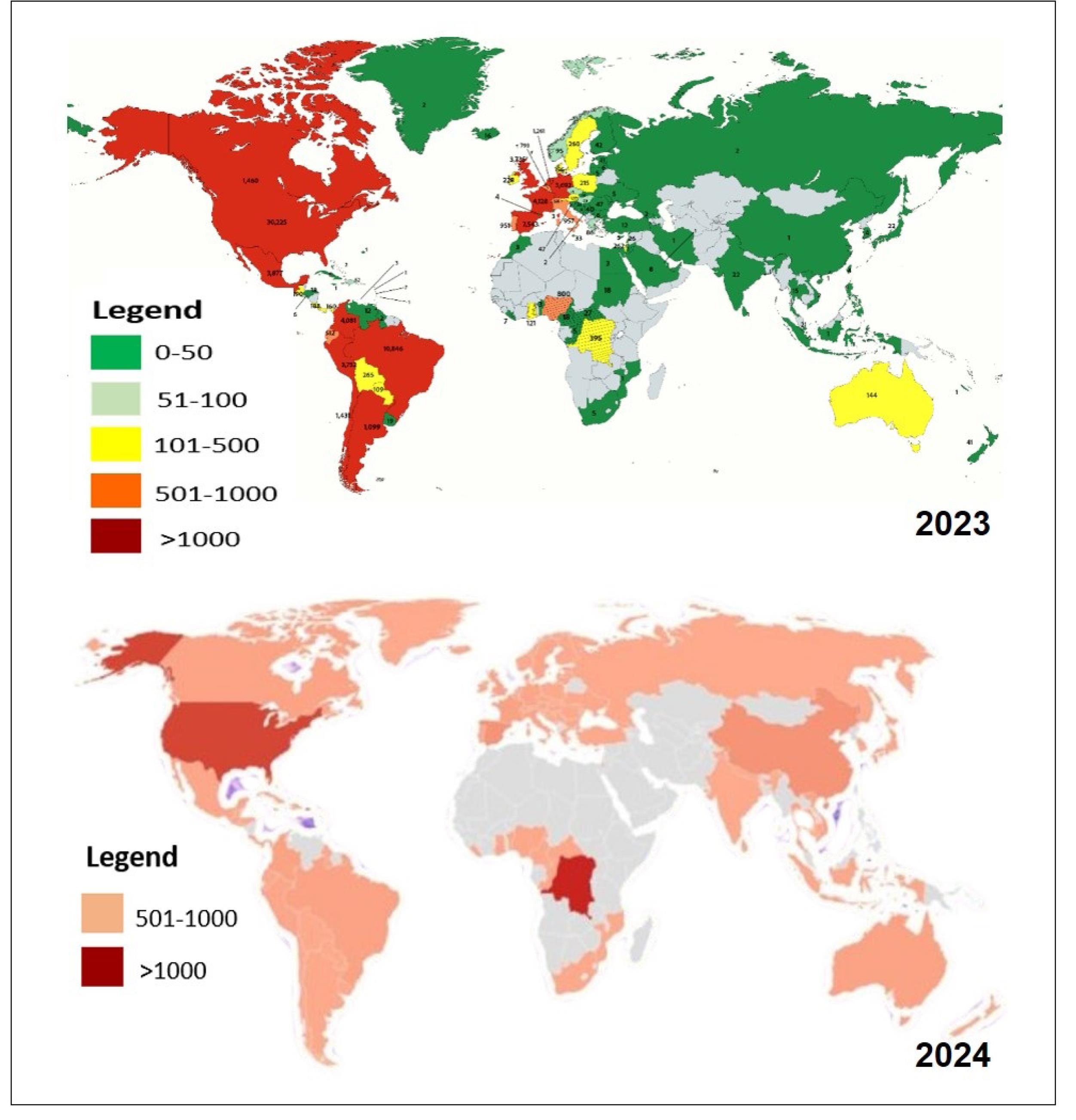
Figure 2.
Global Maps of Mpox Cases in 2022 and 2024. Source. (11,28)
.
Global Maps of Mpox Cases in 2022 and 2024. Source. (11,28)
Figure 3 is a map illustrating African countries with reported laboratory-confirmed cases of Clade 1 Mpox, as well as those with a risk of Clade 1 Mpox exposure. In 2024, the DRC accounted for 96% of Africa’s 17 000 cases, with 1754 confirmed from January to June alone (10,23,26-29). Clade II persists globally, with the United States reporting 1399 cases in early 2024. Wastewater surveillance in the U.S. suggests low levels of detection, and the CDC considers the risk of clade I reaching the United States “very low”. High-density regions and limited healthcare infrastructure remain hotspots, complicating containment efforts (2,26).
Drivers of Global Spread of Mpox
The global resurgence of Mpox is driven by a complex interplay of ecological, social, and economic factors. Understanding these drivers is critical for developing effective interventions.
Ecological Drivers
-
Increased human-animal interactions: Human encroachment into wildlife habitats has increased human-animal contact, raising the risk of zoonotic spillover events. Activities such as hunting bushmeat in Central Africa have been linked to Mpox outbreaks, as handling potentially infected animals facilitates transmission to humans (30).
-
Deforestation and habitat destruction: Deforestation for agriculture, logging, and urbanization displaces wildlife, bringing them into closer contact with humans. This facilitates viral spillover and increases the risk of diseases such as Mpox, as detected in regions such as the Amazon.
Social Drivers
-
Urbanization and population density: Rapid urbanization, particularly in developing nations, leads to densely populated areas with inadequate sanitation and healthcare. In cities such as Lagos, Nigeria, overcrowded conditions and limited public health infrastructure exacerbate Mpox outbreaks (27).
-
Global travel and connectivity: The modern travel era allows infected individuals to spread the virus across continents. The 2022-2023 global Mpox outbreak, which reached over 116 countries, was primarily driven by international travel, highlighting the role of global connectivity in disease spread (27).
Economic Drivers
-
Zoonotic spillover events: Economic activities such as agriculture and mining in biodiverse areas increase human-wildlife contact, facilitating zoonotic spillovers. In West Africa, mining operations have been linked to Mpox outbreaks due to increased human-wildlife interactions.
-
Public health gaps and weak infrastructure: In regions with weak healthcare infrastructure, such as the Democratic Republic of Congo, delayed outbreak detection and limited resources for vaccination have allowed Mpox to spread unchecked.
The drivers mentioned above interact to amplify Mpox transmission. Urbanization contributes to habitat destruction, while global travel spreads the virus to regions with weak public health systems. During the 2022-2023 outbreak, urban centers became hotspots due to dense populations and inadequate healthcare.
Emergence of New Strains and Public Health Implications
The emergence of Mpox clade Ib in the eastern DRC has reshaped the virus’s epidemiology and pathogenicity, leading to its designation as a PHEIC. Genetic mutations in clade Ib enhance sexual transmission and cross-border spread, raising concerns about widespread outbreaks and severe disease. Unlike earlier strains, which primarily spread zoonotically or via respiratory droplets, clade Ib thrives in sexual networks, complicating containment. It is associated with more severe outcomes, including extensive lesions and higher systemic involvement, particularly in immunocompromised individuals (2,3,31,32).
Current vaccines, such as Jynneos and ACAM2000, have been developed for earlier Mpox strains and may have reduced efficacy against clade Ib. While initial data indicate some protection, ongoing research examines whether clade-specific mutations affect vaccine-induced immunity, necessitating potential modifications (20-23). Clade Ib’s enhanced transmission necessitates targeted interventions, including strengthened surveillance and public health campaigns addressing sexual transmission. Education and prevention efforts focus on high-risk populations, while isolation and vaccination strategies are adapted to the strain’s dynamics (24).
The WHO and global stakeholders have intensified efforts to improve surveillance, expedite vaccine development, and coordinate international responses. Challenges include delayed vaccine distribution, logistical barriers, and inconsistent global readiness. The United States has donated 50 000 vaccine doses to the DRC, and WHO frameworks prioritize allocation to high-transmission areas. Strengthening diagnostic capacities, deploying rapid response teams, and enhancing community education remain critical strategies. Lessons from earlier outbreaks, including the 2022 global spread, highlight the need for improved coordination and accelerated vaccine rollout to prevent future pandemics (2,3,5).
Lessons From the Coronavirus Disease 19 Experience
The COVID-19 pandemic has underscored the critical importance of robust global health systems and has provided valuable lessons that can be directly applied to preventing and managing Mpox outbreaks (33). Figure 4 highlights five key lessons from the COVID-19 experience.
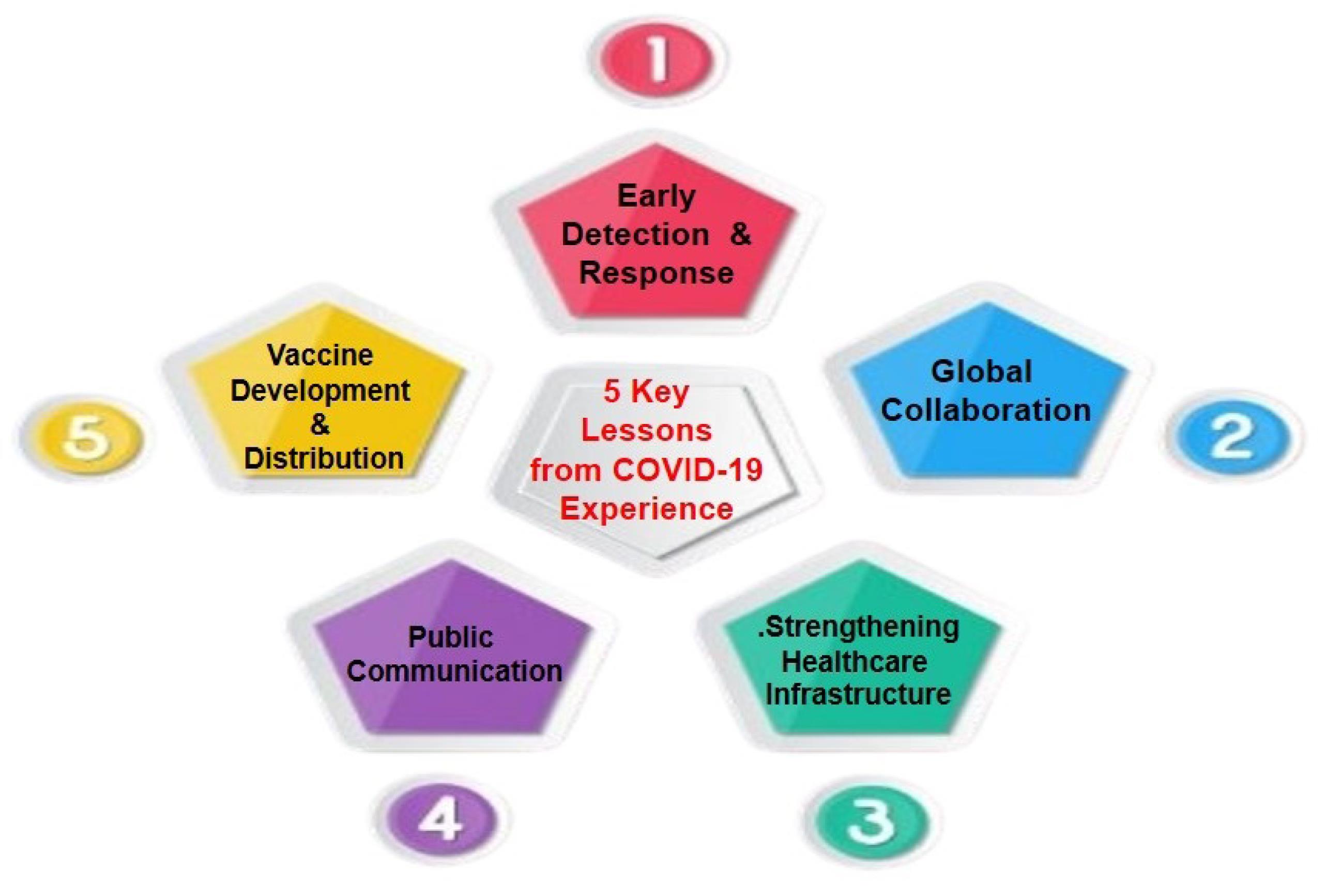
Figure 4.
Five Key Lessons From COVID-19 Experience
.
Five Key Lessons From COVID-19 Experience
The following paragraphs demonstrate how these insights can be leveraged effectively:
1. Early Detection and Response
Lesson learned
During COVID-19, the delay in identifying and responding to the virus allowed it to spread unchecked, leading to a global pandemic. The significance of rapid detection and immediate response became evident as countries with swift interventions fared better in controlling the outbreak (34,35).
Application to Mpox
For Mpox, early detection is equally crucial. Strengthening global surveillance systems modeled after the successful elements of COVID-19 tracking-such as widespread testing and data-sharing platforms-can enable rapid identification of cases. For example, the integration of genomic sequencing, which was critical in tracking COVID-19 variants (36), can be employed to monitor the emergence of new Mpox strains such as clade Ib. Countries should establish protocols for immediate case isolation and contact tracing to prevent Mpox from reaching a critical mass (37).
Actionable recommendation
Develop and deploy Mpox-specific diagnostic tools that can be rapidly distributed to areas at risk. Enhance cross-border surveillance agreements to ensure timely reporting and sharing of data on Mpox cases.
2. Global Collaboration
Lesson learned
The global response to COVID-19 demonstrated that no single country can manage a pandemic alone. Collaborative efforts led to the accelerated development of vaccines and treatments, and shared data were key to understanding and combating the virus (34,38).
Application to Mpox
Mpox requires similar levels of international cooperation. The establishment of global Mpox task forces, akin to those formed for COVID-19, can streamline efforts across borders. For instance, the COVAX initiative, which aimed to provide equitable access to COVID-19 vaccines, can serve as a model for Mpox vaccine distribution (39). Collaborative research efforts, such as pooling resources for clinical trials of antiviral drugs such as tecovirimat, could speed up the development of effective treatments (40).
Actionable recommendation
Create a global Mpox coordination center to facilitate real-time data exchange, resource allocation, and research collaboration, ensuring a unified response to outbreaks.
3. Strengthening Healthcare Infrastructure
Lesson learned
COVID-19 exposed significant weaknesses in healthcare systems worldwide, particularly in the ability to handle surges in patients and provide equitable care. Countries with robust healthcare infrastructure managed the crisis more effectively (33,41).
Application to Mpox
Strengthening healthcare infrastructure, especially in regions where zoonotic diseases such as Mpox are prevalent, is imperative. This includes bolstering hospital capacity, ensuring the availability of medical supplies, and training healthcare workers to recognize and treat Mpox. The setup of specialized Mpox treatment centers in high-risk areas, similar to COVID-19 response centers, could improve patient outcomes and reduce transmission (38).
Actionable recommendation
Invest in the expansion of healthcare infrastructure in vulnerable regions, including mobile clinics and telemedicine services, to ensure rapid response capabilities during Mpox outbreaks.
4. Public Communication
Lesson learned
During the COVID-19 pandemic, clear and consistent communication proved vital in guiding public behavior and ensuring compliance with health measures. Misinformation and mixed messaging, however, led to confusion and resistance in some areas (33,42).
Application to Mpox
A robust public communication strategy is essential for Mpox, particularly given its potential for rapid spread. Governments and health organizations should engage in transparent, fact-based communication about Mpox transmission, symptoms, and preventive measures. Lessons from COVID-19 highlight the importance of using multiple platforms, including social media, to reach diverse populations. Public health campaigns should also address potential stigmatization, especially considering Mpox’s transmission modes, to ensure community cooperation (43-46).
Actionable recommendation
Develop a global Mpox communication plan, including multilingual resources and partnerships with community leaders, to disseminate accurate information and counteract misinformation.
5. Vaccine Development and Distribution
Lesson Learned
The rapid development of COVID-19 vaccines was a monumental achievement, but the unequal distribution highlighted the challenges of global health equity. Ensuring that vaccines reached all populations, particularly in low-income countries, was a significant hurdle (39-42,44).
Application to Mpox
Leveraging advancements in vaccine technology, such as mRNA platforms, could accelerate the development of new Mpox vaccines or improve existing ones. Additionally, ensuring equitable distribution is critical. Lessons from COVAX and other initiatives should inform the creation of a global Mpox vaccine distribution network, ensuring that vaccines reach vulnerable populations promptly (46-48).
Actionable recommendation
Establish a global Mpox vaccine equity program with funding mechanisms to support vaccine access in low-resource settings. This program should prioritize high-risk areas and ensure that logistical challenges are addressed through international cooperation.
By applying these lessons from COVID-19 to Mpox, the global community can enhance its preparedness and response capabilities (33,34). These actionable strategies-early detection, global collaboration, healthcare infrastructure strengthening, effective public communication, and equitable vaccine distribution-are essential to preventing Mpox from becoming a widespread pandemic. Leveraging these insights will help build a more resilient global health system, better equipped to manage emerging infectious diseases such as Mpox (37).
Preventing a Potential Pandemic Outbreak
The upsurge of Mpox is a reminder that pandemics are not a question of “if” but “when”. To effectively prevent a potential pandemic outbreak, we must adopt a multifaceted strategy (Figure 5) that integrates lessons learned from COVID-19 and addresses the various dimensions of pandemic preparedness.
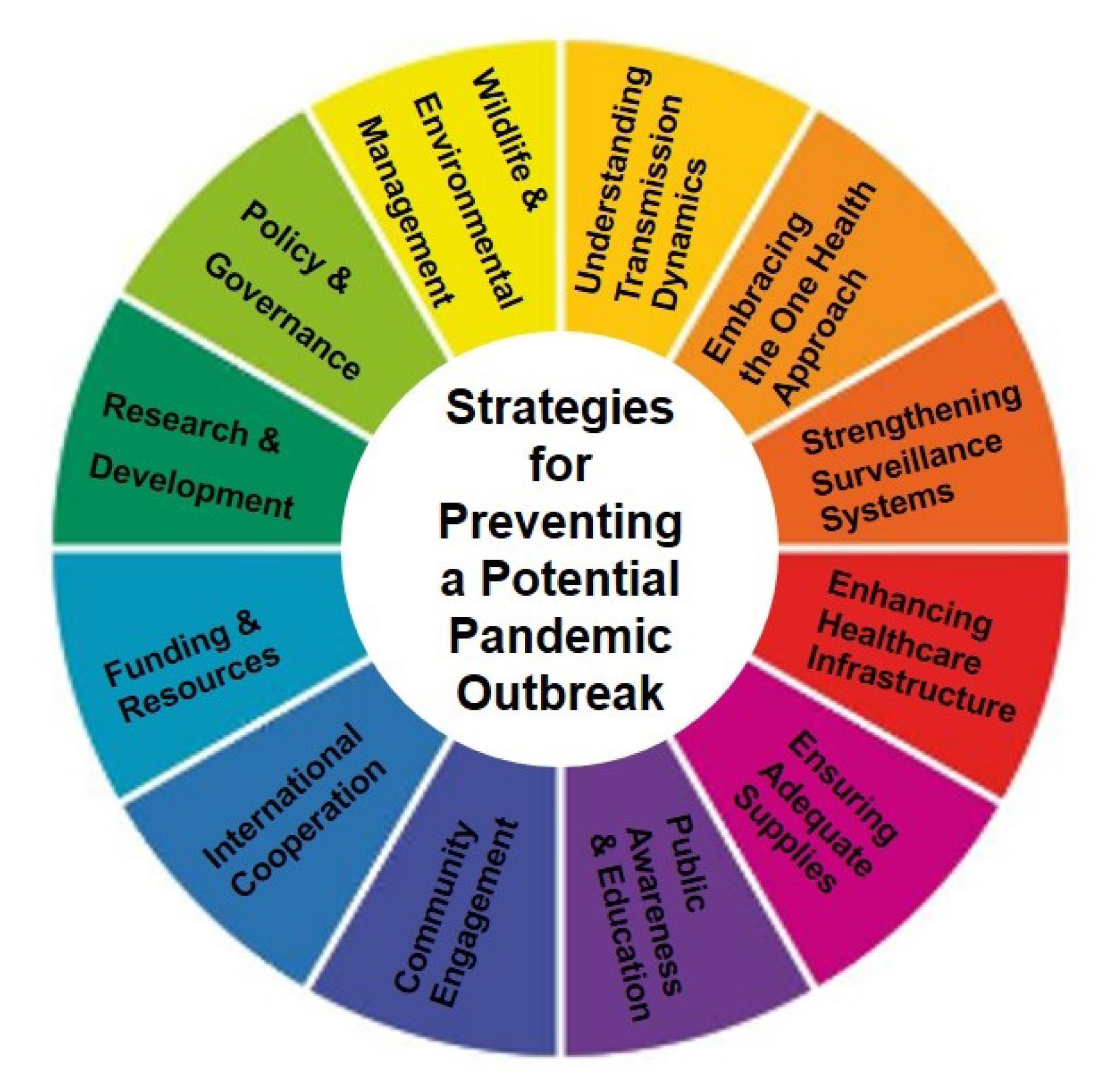
Figure 5.
Strategies for Preventing a Potential Pandemic
.
Strategies for Preventing a Potential Pandemic
1. Understanding Transmission Dynamics
Understanding how Mpox is transmitted within different populations is essential for preventing a widespread epidemic. This knowledge allows for targeted interventions, including vaccination campaigns tailored to specific high-risk groups, such as sex workers and men who have sex with men. The transmission dynamics of Mpox pose a potential risk for broader outbreaks, especially considering factors such as urbanization, deforestation, and international travel, which can facilitate the spread of zoonotic diseases (37,39).
A deeper analysis of specific risk factors influencing Mpox transmission is crucial. For example, behavioral patterns, geographic regions with high human-animal interaction, and socio-economic conditions that exacerbate exposure should be identified and addressed. Tailored interventions should consider these factors, such as localized vaccination drives, educational campaigns in high-risk areas, and targeted public health measures. During the COVID-19 pandemic, for instance, understanding the transmission dynamics in densely populated urban areas led to the development of targeted lockdowns and vaccination efforts that helped curb the spread of the virus (37). Similar approaches could be adapted for Mpox, focusing on regions or communities where the risk of transmission is highest (48,49).
2. Embracing the One Health Approach
The One Health approach recognizes the interconnectedness of human, animal, and environmental health. In the context of Mpox, adopting this approach is crucial for identifying, managing, and mitigating zoonotic threats before they escalate into global pandemics (45). Surveillance programs that monitor wildlife health and environmental changes have proven effective in the early detection of zoonotic diseases. For instance, programs that track disease outbreaks in wildlife populations can provide early warnings and allow for preemptive public health interventions. In Tanzania, for example, the monitoring of wildlife diseases has led to the early detection and containment of potential zoonotic outbreaks, preventing them from spreading to human populations (46). The One Health approach supports the strengthening of surveillance systems by providing a more comprehensive view of potential health threats. By integrating animal health data with human health monitoring, it is possible to improve our ability to predict and respond to outbreaks. Collaboration between veterinarians, ecologists, and public health officials is essential for effective implementation (45,46).
3. Strengthening Surveillance Systems
Robust surveillance systems are critical for the early detection and response to potential Mpox outbreaks. However, many regions currently lack adequate surveillance, leading to gaps in understanding the virus’s spread and impact. To address these gaps, investments in technology and training are necessary. For example, deploying AI-driven data analysis tools can help identify emerging patterns and predict outbreaks more accurately. Additionally, healthcare workers should receive continuous training in recognizing unusual disease patterns and effectively reporting them. In regions with limited resources, implementing low-cost surveillance solutions, such as community-based reporting systems and mobile health technologies, can enhance local capacity to detect and respond to outbreaks (49,50).
4. Enhancing Healthcare Infrastructure
Strengthening healthcare infrastructure is crucial to preventing Mpox from escalating into a pandemic. Improved infrastructure allows for better disease surveillance, early detection, rapid response, and effective containment of the virus. Specific infrastructure improvements, such as establishing more diagnostic laboratories, increasing the availability of isolation units, and ensuring the supply of essential medical equipment, are necessary. For example, during the COVID-19 pandemic, the rapid construction of temporary hospitals and the expansion of intensive care unit capacities in countries such as China and Italy were critical in managing the surge of cases (36,51). Similar strategies could be employed to prepare for potential Mpox outbreaks. Similarly, in response to the Ebola outbreak in West Africa, investments in healthcare infrastructure, such as the establishment of treatment centers and the training of local healthcare workers, played a crucial role in containing the virus. These lessons can inform efforts to enhance infrastructure in regions at risk of Mpox outbreaks (36,39).
5. Ensuring Adequate Supplies
Ensuring a sufficient stockpile of medical supplies, including vaccines, antiviral treatments, personal protective equipment, and diagnostic tools, is vital for a rapid and effective response to Mpox. Effective management of supply chains is essential to ensure that these supplies are available where and when they are necessary. Strategies such as establishing partnerships with manufacturers, implementing just-in-time supply models, and utilizing digital tracking systems can enhance the efficiency of supply distribution. The Strategic National Stockpile in the U.S., which maintains a reserve of critical medical supplies for emergencies, has been a model for ensuring readiness during health crises. Similar stockpiles should be established and maintained globally, with a focus on equitable distribution (37,42).
6. Public Awareness and Education
Raising public awareness about Mpox, its symptoms, and modes of transmission is crucial for preventing the spread of the virus (52). Public health campaigns that use clear, consistent messaging across multiple platforms have proven effective during past outbreaks. For example, during the Zika virus outbreak, targeted communication campaigns in Brazil and other affected countries played a key role in educating the public and reducing transmission. Messaging should be tailored to different populations, considering factors such as cultural beliefs, literacy levels, and access to information. This ensures that public health messages are understood and followed by all segments of the population (53).
7. Community Engagement
Active community engagement is essential for effective pandemic preparedness and response. Engaging communities fosters trust, compliance, and resilience, which are critical for the successful implementation of public health measures (54). Examples of successful community engagement include the Ebola response in West Africa. In Nigeria, for instance, local leaders and community members were actively involved in surveillance, education, and response efforts. This approach helped constrain the outbreak and build trust between communities and health authorities. Specific strategies for engaging communities could include establishing community health committees, conducting workshops to educate local leaders, and creating platforms for regular dialogue between health authorities and community members. These initiatives ensure that community voices are heard and that public health measures are culturally sensitive and widely accepted (39).
8. International Cooperation
The declaration of a PHEIC underscores the importance of international cooperation in preventing a pandemic. Countries must work together to share information, resources, and strategies to combat Mpox (55). Joint task forces, shared databases, and international agreements are essential mechanisms for fostering collaboration. For example, the Global Outbreak Alert and Response Network has successfully coordinated international responses to various outbreaks, including SARS and H1N1. Such mechanisms should be strengthened and expanded to ensure a coordinated global response to Mpox. The international collaboration during the COVID-19 pandemic, particularly through vaccine development and distribution efforts such as COVAX, demonstrates the importance of global solidarity in addressing health crises. These experiences can inform efforts to enhance cooperation for Mpox prevention (39).
9. Funding and Resources
Sustainable funding is indispensable for effective pandemic preparedness. Consistent and substantial investments are needed in healthcare infrastructure, emergency response systems, and research initiatives (56). Innovative funding mechanisms, such as the Pandemic Emergency Financing Facility created by the World Bank, provide financial resources to countries during health crises. These mechanisms should be expanded and tailored to address specific needs related to Mpox. Effective allocation of resources involves prioritizing areas with the highest risk of outbreaks and ensuring that funds are used efficiently. Transparent reporting and accountability measures are essential to maintaining the integrity of funding initiatives.
10. Research and Development
Ongoing investment in research and development is crucial for staying ahead of potential health crises (57). This includes advancing diagnostic tools, treatments, and vaccines for Mpox. Research initiatives such as the Coalition for Epidemic Preparedness Innovations have been instrumental in accelerating the development of vaccines for emerging infectious diseases. Similar efforts should be directed toward Mpox, focusing on developing effective vaccines and treatments that can be rapidly deployed in the event of an outbreak. Recent innovations in mRNA vaccine technology, which played a pivotal role in the COVID-19 response, could be adapted for Mpox. Collaborative efforts between governments, research institutions, and the private sector are essential for driving these innovations forward (50,57).
11. Policy and Governance
Robust policy and governance structures are necessary to coordinate pandemic preparedness efforts effectively (58). Clear policies and regulatory frameworks guide pandemic response activities and ensure that efforts are streamlined across sectors. The International Health Regulations, which provide a legal framework for preventing and responding to global health threats, are an example of effective governance in action. These regulations should be regularly reviewed and updated based on lessons learned from recent outbreaks, including Mpox. Establishing governance structures that facilitate cross-sector coordination and collaboration is crucial for a cohesive response to health emergencies. For example, interagency task forces that include representatives from health, finance, and education sectors can ensure that resources are allocated effectively and that response activities are well-coordinated (39,40).
12. Wildlife and Environmental Management
Effective wildlife and environmental management are key to preventing Mpox pandemics by addressing the root causes of zoonotic spillover (59). Implementing policies that limit human encroachment into wildlife habitats, such as regulating land use and controlling illegal wildlife trade, is essential for reducing the risk of zoonotic spillover. For example, efforts to curb deforestation in the Amazon have been linked to reduced outbreaks of diseases such as malaria and dengue, highlighting the importance of preserving natural habitats. Sustainable development initiatives that balance human needs with environmental conservation can also contribute to reducing the risk of zoonotic diseases. For instance, promoting sustainable agriculture practices that minimize habitat disruption can reduce the likelihood of human-wildlife interactions that lead to disease transmission (60).
Questions Seeking Answers
Amidst the global upsurge of Mpox, several critical questions remain unanswered, requiring ongoing research and exploration. Here are some of the most pressing questions:
-
What are the primary factors driving the resurgence of Mpox in non-endemic regions? Is it due to changes in the virus’s genetic structure, increased human-animal interaction, or other ecological shifts?
-
How is the Mpox virus evolving? What are the implications for its transmissibility and virulence?
-
What is the long-term efficacy of existing Mpox vaccines? How do they perform against different viral clades?
-
How safe are Mpox vaccines for different populations, including immunocompromised individuals, pregnant women, and children?
-
What are the most effective antiviral treatments for Mpox? How can they be optimized?
-
How can we improve supportive care for Mpox patients, particularly in severe cases?
-
Are there emerging drug-resistant strains of Mpox? How should they be managed?
Addressing these questions requires a multidisciplinary approach, involving virologists, epidemiologists, public health experts, clinicians, and policymakers. Global collaboration and sustained investment in research, vaccine development, and healthcare infrastructure are essential to managing the ongoing Mpox threat and preventing future outbreaks.
Future Research Direction
Primary Factors Driving the Resurgence of Mpox in Non-Endemic Regions
Comparative genomic studies of Mpox strains from endemic and non-endemic regions could reveal genetic mutations that may be responsible for increased transmissibility or virulence. Additionally, ecological studies investigating changes in human-animal interactions, such as increased contact with reservoir species or shifts in land use, could identify environmental factors contributing to the spread.Longitudinal studies monitoring genetic changes in Mpox strains over time, combined with ecological surveillance in high-risk areas, could be supplemented with spatial modeling to predict future outbreaks based on identified risk factors (51).
Evolution of the Mpox Virus and its Implications for Transmissibility and Virulence
In-depth phylogenetic analysis to track viral evolution, alongside studies of viral fitness and replication in various hosts, could help understand how the virus is adapting. Research could also explore the role of recombination and mutation rates in viral evolution. Laboratory-based studies using cell culture and animal models to assess changes in viral behavior are supported by bioinformatic approaches to analyze large-scale genomic data (6).
Long-term Efficacy of Existing Mpox Vaccines
Studies are needed to evaluate the durability of immune responses elicited by Mpox vaccines over time and across different viral clades. This could also include the development of next-generation vaccines that offer broader protection. Longitudinal cohort studies tracking vaccinated individuals over several years can be performed, with periodic assessments of antibody titers, T-cell responses, and vaccine efficacy against circulating strains. Additionally, vaccine challenge studies in animal models could provide insights into cross-clade protection (24).
Safety of Mpox Vaccines for Different Populations
Rigorous clinical trials and post-marketing surveillance should be conducted to assess vaccine safety in special populations, including immunocompromised individuals, pregnant women, and children. This research could also explore the immune correlates of protection in these groups. Phase IV clinical trials with large and diverse participant pools, coupled with observational studies and registries that monitor adverse events in real-world settings, can also be performed in this regard (57).
Most Effective Antiviral Treatments for Mpox
Antiviral drugs, either repurposed or novel, can be identified and optimized for treating Mpox. This includes determining the efficacy of existing antivirals against current strains and exploring combination therapies. In vitro drug screening against multiple Mpox strains can be conducted, followed by animal model testing and randomized controlled trials in human patients. Pharmacokinetic and pharmacodynamic studies would also be necessary to optimize dosing regimens (26,37).
Improving Supportive Care for Severe Mpox Cases
Standardized care protocols can be developed for severe Mpox, focusing on managing complications such as secondary infections, respiratory distress, and systemic inflammation. Research could also explore the role of adjunctive therapies, such as immunomodulators. Clinical trials and observational studies can be performed to evaluate the effectiveness of various supportive care strategies. This should include the creation of care registries to collect data on outcomes and refine treatment protocols (25).
Emerging Drug-Resistant Strains of Mpox
Surveillance for antiviral resistance markers in circulating Mpox strains can be started, with parallel studies to understand the mechanisms driving resistance. Research could also focus on developing second-line therapies to combat resistant strains. Genomic surveillance of viral isolates from treated patients should be initiated, coupled with in vitro resistance testing. Studies on resistance mechanisms at the molecular level, possibly supported by structural biology techniques, should be conducted to design inhibitors that can overcome resistance (22,25).
Each of these research areas is crucial for developing a comprehensive understanding of Mpox and ensuring effective public health responses. Collaboration across international borders and between academic, governmental, and private institutions will be essential to advance these research agendas.
Conclusion
The WHO’s designation of Mpox as a Public Health Emergency of International Concern underscores the urgent need to address the resurgence of this zoonotic disease. Lessons from COVID-19 emphasize the critical importance of strengthening surveillance, advancing vaccine deployment, and fostering global collaboration to mitigate future threats. Enhanced surveillance systems are vital for the early detection of emerging strains and a better understanding of transmission dynamics. Investments in genomic monitoring and data-sharing platforms will allow for real-time tracking of viral evolution. Equally essential is the expansion of healthcare infrastructure, particularly in endemic and resource-limited regions, to facilitate rapid outbreak response and equitable access to care. Vaccination strategies must prioritize long-term efficacy studies, safety evaluations in vulnerable populations, and streamlined deployment mechanisms to reduce disparities. Global vaccine stockpiles, supported by robust distribution networks, can ensure timely delivery during outbreaks. Addressing public concerns through transparent communication and community engagement is necessary to build trust and increase vaccine acceptance. Policy implications extend to the development of integrated global health frameworks that prioritize preparedness funding, equitable resource allocation, and interdisciplinary research. Future research should focus on optimized antiviral regimens, standardized treatment protocols for severe Mpox cases, and the containment of drug-resistant strains. Decisive action and sustained international collaboration are essential to prevent Mpox from becoming a global pandemic. By acting now, the global community can build a resilient health system capable of protecting populations from Mpox and future infectious disease threats.
Authors’ Contribution
Conceptualization: Seyi Samson Enitan.
Data curation: Seyi Samson Enitan, Richard Yomi Akele, Banenat Bajehson Dogonyaro, Seto Saint Tunrayo Aladenika, Grace Eleojo Itodo, Grace Amarachi John- Ugwuanya, Oluwanifemi Adefiyinfoluwa Ajayi, Ayomide Oluwatobiloba Okuneye, Temitayo Oluwatomisin Ajayi.
Formal analysis: Seyi Samson Enitan, Richard Yomi Akele & Michael Unata Iduh.
Investigation: Seyi Samson Enitan, Richard Yomi Akele, Adesuyi Ayodeji Omoare, Banenat Bajehson Dogonyaro, Michael Unata Iduh, Seto Saint Tunrayo Aladenika, Grace Eleojo Itodo.
Methodology: Seyi Samson Enitan, Richard Yomi Akele, Adesuyi Ayodeji Omoare, Michael Unata Iduh, Kester Awharentomah Digban, Seto Saint Tunrayo Aladenika, Grace Eleojo Itodo, Grace Amarachi John-Ugwuanya, Okeoghene Marcel Edafetanure-Ibeh, Ameh Raphael Adole, Agbolahan Adegboyega Adebanjo.
Project administration: Seyi Samson Enitan, Richard Yomi Akele
Resources: Seyi Samson Enitan, Okeoghene Marcel Edafetanure-Ibeh, Richard Yomi Akele.
Software: Seyi Samson Enitan, Banenat Bajehson Dogonyaro, Seto Saint Tunrayo Aladenika.
Supervision: Seyi Samson Enitan, Grace Eleojo Itodo.
Validation: Richard Yomi Akele, Michael Unata Iduh.
Visualization: Seyi Samson Enitan.
Writing–original draft: Seyi Samson Enitan, Richard Yomi Akele, Adesuyi Ayodeji Omoare, Banenat Bajehson Dogonyaro, Michael Unata Iduh, Seto Saint Tunrayo Aladenika.
Writing–review & editing: Seyi Samson Enitan, Kester Awharentomah Digban, Grace Eleojo Itodo, Grace Amarachi John-Ugwuanya, Okeoghene Marcel Edafetanure-Ibeh, Ameh Raphael Adole, Agbolahan Adegboyega Adebanjo, Oluwanifemi Adefiyinfoluwa Ajayi, Ayomide Oluwatobiloba Okuneye, Temitayo Oluwatomisin Ajayi.
Competing Interests
The authors declare no competing interests.
Data Availability Statement
Not applicable.
Ethical Approval
Not applicable.
Funding
None.
References
- Shanmugaraj B, Khorattanakulchai N, Phoolcharoen W. Emergence of monkeypox: another concern amidst COVID-19 crisis. Asian Pac J Trop Med 2022; 15(5):193-5. doi: 10.4103/1995-7645.346081 [Crossref] [ Google Scholar]
- World Health Organization (WHO). WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. WHO; 2024. Available from: https://www.who.int/news/item/14-08-2024-who-director-general-declares-Mpox-outbreak-a-public-health-emergency-of-international-concern.
- Kozlov M. Growing mpox outbreak prompts WHO to declare global health emergency. Nature 2024; 632(8026):718-9. doi: 10.1038/d41586-024-02607-y [Crossref] [ Google Scholar]
- Chaudhari S, Treffeisen L, Virk J, Parikh T, Gopalakrishnan Ravikumar NP, Goti AM. The 2022 monkeypox epidemic and what has led to the current state of the disease in the US: a systematic review. Cureus 2023; 15(1):e33515. doi: 10.7759/cureus.33515 [Crossref] [ Google Scholar]
- Sberna G, Rozera G, Minosse C, Bordi L, Mazzotta V, D’Abramo A. Role of direct sexual contact in human transmission of monkeypox virus, Italy. Emerg Infect Dis 2024; 30(9):1829-33. doi: 10.3201/eid3009.240075 [Crossref] [ Google Scholar]
- Krishna S, Kurrey C, Yadav M, Mahilkar S, Sonkar SC, Vishvakarma NK. Insights into the emergence and evolution of monkeypox virus: historical perspectives, epidemiology, genetic diversity, transmission, and preventative measures. Infect Med (Beijing) 2024; 3(2):100105. doi: 10.1016/j.imj.2024.100105 [Crossref] [ Google Scholar]
- Abdullah Abdullah, Ali S, Cançado F, de Oliveira CA. The emergence of monkeypox virus, new challenges to the healthcare settings in Pakistan. J Med Virol 2023; 95(1):e27899. doi: 10.1002/jmv.27899 [Crossref] [ Google Scholar]
- Ayorinde TA, Olufadewa II, Adesina MA, Oladele RI, Oladoye MJ, Adene T. The reemergence of the human monkeypox: strengthening Africa’s epidemic preparedness and response system. Ann Med Surg (Lond) 2023; 85(1):24-7. doi: 10.1097/ms9.0000000000000039 [Crossref] [ Google Scholar]
- León-Figueroa DA, Bonilla-Aldana DK, Pachar M, Romaní L, Saldaña-Cumpa HM, Anchay-Zuloeta C. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med Infect Dis 2022; 49:102362. doi: 10.1016/j.tmaid.2022.102362 [Crossref] [ Google Scholar]
- Begum JP, Ngangom L, Semwal P, Painuli S, Sharma R, Gupta A. Emergence of monkeypox: a worldwide public health crisis. Hum Cell 2023; 36(3):877-93. doi: 10.1007/s13577-023-00870-1 [Crossref] [ Google Scholar]
- Kumar S, Guruparan D, Karuppanan K. Recent advances in monkeypox (mpox): characterization, diagnosis, and therapeutics-a multidimensional review. Authorea [Preprint]. July 4, 2023. Available from: https://www.techrxiv.org/doi/full/10.22541/au.168846306.66701439.
- Fowotade A, Fasuyi TO, Bakare RA. Re-emergence of monkeypox in Nigeria: a cause for concern and public enlightenment. Afr J Clin Exp Microbiol 2018; 19(4):307-13. doi: 10.4314/ajcem.v19i4.9 [Crossref] [ Google Scholar]
- Precious ND, Agboola P, Oluwatimilehin O, Olakunle OK, Olaniyi P, Adiatu AI. Re-emergence of monkeypox virus outbreak in Nigeria: epidemic preparedness and response (review-commentary). Ann Med Surg (Lond) 2023; 85(8):3990-6. doi: 10.1097/ms9.0000000000001069 [Crossref] [ Google Scholar]
- Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl Trop Dis 2022; 16(2):e0010141. doi: 10.1371/journal.pntd.0010141 [Crossref] [ Google Scholar]
- Dabie K, Adulley F, Jonathan E, Ababio BA, Peprah-Yamoah E, Osman M. Recent status and knowledge on the re-emergence of monkeypox disease. Sci Afr 2023; 21:e01849. doi: 10.1016/j.sciaf.2023.e01849 [Crossref] [ Google Scholar]
- Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S. Emergence of monkeypox - west and central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep 2018; 67(10):306-10. doi: 10.15585/mmwr.mm6710a5 [Crossref] [ Google Scholar]
- Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther 2022; 7(1):373. doi: 10.1038/s41392-022-01215-4 [Crossref] [ Google Scholar]
- Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus 2022; 14(7):e26531. doi: 10.7759/cureus.26531 [Crossref] [ Google Scholar]
- Ejaz H, Junaid K, Younas S, Abdalla AE, Bukhari SN, Abosalif KO. Emergence and dissemination of monkeypox, an intimidating global public health problem. J Infect Public Health 2022; 15(10):1156-65. doi: 10.1016/j.jiph.2022.09.008 [Crossref] [ Google Scholar]
- Shafaati M, Zandi M. Human monkeypox (hMPXV) re-emergence: host immunity status and current vaccines landscape. J Med Virol 2023; 95(1):e28251. doi: 10.1002/jmv.28251 [Crossref] [ Google Scholar]
- Payne AB, Ray LC, Kugeler KJ, Fothergill A, White EB, Canning M. Incidence of monkeypox among unvaccinated persons compared with persons receiving ≥ 1 JYNNEOS vaccine dose - 32 US jurisdictions, July 31-September 3, 2022. MMWR Morb Mortal Wkly Rep 2022; 71(40):1278-82. doi: 10.15585/mmwr.mm7140e3 [Crossref] [ Google Scholar]
- Shamim MA, Satapathy P, Padhi BK, Veeramachaneni SD, Akhtar N, Pradhan A. Pharmacological treatment and vaccines in monkeypox virus: a narrative review and bibliometric analysis. Front Pharmacol 2023; 14:1149909. doi: 10.3389/fphar.2023.1149909 [Crossref] [ Google Scholar]
- Meo SA, Al-Masri AA, Klonoff DC, Alshahrani AN, Al-Khlaiwi T. Comparison of biological, pharmacological characteristics, indications, contraindications and adverse effects of JYNNEOS and ACAM2000 monkeypox vaccines. Vaccines (Basel) 2022; 10(11):1971. doi: 10.3390/vaccines10111971 [Crossref] [ Google Scholar]
- Sun WM. Monkeypox, smallpox, FDA, and accelerated approval of vaccines - a regulatory perspective. Vaccine 2023; 41(25):3681-2. doi: 10.1016/j.vaccine.2023.05.008 [Crossref] [ Google Scholar]
- Centers for Disease Control and Prevention (CDC). Mpox Treatment Information for Healthcare Professionals. 2024. Available from: https://www.cdc.gov/poxvirus/Mpox/clinicians/treatment.html. Accessed August 16, 2024.
- National Institute of Health (NIH). Mpox (Formerly Monkeypox) Treatment. 2024. Available from: https://www.niaid.nih.gov/diseases-conditions/Mpox-treatment. Accessed February 5, 2024.
- Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med 2022; 387(8):679-91. doi: 10.1056/NEJMoa2207323 [Crossref] [ Google Scholar]
- Jeyaraman M, Selvaraj P, Halesh MB, Jeyaraman N, Nallakumarasamy A, Gupta M. Monkeypox: an emerging global public health emergency. Life (Basel) 2022; 12(10):1590. doi: 10.3390/life12101590 [Crossref] [ Google Scholar]
- Mpox Update as Map Reveals Global Cases This Year. 2024. Available from: https://dnyuz.com/2024/08/20/Mpox-update-as-map-reveals-global-cases-this-year/. Accessed August 20, 2024.
- Hakim MS, Widyaningsih SA. The recent re-emergence of human monkeypox: would it become endemic beyond Africa?. J Infect Public Health 2023; 16(3):332-40. doi: 10.1016/j.jiph.2023.01.011 [Crossref] [ Google Scholar]
- Karama RS, Akinola A, Kama J. Re-emergence of human monkeypox 2022: its ecology and public health significance-short review article. Int J Community Med Public Health 2023; 10(4):1609-15. doi: 10.18203/2394-6040.ijcmph20230951 [Crossref] [ Google Scholar]
- Obermeier PE, Plinke CF, Brinkmann A, Lachmann R, Melchert J, Corman VM. Reemergence of Clade IIb-associated mpox, Germany, July-December 2023. Emerg Infect Dis 2024; 30(7):1416-9. doi: 10.3201/eid3007.240092 [Crossref] [ Google Scholar]
- Sachs JD, Karim SSA, Aknin L, Allen J, Brosbøl K, Colombo F. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet 2022; 400(10359):1224-80. doi: 10.1016/s0140-6736(22)01585-9 [Crossref] [ Google Scholar]
- Gostin LO. COVID-19 reveals urgent need to strengthen the World Health Organization. Jama 2020; 323(23):2361-2. doi: 10.1001/jama.2020.8486 [Crossref] [ Google Scholar]
- Lal A, Ashworth HC, Dada S, Hoemeke L, Tambo E. Optimizing pandemic preparedness and response through health information systems: lessons learned from Ebola to COVID-19. Disaster Med Public Health Prep 2022; 16(1):333-40. doi: 10.1017/dmp.2020.361 [Crossref] [ Google Scholar]
-
Karimi-Maleh H, Dragoi EN, Lichtfouse E. How the COVID-19 pandemic has changed research? Environ Chem Lett. 2022:1-4. doi: 10.1007/s10311-022-01536-4.
- Lu J, Xing H, Wang C, Tang M, Wu C, Ye F. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther 2023; 8(1):458. doi: 10.1038/s41392-023-01675-2 [Crossref] [ Google Scholar]
- World Health Organization (WHO). COVID-19 Strategic Preparedness and Response Plan: 2023 and Beyond [Internet]. Geneva: WHO; 2023. Available from: https://applications.emro.who.int/docs/WHOEMCSR513E-eng.pdf. Accessed November 23, 2024.
- Lu T, Wu Z, Jiang S, Lu L, Liu H. The current emergence of monkeypox: the recurrence of another smallpox?. Biosaf Health 2022; 4(6):369-75. doi: 10.1016/j.bsheal.2022.09.004 [Crossref] [ Google Scholar]
- Kofman A, Kantor R, Adashi EY. Potential COVID-19 endgame scenarios: eradication, elimination, cohabitation, or conflagration?. JAMA 2021; 326(4):303-4. doi: 10.1001/jama.2021.11042 [Crossref] [ Google Scholar]
- Yamey G, Garcia P, Hassan F, Mao W, McDade KK, Pai M. It is not too late to achieve global COVID-19 vaccine equity. BMJ 2022; 376:e070650. doi: 10.1136/bmj-2022-070650 [Crossref] [ Google Scholar]
- Sah R, Mohanty A, Hada V, Singh P, Govindaswamy A, Siddiq A. The emergence of monkeypox: a global health threat. Cureus 2022; 14(9):e29304. doi: 10.7759/cureus.29304 [Crossref] [ Google Scholar]
- Najjar M, Albuaini S, Fadel M, Mohsen F, Najjar G, Assaf D. COVID-19 vaccination reported side effects and hesitancy among the Syrian population: a cross-sectional study. Ann Med 2023; 55(2):2241351. doi: 10.1080/07853890.2023.2241351 [Crossref] [ Google Scholar]
- Subramanian SV, Kumar A. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur J Epidemiol 2021; 36(12):1237-40. doi: 10.1007/s10654-021-00808-7 [Crossref] [ Google Scholar]
- Ogunleye SC, Akinsulie OC, Aborode AT, Olorunshola MM, Gbore D, Oladoye M. The re-emergence and transmission of monkeypox virus in Nigeria: the role of one health. Front Public Health 2023; 11:1334238. doi: 10.3389/fpubh.2023.1334238 [Crossref] [ Google Scholar]
- Reynolds MG, Doty JB, McCollum AM, Olson VA, Nakazawa Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One Health. Expert Rev Anti Infect Ther 2019; 17(2):129-39. doi: 10.1080/14787210.2019.1567330 [Crossref] [ Google Scholar]
- Heymann DL, Shindo N. COVID-19: what is next for public health?. Lancet 2020; 395(10224):542-5. doi: 10.1016/s0140-6736(20)30374-3 [Crossref] [ Google Scholar]
- Bert F, Lo Moro G, Calabrese F, Barattero V, Peano A, Scaioli G. Assessment of Italian population awareness on One-Health, zoonoses and the mpox vaccine: a nationwide cross-sectional study. Vaccines (Basel) 2024; 12(3):258. doi: 10.3390/vaccines12030258 [Crossref] [ Google Scholar]
- Raman H, Jamil A, Rasheed A, Abdulrahman Jairoun A, Lua PL, Ibrahim UI. Knowledge of medical students towards the re-emergence of human monkeypox virus. Cureus 2023; 15(10):e46761. doi: 10.7759/cureus.46761 [Crossref] [ Google Scholar]
- Shafaati M, Zandi M. State-of-the-art on monkeypox virus: an emerging zoonotic disease. Infection 2022; 50(6):1425-30. doi: 10.1007/s15010-022-01935-3 [Crossref] [ Google Scholar]
- Rivers C, Watson C, Phelan AL. The resurgence of mpox in Africa. JAMA 2024; 332(13):1045-6. doi: 10.1001/jama.2024.17829 [Crossref] [ Google Scholar]
- Paparini S, Whelan I, Mwendera C, Hayes R, Maatouk I, Lewis R. Prevention of sexual transmission of mpox: a systematic review and qualitative evidence synthesis of approaches. Infect Dis (Lond) 2024; 56(8):589-605. doi: 10.1080/23744235.2024.2364801 [Crossref] [ Google Scholar]
- Gallo P, Galea N, Colucci A, Valli R, Schwarz M, Fanales Belasio E. Mpox: awareness, knowledge and information channels used by individuals accessing a sexually transmitted infections Helpline. Ann Ig 2024; 36(6):626-35. doi: 10.7416/ai.2024.2637 [Crossref] [ Google Scholar]
- Branda F, Ceccarelli G, Ciccozzi M, Scarpa F. Strengthening community resilience: lessons from COVID-19 for mpox prevention. Lancet 2024; 404(10456):929. doi: 10.1016/s0140-6736(24)01752-5 [Crossref] [ Google Scholar]
-
Ndembi N, Ngongo N, Foláyan MO, Yameogo JM, Braka F, Gueye SA, et al. Africa’s mpox strategic preparedness and response plan: a coordinated continental effort to boost health security. Lancet Glob Health. 2024. doi: 10.1016/s2214-109x(24)00464-9.
- Anderer S. WHO announces mpox global plan, appeals for funding. JAMA 2024; 332(15):1228. doi: 10.1001/jama.2024.19128 [Crossref] [ Google Scholar]
- Tuttle KR. Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol 2020; 16(10):562-4. doi: 10.1038/s41581-020-00336-9 [Crossref] [ Google Scholar]
- Abubakar I, Lutwama J, Kyobutungi C, Sankoh O. Mpox global emergency: strengthening African leadership. Lancet 2024; 404(10460):1286-8. doi: 10.1016/s0140-6736(24)02068-3 [Crossref] [ Google Scholar]
- Tran T, Xie S. Mitigating wildlife spillover in the clinical setting: how physicians and veterinarians can help prevent future disease outbreaks. AJPM Focus 2024; 3(2):100193. doi: 10.1016/j.focus.2024.100193 [Crossref] [ Google Scholar]
- Chen F, Jiang F, Ma J, Alghamdi MA, Zhu Y, Yong JW. Intersecting planetary health: Exploring the impacts of environmental stressors on wildlife and human health. Ecotoxicol Environ Saf 2024; 283:116848. doi: 10.1016/j.ecoenv.2024.116848 [Crossref] [ Google Scholar]