Avicenna Journal of Clinical Microbiology and Infection. 11(4):182-190.
doi: 10.34172/ajcmi.3563
Review Article
Advancing Toward a Hepatitis C-Free World: Emerging Therapies, Persistent Challenges, and Future Prospects
Seyi Samson Enitan 1, *  , Princess Hephzibah Soboyejo 1
, Princess Hephzibah Soboyejo 1  , Kester Awharentomah Digban 2
, Kester Awharentomah Digban 2  , Michael Unata Iduh 3
, Michael Unata Iduh 3  , Ifeoluwapo Oyebola Asekun-Olarinmoye 4
, Ifeoluwapo Oyebola Asekun-Olarinmoye 4  , Chidimma Anthonia Azike 5
, Chidimma Anthonia Azike 5  , Grace Eleojo Itodo 6
, Grace Eleojo Itodo 6  , Ameh Raphael Adole 7
, Ameh Raphael Adole 7  , Ayomide Oluwatobiloba Okuneye 1
, Ayomide Oluwatobiloba Okuneye 1 
Author information:
1Department of Medical Laboratory Science, School of Public and Allied Health, Babcock University, Ilishan-Remo 121109, Ogun State, Nigeria
2Department of Medical Laboratory Science, College of Medical & Health Sciences, Novena University, Ogume, Kwale 322107, Delta State, Nigeria
3Department of Medical Microbiology, School of Medical Laboratory Science, Usmanu Danfodiyo University, Sokoto, Sokoto State Nigeria
4Faculty of Basic Medical Sciences, University of Ilesa, Ilesa, Osun State, Nigeria
5Department of Medical Laboratory Science, Faculty of Medical Laboratory Science, Rivers State University, Eagle Island, Port Harcourt 500101, Rivers State, Nigeria
6Department of Microbiology, Federal Teaching Hospital, Lokoja, Kogi State, Nigeria
7Department of Infectious disease, Clinton Health Access Initiative, FCT-Abuja, Nigeria
Abstract
Recent advancements in the treatment of hepatitis C virus (HCV) infection have significantly advanced the goal of achieving a hepatitis C-free world. The introduction of direct-acting antivirals (DAAs) has revolutionized treatment, offering over 95% cure rates, shorter treatment durations, and fewer side effects. Pan-genotypic regimens, such as sofosbuvir/velpatasvir, have further simplified treatment by being effective across all HCV genotypes. Despite these advancements, substantial challenges persist globally. An estimated 50 million people are living with chronic HCV worldwide, yet many remain undiagnosed, particularly in low- and middle-income countries, where there is limited healthcare infrastructure. High treatment costs further restrict access to these life-saving therapies. Reinfection rates remain high among certain populations, such as people who inject drugs (PWIDs), and stigma continues to deter individuals from seeking testing and treatment. To advance HCV elimination, future efforts must prioritize universal screening, affordable treatment, improved diagnostic technologies, and intensified research into vaccine development. Strengthened global and local collaboration is essential to overcome these challenges and reduce the global HCV burden.
Keywords: Hepatitis C, Therapies, Direct-acting antivirals, Pan-genotypic regimens, Treatment access, HCV vaccine development
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Enitan SS, Soboyejo PH, Digban KA, Iduh MU, Asekun-Olarinmoye IO, Azike CA, et al. Advancing toward a hepatitis C-free world: emerging therapies, persistent challenges, and future prospects. Avicenna J Clin Microbiol Infect. 2024; 11(4):182-190. doi:10.34172/ajcmi.3563
Introduction
Hepatitis C virus (HCV) infection remains a significant global health challenge (1). This bloodborne RNA virus, belonging to the Flaviviridae family, primarily targets the liver and is characterized by its genetic diversity, with multiple genotypes and subtypes identified (2). The persistent nature of HCV infection, coupled with its ability to remain asymptomatic in its early stages, contributes to its status as a major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma (3).
According to the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC), about 0.7% of the world’s population (50 million) are infected with HCV, with 1 million new cases and 240 000 deaths reported annually. Only 36.4% of people living with chronic HCV are diagnosed, and merely about 20% have access to treatment (4,5). The epidemiology of HCV is marked by significant geographical and demographic variations. Recent data indicate that the highest prevalence rates are observed in Eastern Europe, Central Asia, and parts of Africa and the Middle East (6). The global distribution of HCV genotypes further complicates the epidemiological landscape, with genotype 1 being the most prevalent globally, while genotypes 3 and 4 dominate in specific regions (7).
The transmission of HCV primarily occurs through exposure to infected blood, with injection drug use representing the most significant risk factor in many countries (8). Other modes of transmission include unsafe medical procedures, particularly in resource-limited settings, and, to a lesser extent, sexual contact and vertical transmission from the mother to the child (9). The relative contribution of these transmission routes varies considerably across different populations and geographical regions, necessitating tailored prevention strategies.
Imagine a world free from the silent threat of hepatitis C, a vision that seemed impossible just a decade ago. Today, we stand on the cusp of transforming this dream into reality. The pursuit of a hepatitis C-free world is one of resilience, scientific triumph, and human determination. The advent of direct-acting antivirals (DAAs) has revolutionized the treatment landscape for HCV, offering cure rates exceeding 95% with shorter treatment durations and improved tolerability compared to interferon-based regimens. This therapeutic breakthrough has transformed the prognosis for individuals with chronic HCV infection and has been pivotal in shaping global elimination strategies. However, the high cost of DAAs and limited access in many low- and middle-income countries continue to pose significant barriers to universal treatment (10).
In response to these challenges, the WHO has set ambitious targets to eliminate HCV as a public health threat by 2030, aiming for a 90% reduction in new infections and a 65% reduction in mortality (11). Achieving these goals requires a multifaceted approach encompassing enhanced surveillance, expanded screening programs, and improved access to treatment. Several countries have implemented comprehensive national strategies, with notable success stories emerging from Australia, Egypt, and Georgia (12).
Despite these advancements, significant obstacles persist in the global effort to combat HCV. Key challenges include the lack of a prophylactic vaccine, the emergence of antiviral resistance, and the disproportionate burden of infection among marginalized populations. Moreover, the coronavirus disease 19 pandemic has disrupted HCV elimination efforts in many regions, highlighting the vulnerability of public health initiatives to external shocks (13).
Recent research has focused on developing pan-genotypic DAA regimens, exploring shorter treatment durations, and investigating novel approaches to reach underserved populations. Additionally, advancements in point-of-care diagnostics and non-invasive assessment of liver fibrosis are enhancing the capacity for decentralized care models, particularly crucial in resource-limited settings (14).
The economic impact of HCV infection and the cost-effectiveness of treatment strategies have garnered increased attention. Studies demonstrate that while the initial investment in DAAs is substantial, the long-term benefits in reducing liver-related morbidity and mortality result in overall cost savings to healthcare systems (15). This economic rationale has been instrumental in advocating for expanded access to treatment at the policy level.
As the field progresses toward HCV elimination, several key areas require further investigation. They include optimizing screening strategies to identify the millions of individuals unaware of their HCV status, developing effective models of care for high-risk populations, and addressing the growing burden of HCV-related liver cancer in the context of improved antiviral therapy (16).
This narrative review aims to provide a comprehensive overview of the current state of HCV research, examining emerging therapies, persistent challenges, and prospects in the global effort to eliminate hepatitis C. By synthesizing recent epidemiological data, treatment advancements, and public health strategies, this study seeks to elucidate the complex landscape of HCV management and inform future directions in research and policy.
Global Burden of Hepatitis C
According to the WHO’s latest report (11), an estimated 50 million individuals worldwide live with chronic hepatitis C infection, with approximately 1 million new infections occurring annually. Hepatitis C-related mortality reached about 240 000 deaths in 2022, primarily due to cirrhosis and liver cancer complications (Figure 1 is a global map showing the prevalent cases of chronic hepatitis C by the WHO region in 2022). Despite highly effective DAA treatments that can cure over 95% of cases, access to diagnosis and treatment remains challenging (4,11).
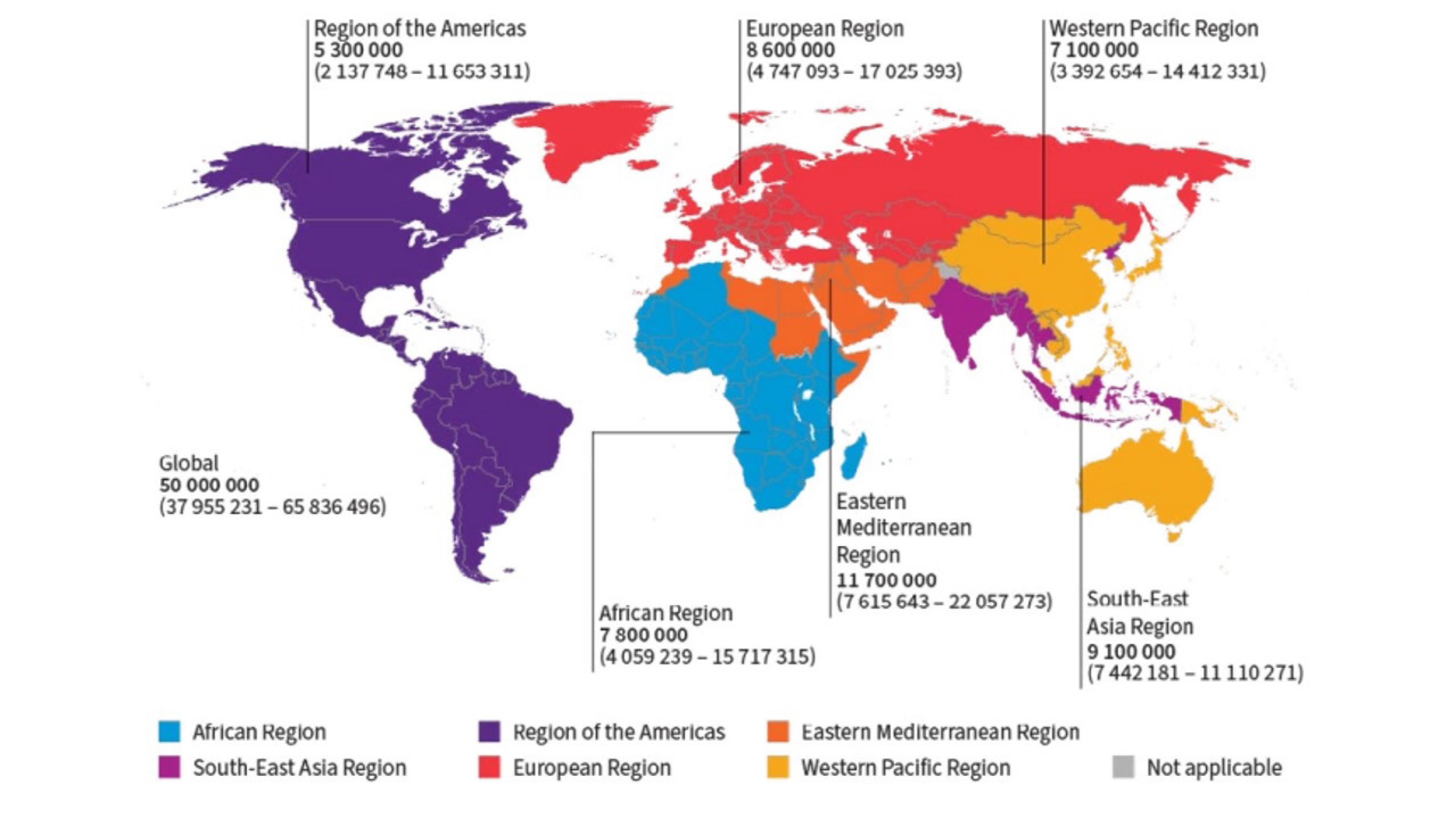
Figure 1.
Prevalent Cases of Chronic Hepatitis C by the World Health Organization Region (2022). Source: The World Health Organization (11)
.
Prevalent Cases of Chronic Hepatitis C by the World Health Organization Region (2022). Source: The World Health Organization (11)
Only 36.4% of infected individuals were aware of their diagnosis, and just 20% of those diagnosed had received treatment by the end of 2022. The chronicity rate of HCV infection is notably high, with a significant proportion of acute infections progressing to chronic status. Transmission dynamics vary across populations, with the primary route being blood-to-blood contact. They include unsafe injection practices, inadequate sterilization of medical equipment, and transfusion of unscreened blood products (11,17-19).
Furthermore, according to the CDC’s 2022 Viral Hepatitis Surveillance Report, HCV remains a significant public health concern in the United States (5,18-20). In 2022, 5,023 acute HCV cases were reported, translating to an estimated 66 900 actual new infections when accounting for under-reporting. Additionally, 104 729 chronic HCV cases were reported. The median age for acute HCV cases was 38 years, with 63.9% occurring among individuals aged 20-39 years and 62.2% in males. Injection drug use was reported as a risk factor in 63.7% of acute cases. Mortality data from 2021 indicated 14 847 deaths with hepatitis C as an underlying or contributing cause. Alarmingly, reported acute HCV cases increased by 41.5% from 2018 to 2022, with the highest rates observed in the West and Appalachian regions. These statistics underscore the urgent need for expanded testing and treatment coverage, particularly in high-prevalence settings and among high-risk populations (18-20).
Latest Therapies
Over the past decade, the treatment landscape for hepatitis C has undergone a revolutionary transformation primarily due to the advent of DAAs (21,22). This shift has been characterized by significantly higher cure rates, commonly referred to as sustained virologic response (SVR), alongside shorter treatment durations and reduced side effects compared to older therapies such as interferon-based treatments. In pivotal studies, DAAs have demonstrated cure rates exceeding 95%, with treatment courses often reduced to 8-12 weeks (23,24). The revolutionary transformation brought about by DAAs is largely due to their mechanism of action. Understanding the mechanisms of action and potential limitations of these therapies is crucial.
Direct-Acting Antivirals
DAAs have revolutionized the treatment of hepatitis C by directly targeting and inhibiting essential viral proteins, thereby halting the replication of the virus (25,26). DAAs are categorized into three main classes, namely, NS3/4A protease inhibitors, NS5A inhibitors, and NS5B polymerase inhibitors, each targeting different stages of the HCV life cycle (27).
-
NS3/4A protease inhibitors: These drugs inhibit the NS3/4A serine protease, an enzyme essential for viral replication. By blocking this enzyme, NS3/4A protease inhibitors, such as glecaprevir, prevent the virus from processing its polyprotein into functional units, thereby halting replication (28,29).
-
NS5A inhibitors: NS5A inhibitors, such as ledipasvir and velpatasvir, target the NS5A protein, which plays a key role in viral replication and assembly. These inhibitors disrupt the replication complex and the assembly of new virions, making them a critical component of DAA regimens (29,30).
-
NS5B polymerase inhibitors: The NS5B polymerase is an RNA-dependent RNA polymerase responsible for copying the viral RNA genome. NS5B inhibitors, such as sofosbuvir, come in two types; that is, nucleoside analogs that mimic the natural substrates of the polymerase and non-nucleoside inhibitors that bind to the enzyme and inhibit its activity. Both types effectively stop viral replication (30,31).
The characteristics of DAAs are presented in Table 1. These drugs have significantly shortened treatment durations to 8-12 weeks. DAAs offer fewer side effects compared to older therapies, making them a cornerstone of modern HCV treatment. Combinations of these drugs, such as sofosbuvir/ledipasvir and glecaprevir/pibrentasvir, have demonstrated cure rates exceeding 95% across various genotypes, both in clinical trials and real-world settings. For instance, studies have shown that sofosbuvir-based regimens achieve SVR rates of 95-99%, even in patients with advanced liver disease (32).
Table 1.
Direct-Acting Antivirals and Their Characteristics
|
DAA Class
|
Example Drugs
|
Targeted Viral Protein
|
Mechanism of action
|
Cure Rate
|
Typical Treatment Duration
|
| NS3/4A protease inhibitors |
Glecaprevir, voxilaprevir |
NS3/4A protease |
Inhibits viral replication by blocking the cleavage of the HCV polyprotein. |
> 95% |
8-12 weeks |
| NS5A inhibitors |
Ledipasvir, velpatasvir |
NS5A protein |
Disrupts viral replication and assembly of new virions. |
> 95% |
8-12 weeks |
| NS5B polymerase inhibitors |
Sofosbuvir, dasabuvir |
NS5B RNA polymerase |
Inhibits viral RNA replication and can be nucleoside or non-nucleoside. |
> 95% |
8-12 weeks |
Note. Direct-acting antivirals; HCV: Hepatitis C virus.
Source. (33,34).
Canonical Protease Inhibitors
Canonical protease inhibitors target the NS3/4A protease, which is a crucial enzyme in the HCV lifecycle and is responsible for cleaving the viral polyprotein into components essential for replication. By inhibiting this enzyme, these drugs disrupt the replication process. First-generation protease inhibitors, such as boceprevir and telaprevir, were significant advancements in HCV treatment, and offered improved outcomes compared to interferon-based therapies. However, their use was constrained by notable side effects, complex dosing regimens, and limited efficacy across HCV genotypes. DAAs have since replaced canonical protease inhibitors, offering superior efficacy, broader genotype coverage, shorter treatment durations, and enhanced safety. While canonical protease inhibitors have been phased out, they laid the groundwork for the development of targeted HCV therapies and hence remain a milestone in antiviral treatment. Unlike canonical protease inhibitors, which act exclusively on the NS3/4A protease, DAAs target multiple viral proteins, including NS5A and NS5B. This multi-target approach increases the antiviral potency and reduces the likelihood of resistance, thereby making DAAs a more effective solution for HCV treatment (35).
Additionally, DAAs offer a more favorable safety profile compared to older treatments, which often involved significant side effects such as flu-like symptoms and depression. The improved tolerability of DAAs has made them accessible to a broader patient population, including those with comorbid conditions (36).
Pan-Genotypic Regimens
Pan-genotypic DAAs are effective against all HCV genotypes, simplifying treatment protocols and eliminating the need for genotype testing. These regimens, such as sofosbuvir/velpatasvir and glecaprevir/pibrentasvir, have been pivotal in expanding access to treatment, especially in resource-limited settings where genotype testing may not be readily available (37). The efficacy of these regimens has been demonstrated in a broad range of patients, including those with cirrhosis and co-infections like HIV. For example, the ASTRAL-1 trial showed that sofosbuvir/velpatasvir achieved a 99% SVR rate across all HCV genotypes, underscoring the potential for these regimens to be universally applied (38).
Shortened Treatment Durations
Advances in DAA therapy have reduced treatment durations from 24-48 weeks with older therapies to as short as 8-12 weeks with DAAs. This reduction not only improves patient adherence but also lowers the overall burden of treatment. Shorter regimens have been shown to be highly effective, with studies reporting similar SVR rates compared to longer courses, even in treatment-experienced patients (39).
Limitations and Side Effects
Despite the remarkable success of DAAs, they are not without limitations. Potential side effects can include fatigue, headache, and nausea, though these are generally mild ones compared to the side effects of older interferon-based therapies. In some cases, patients with advanced liver disease or co-infections may experience more severe side effects or reduced efficacy (40).
Furthermore, while DAAs have shown high cure rates, there is a small risk of viral resistance, particularly in patients who do not achieve SVR. This has led to ongoing research into optimizing treatment regimens and developing new DAAs to overcome resistance. While DAAs represent a significant advancement in HCV treatment, ongoing monitoring for side effects and resistance, particularly in vulnerable populations, remains essential. Continued research and clinical trials will be crucial in refining these therapies and ensuring their efficacy across diverse patient groups (41).
Current Challenges
Despite the remarkable progress in hepatitis C treatment, several challenges hinder the path to a hepatitis C-free world.
Diagnosis and Screening
A significant proportion of individuals with hepatitis C yet remain undiagnosed, which presents a major barrier to eradicating the disease. The WHO estimates that nearly 80% of the global population infected with HCV remains unaware of their status. In regions such as Sub-Saharan Africa and Southeast Asia, where healthcare infrastructure is limited, this figure can be even higher. Increasing awareness and implementing widespread screening programs, particularly in high-risk populations such as people who inject drugs (PWIDs) and individuals in correctional facilities, are highly required for identifying and treating infected individuals. For example, Egypt’s national screening program, which has been tested over 60 million people, has been a model of how large-scale screening can drastically reduce the undiagnosed burden (42,43).
Access to Treatment
While DAAs are highly effective, their high cost remains a significant barrier to accessibility, particularly in low- and middle-income countries (44). Sub-Saharan Africa faces significant barriers to treatment, including high costs of DAAs and limited healthcare infrastructure. These factors contribute to low treatment rates despite high HCV prevalence. South Asia has witnessed improvements in access through the availability of generics, but high drug prices and limited healthcare resources still restrict widespread treatment. In some regions, such as Central Asia and parts of Africa, the cost of a full course of DAA treatment can exceed the average annual income, making it unattainable for many. Efforts to negotiate lower prices, such as those led by organizations such as Médecins Sans Frontières and the Coalition for Global Hepatitis Elimination, have been crucial in promoting generic formulations and enhancing healthcare infrastructure (45). Countries such as India have successfully negotiated lower prices for DAAs, leading to broader access and serving as a model for other nations grappling with similar challenges. Eastern Europe and Central Asia experience challenges with both high prevalence and limited access to DAAs. Efforts are being made to improve accessibility, but progress is uneven. Western Europe and North America have more robust healthcare systems and broader access to hepatitis C treatment, thanks to national screening programs and public health policies (46). These regions benefit from higher treatment rates and more effective management of the disease. The global prevalence and access to hepatitis C treatment by region is provided in Table 2. In addition, challenges and proposed strategies for overcoming hepatitis C treatment barriers are presented in Table 3.
Table 2.
Global Prevalence and Access to Hepatitis C Treatment by Region
|
Region
|
Estimated HCV Prevalence (%)
|
Undiagnosed Cases (%)
|
Access to DAAs (%)
|
Main Barriers to Access
|
| Sub-Saharan Africa |
3.5 |
85 |
< 5 |
High cost and limited healthcare infrastructure |
| Eastern Europe and Central Asia |
3 |
80 |
10-20 |
Stigma and inadequate harm reduction services |
| Middle East and North Africa |
2.9 |
75 |
20-30 |
Political instability and high drug prices |
| Western Europe and North America |
0.5 |
20 |
> 50 |
Cost and insurance coverage |
| East Asia and Pacific |
1.7 |
60 |
30-40 |
High drug costs and limited public health campaigns |
Note. Direct-acting antivirals; HCV: Hepatitis C virus.
Source. (47,48).
Table 3.
Challenges and Strategies for Overcoming Hepatitis C Treatment Barriers
|
Challenge
|
Description
|
Proposed Strategy
|
Example/Case Study
|
| High cost of DAAs |
The high cost of DAAs limits access in low- and middle-income countries. |
Promoting the use of generic formulations and negotiating lower prices through international organizations. |
India’s successful negotiation for lower DAA prices, increasing accessibility. |
| Reinfection among high-risk groups |
Reinfection rates are high among PWIDs and other high-risk groups, undermining treatment efforts. |
Implementing comprehensive harm reduction strategies, including needle exchange and opioid substitution therapy. |
Australia’s national harm reduction program significantly reduced reinfection rates. |
| Stigma and discrimination |
The stigma associated with hepatitis C, particularly among marginalized groups, deters individuals from seeking testing and treatment. |
Conducting public health campaigns to reduce stigma and promote understanding of hepatitis C. |
“Know More Hepatitis” campaign in the U.S., which increased testing and treatment uptake among affected populations. |
| Limited healthcare infrastructure |
In many regions, healthcare infrastructure is inadequate to support widespread screening, diagnosis, and treatment. |
Investing in healthcare infrastructure and training, particularly in low-resource settings. |
Egypt’s national screening program drastically reduced the undiagnosed HCV burden. |
Note. DAA, Direct-acting antiviral; HCV, Hepatitis C virus; PWIDs, people who inject drugs.
Source. (49-51).
Reinfection
Reinfection after successful treatment remains a significant concern, particularly among high-risk groups such as PWIDs. Studies have shown that reinfection rates in PWIDs can range from 2% to 10% per year, depending on the region and the availability of harm reduction services. Comprehensive harm reduction strategies, including needle exchange programs and opioid substitution therapy, are vital to preventing reinfection. In countries like Australia and Canada, where harm reduction programs are well-established, reinfection rates have been significantly reduced, demonstrating the effectiveness of these approaches. However, in regions where harm reduction services are scarce or stigmatized, reinfection continues to undermine treatment efforts (51).
Stigma and Discrimination
Stigma and discrimination associated with hepatitis C can deter individuals from seeking testing and treatment, perpetuating the cycle of infection. This is particularly problematic in regions where hepatitis C is associated with marginalized populations, such as PWIDs or men who have sex with men. In Eastern Europe, for example, hepatitis C-related stigma is compounded by the criminalization of drug use, leading to widespread discrimination and reluctance to access healthcare. Public health campaigns aiming at reducing stigma and promoting understanding of hepatitis C are necessary to encourage affected individuals to seek care. Successful initiatives, such as the “Know More Hepatitis” campaign in the United States, have shown that targeted education can reduce stigma and increase testing and treatment uptake (52).
Therapeutic Failures and the Potential Emergence of Antiviral Resistance
While current antiviral therapies for hepatitis C are highly effective, achieving cure rates exceeding 95% with DAAs, there remain instances of therapeutic failure and the potential for antiviral resistance. Therapeutic failure is relatively uncommon and typically associated with specific patient factors, including advanced liver disease, high baseline viral load, or prior treatment experience. In these cases, viral persistence despite DAA treatment can result from limited immune control and suboptimal response to antiviral drugs. Patients with cirrhosis or those who have previously undergone treatment with DAAs are at a slightly higher risk of experiencing relapse or non-response, necessitating careful monitoring and possibly retreatment with alternative regimens (11,12).
The emergence of antiviral resistance, although rare, is a critical consideration in hepatitis C treatment. Resistance-associated substitutions can develop when the virus mutates, allowing it to evade the action of DAAs. These RASs are more commonly observed in specific HCV genotypes, such as genotype 1a, and can reduce the effectiveness of certain classes of DAAs, particularly NS5A inhibitors. Resistance may emerge if patients do not adhere to their prescribed treatment or if incomplete viral suppression allows for the selection of resistant variants. While newer, pan-genotypic DAAs have improved efficacy against RASs, the risk of resistance remains, especially in patients with prior DAA exposure (17).
To manage these challenges, current guidelines recommend resistance testing for patients who have experienced previous DAA failure or are at risk of resistance-related relapse. This testing helps in selecting an appropriate second-line regimen to overcome resistance barriers. By tailoring treatment approaches based on individual resistance profiles and clinical factors, healthcare providers can further optimize outcomes and sustain the efficacy of antiviral therapies. Continued research and vigilant clinical practices are essential to prevent the spread of resistant HCV strains and to maintain the high success rates of DAA therapies in diverse patient populations (18).
Lack of Effective Hepatitis C Vaccine Candidate
The development of an effective HCV has encountered multiple scientific and clinical challenges, particularly regarding the prevention of chronic infection in adults. One of the most critical obstacles is HCV’s remarkable genetic diversity. With seven major genotypes and numerous subtypes, the virus mutates rapidly, making it challenging to create a single vaccine that elicits a broad and durable immune response across all HCV variants. This genetic variability enables the virus to escape immune detection, allowing it to establish persistent infection even when the host’s immune system initially responds to vaccination (52,53).
Moreover, unlike some other viral infections, natural immunity to HCV is often incomplete, as individuals can be re-infected with different HCV genotypes after clearing an initial infection. This indicates that the immune response to HCV, whether acquired through natural exposure or vaccine-induced, may not provide full protection. Vaccine candidates designed to elicit T-cell responses—targeting viral proteins such as NS3, NS5A, and NS5B—have shown some promise in generating cellular immunity, which is crucial for controlling HCV infection. However, these responses have not consistently prevented the establishment of chronic infection in clinical trials, especially in high-risk groups (54,55).
Further complicating the development process are the immune evasion strategies employed by HCV, including the suppression of interferon signaling and modulation of host immune pathways, which reduce the effectiveness of both natural and vaccine-induced immune responses. While recent studies have explored novel vaccine platforms, such as viral vector-based vaccines and peptide vaccines, the inability to consistently prevent chronic infection in adults remains a significant limitation. Addressing these challenges will require continued research into the mechanisms of HCV immunity, improved vaccine designs capable of inducing robust, cross-genotype protection, and strategies that can overcome the virus’s immune-evasion tactics (53,55).
Future Prospects of Hepatitis C Vaccine Eradication
The future of HCV eradication is promising, with several avenues of research and policy initiatives offering hope. The following pillars are cardinal to HCV eradication (Figure 2):
Figure 2.
Four Pillars of Hepatitis C Eradication
.
Four Pillars of Hepatitis C Eradication
Universal Screening and Treatment
Achieving universal screening and treatment is a critical step toward eradicating HCV. Integrating HCV screening into routine healthcare services, particularly in primary care settings, can help identify infections early, allowing for timely intervention. Countries such as Australia and Iceland have made significant strides in this area by implementing nationwide screening programs coupled with free access to DAAs, resulting in substantial reductions in HCV prevalence. For universal screening and treatment to become a global reality, robust policy frameworks and sustained funding will be necessary to overcome barriers to healthcare access, particularly in low- and middle-income countries (50,51).
Improved Diagnostics
Advances in diagnostic technologies are pivotal to early detection and effective management of HCV. Recent developments in point-of-care testing, such as the Xpert hepatitis C Viral Load Fingerstick test, offer rapid and accurate results with minimal invasiveness, making them ideal for use in resource-limited settings. Additionally, non-invasive liver fibrosis assessments, such as transient elastography (FibroScan), are replacing liver biopsies, enabling safer and more accessible monitoring of liver health in HCV patients. These innovations not only improve patient outcomes but also streamline the diagnostic process, making it more feasible to implement widespread screening programs (51).
Vaccination
Developing an effective vaccine for HCV remains one of the most formidable challenges in the fight against the virus. The genetic variability of HCV, with its multiple genotypes and rapid mutation rate, complicates vaccine development (52). Moreover, the virus’s ability to evade the immune system presents significant hurdles in eliciting a robust and durable immune response. Despite these challenges, ongoing research is making progress. Experimental vaccines, such as those using viral vectors or nanoparticle-based approaches, are currently in clinical trials. While a successful vaccine is still on the horizon, its development would represent a monumental step toward preventing new infections and ultimately achieving global eradication (53-56).
Global and Local Collaboration
Effective global and local collaboration is crucial for addressing the HCV epidemic and scaling up elimination efforts. Partnerships among governments, global organizations, non-governmental organizations (NGOs), and the private sector are essential to mobilize resources, share expertise, and develop the necessary infrastructure for implementing hepatitis C interventions. The WHO, through its Global Health Sector Strategy on Viral Hepatitis 2016-2021, has set targets to reduce new HCV infections by 90% and related mortality by 65% by 2030. Achieving these goals requires coordinated action, robust political commitment, and active involvement from both global and local organizations (54,55).
Global organizations such as the WHO and the Coalition for Global Hepatitis Elimination support countries by establishing standards, providing technical guidance, and aiding in the development of national strategies. NGOs such as Médecins Sans Frontières and the Clinton Health Access Initiative also play vital roles, facilitating access to treatment, expanding testing, and building healthcare capacity, particularly in low-resource settings. At national levels, partnerships between governments, healthcare providers, and local NGOs ensure interventions meet specific population needs. Successful national programs in countries such as Egypt, France, Canada, and the US serve as models for other nations, demonstrating the impact of coordinated public health efforts on reducing HCV prevalence (42,57).
Conclusion
The journey toward a hepatitis C-free world is within reach, thanks to significant advancements in therapy and the potential for future innovations. However, achieving this goal requires a unified and strategic approach from all stakeholders. Governments must commit to integrating HCV screening into national healthcare systems and ensuring that treatment is accessible to all, especially in low- and middle-income countries. Healthcare providers play a crucial role in implementing these strategies and reducing barriers to care. International organizations and non-governmental entities must continue to drive global collaboration, focusing on research, resource allocation, and policy implementation. By prioritizing these actions and fostering strong partnerships across borders, it is possible to reduce the global burden of hepatitis C and move decisively toward its elimination as a public health threat. The time to act is now, and with collective effort, a hepatitis C-free world can become a reality.
Key Implication
This review underscores the necessity of local and global collaboration and policy innovation to enhance universal access to hepatitis C treatment. Addressing barriers such as high drug costs, inadequate healthcare infrastructure, and persistent stigma is crucial for reducing HCV prevalence and moving toward its global eradication.
Authors’ Contribution
Conceptualization: Seyi Samson Enitan, Princess Hephzibah Soboyejo.
Data curation: Seyi Samson Enitan, Princess Hephzibah Soboyejo, Kester Awharentomah Digban, Ayomide Oluwatobiloba Okuneye.
Formal analysis: Seyi Samson Enitan, Princess Hephzibah Soboyejo.
Investigation: Seyi Samson Enitan, Princess Hephzibah Soboyejo, Kester Awharentomah Digban.
Methodology: Seyi Samson Enitan, Princess Hephzibah Soboyejo, Michael Unata Iduh.
Project administration: Seyi Samson Enitan, Kester Awharentomah Digban.
Resources: Seyi Samson Enitan, Princess Hephzibah Soboyejo
Software: Seyi Samson Enitan, Ifeoluwapo Oyebola Asekun-Olarinmoye.
Supervision: Seyi Samson Enitan, Michael Unata Iduh.
Validation: Seyi Samson Enitan, Grace Eleojo Itodo, Chidimma Anthonia Azike, Ameh Raphael Adole.
Visualization: Seyi Samson Enitan.
Writing–original draft: Seyi Samson Enitan, Princess Hephzibah Soboyejo, Kester Awharentomah Digban, Michael Unata Iduh, Ifeoluwapo Oyebola Asekun-Olarinmoye.
Writing–review & editing: Seyi Samson Enitan, Kester Awharentomah Digban, Chidimma Anthonia Azike, Grace Eleojo Itodo, Ameh Raphael Adole, Ayomide Oluwatobiloba Okuneye.
Competing Interests
The authors declare no competing interests.
Consent
Not applicable.
Data Availability Statement
Not applicable.
Ethical Approval
Not applicable.
Funding
None.
References
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345(1):41-52. doi: 10.1056/nejm200107053450107 [Crossref] [ Google Scholar]
- Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol 2007; 13(17):2436-41. doi: 10.3748/wjg.v13.i17.2436 [Crossref] [ Google Scholar]
- Purcell R. The hepatitis C virus: overview. Hepatology 1997; 26(3 Suppl 1):11s-4s. doi: 10.1002/hep.510260702 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Chronic Viral Hepatitis. 2022. Available from: https://www.who.int/data/gho/data/themes/chronic-viral-hepatitis. Accessed November 15, 2024.
- Centers for Disease Control and Prevention (CDC). Viral Hepatitis Surveillance Report – Hepatitis C. 2022. Available from: https://www.cdc.gov/hepatitis/statistics/2022surveillance/hepatitis-c.htm. Accessed November 15, 2024.
- Flisiak R, Jaroszewicz J, Parfieniuk-Kowerda A. Emerging treatments for hepatitis C. Expert Opin Emerg Drugs 2013; 18(4):461-75. doi: 10.1517/14728214.2013.847089 [Crossref] [ Google Scholar]
- Papatheodoridis G, Hatzakis A. Public health issues of hepatitis C virus infection. Best Pract Res Clin Gastroenterol 2012; 26(4):371-80. doi: 10.1016/j.bpg.2012.09.012 [Crossref] [ Google Scholar]
- Hellard M, Sacks-Davis R, Doyle J. Hepatitis C elimination by 2030 through treatment and prevention: think global, act in local networks. J Epidemiol Community Health 2016; 70(12):1151-4. doi: 10.1136/jech-2015-205454 [Crossref] [ Google Scholar]
- Nasrullah M, Sergeenko D, Gvinjilia L, Gamkrelidze A, Tsertsvadze T, Butsashvili M. The role of screening and treatment in national progress toward hepatitis C elimination - Georgia, 2015-2016. MMWR Morb Mortal Wkly Rep 2017; 66(29):773-6. doi: 10.15585/mmwr.mm6629a2 [Crossref] [ Google Scholar]
- Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nat Med 2013; 19(7):850-8. doi: 10.1038/nm.3184 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. WHO; 2024. Available from: https://www.who.int/publications/i/item/9789240091672. Accessed November 15, 2024.
- Taha G, Ezra L, Abu-Freha N. Hepatitis C elimination: opportunities and challenges in 2023. Viruses 2023; 15(7):1413. doi: 10.3390/v15071413 [Crossref] [ Google Scholar]
- Shahid I, Alzahrani AR, Al-Ghamdi SS, Alanazi IM, Rehman S, Hassan S. Hepatitis C diagnosis: simplified solutions, predictive barriers, and future promises. Diagnostics (Basel) 2021; 11(7):1253. doi: 10.3390/diagnostics11071253 [Crossref] [ Google Scholar]
- Houghton M. The long and winding road leading to the identification of the hepatitis C virus. J Hepatol 2009; 51(5):939-48. doi: 10.1016/j.jhep.2009.08.004 [Crossref] [ Google Scholar]
- Lancaster K, Rhodes T. Futuring a world without disease: visualising the elimination of hepatitis C. Critical Public Health 2022; 32(2):153-67. doi: 10.1080/09581596.2020.1787347 [Crossref] [ Google Scholar]
- Franco RA, Galbraith JW, Overton ET, Saag MS. Direct-acting antivirals and chronic hepatitis C: towards elimination. Hepatoma Res 2018; 4:74. doi: 10.20517/2394-5079.2018.94 [Crossref] [ Google Scholar]
- Centers for Disease Control and Prevention (CDC). Global Viral Hepatitis. 2024. Available from: https://www.cdc.gov/hepatitis/global/index.html. Accessed November 15, 2024.
- Centers for Disease Control and Prevention (CDC). Hepatitis C Surveillance. 2022. Available from: https://www.cdc.gov/hepatitis/statistics/2022surveillance/hepatitis-c.htm. Accessed November 15, 2024.
- Centers for Disease Control and Prevention (CDC). Clinical Overview of Hepatitis C. 2023. Available from: https://www.cdc.gov/hepatitis-c/hcp/clinical-overview/?CDC_AAref_Val=https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed November 15, 2024.
- Soriano V, Peters MG, Zeuzem S. New therapies for hepatitis C virus infection. Clin Infect Dis 2009; 48(3):313-20. doi: 10.1086/595848 [Crossref] [ Google Scholar]
- Schröeder SE, Pedrana A, Scott N, Wilson D, Kuschel C, Aufegger L. Innovative strategies for the elimination of viral hepatitis at a national level: a country case series. Liver Int 2019; 39(10):1818-36. doi: 10.1111/liv.14222 [Crossref] [ Google Scholar]
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370(20):1889-98. doi: 10.1056/NEJMoa1402454 [Crossref] [ Google Scholar]
- Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015; 373(27):2599-607. doi: 10.1056/NEJMoa1512610 [Crossref] [ Google Scholar]
- Hügle T, Cerny A. Current therapy and new molecular approaches to antiviral treatment and prevention of hepatitis C. Rev Med Virol 2003; 13(6):361-71. doi: 10.1002/rmv.397 [Crossref] [ Google Scholar]
- Horsley-Silva JL, Vargas HE. New therapies for hepatitis C virus infection. Gastroenterol Hepatol (N Y) 2017; 13(1):22-31. [ Google Scholar]
- Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med 2017; 376(22):2134-46. doi: 10.1056/NEJMoa1613512 [Crossref] [ Google Scholar]
- Zoulim F, Liang TJ, Gerbes AL, Aghemo A, Deuffic-Burban S, Dusheiko G. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut 2015; 64(11):1824-33. doi: 10.1136/gutjnl-2015-310421 [Crossref] [ Google Scholar]
- Toniutto P, Fabris C, Pirisi M. Antiviral treatment of hepatitis C. Expert Opin Pharmacother 2006; 7(15):2025-35. doi: 10.1517/14656566.7.15.2025 [Crossref] [ Google Scholar]
- Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G. Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol 2014; 20(29):9633-52. doi: 10.3748/wjg.v20.i29.9633 [Crossref] [ Google Scholar]
- Schalm SW, Brouwer JT, Bekkering FC, van Rossum TG. New treatment strategies in non-responder patients with chronic hepatitis C. J Hepatol 1999; 31 Suppl 1:184-8. doi: 10.1016/s0168-8278(99)80398-5 [Crossref] [ Google Scholar]
- Puchades Renau L, Berenguer M. Introduction to hepatitis C virus infection: overview and history of hepatitis C virus therapies. Hemodial Int 2018; 22 Suppl 1:S8-21. doi: 10.1111/hdi.12647 [Crossref] [ Google Scholar]
- Brouwer JT, Nevens F, Kleter B, Elewaut A, Adler M, Brenard R. Efficacy of interferon dose and prediction of response in chronic hepatitis C: Benelux study in 336 patients. J Hepatol 1998; 28(6):951-9. doi: 10.1016/s0168-8278(98)80342-5 [Crossref] [ Google Scholar]
- Craxì A, Almasio P, Schalm S. Evaluation of efficacy of antiviral therapy for chronic hepatitis C: a EUROHEP Consensus Report on response criteria. J Viral Hepat 1996; 3(5):273-6. doi: 10.1111/j.1365-2893.1996.tb00055.x [Crossref] [ Google Scholar]
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014; 146(5):1176-92. doi: 10.1053/j.gastro.2014.03.003 [Crossref] [ Google Scholar]
- Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370(21):1993-2001. doi: 10.1056/NEJMoa1316145 [Crossref] [ Google Scholar]
- Alshuwaykh O, Kwo PY. Current and future strategies for the treatment of chronic hepatitis C. Clin Mol Hepatol 2021; 27(2):246-56. doi: 10.3350/cmh.2020.0230 [Crossref] [ Google Scholar]
- Lemoine M, Thursz M. Hepatitis C, a global issue: access to care and new therapeutic and preventive approaches in resource-constrained areas. Semin Liver Dis 2014; 34(1):89-97. doi: 10.1055/s-0034-1371082 [Crossref] [ Google Scholar]
-
Abutaleb A, Kottilil S, Rosenthal E. Hepatitis C virus. In: Kaslow RA, Stanberry LR, Powers AM, eds. Viral Infections of Humans: Epidemiology and Control. New York, NY: Springer; 2020. p. 1-28. doi: 10.1007/978-1-4939-9544-8_64-1.
- Stasi C, Milli C, Voller F, Silvestri C. The epidemiology of chronic hepatitis C: where we are now. Livers 2024; 4(2):172-81. doi: 10.3390/livers4020013 [Crossref] [ Google Scholar]
- Handanagic S, Shadaker S, Drobeniuc J, Tsereteli M, Alkhazashvili M, Adesigbin C. Lessons learned from global hepatitis C elimination programs. J Infect Dis 2024; 229(Suppl 3):S334-41. doi: 10.1093/infdis/jiad198 [Crossref] [ Google Scholar]
- Manns MP, Maasoumy B. Breakthroughs in hepatitis C research: from discovery to cure. Nat Rev Gastroenterol Hepatol 2022; 19(8):533-50. doi: 10.1038/s41575-022-00608-8 [Crossref] [ Google Scholar]
- Cox AL, El-Sayed MH, Kao JH, Lazarus JV, Lemoine M, Lok AS. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol 2020; 17(9):533-42. doi: 10.1038/s41575-020-0332-6 [Crossref] [ Google Scholar]
- Schiano Moriello N, Pinchera B, Gentile I. Personalized care approaches to hepatitis C therapy: recent advances and future directions. Expert Rev Anti Infect Ther 2024; 22(4):139-51. doi: 10.1080/14787210.2024.2328336 [Crossref] [ Google Scholar]
- Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis 2014; 58(7):928-36. doi: 10.1093/cid/ciu012 [Crossref] [ Google Scholar]
- Bernal LA, Soti V. Hepatitis C virus: insights into its history, treatment, challenges, and future directions. Cureus 2023; 15(8):e43924. doi: 10.7759/cureus.43924 [Crossref] [ Google Scholar]
- Basyte-Bacevice V, Kupcinskas L. Viral hepatitis C: from unraveling the nature of disease to cure and global elimination. Dig Dis 2024; 42(5):486-95. doi: 10.1159/000539210 [Crossref] [ Google Scholar]
- Fricker GP, Ghany MG, Mera J, Pinsky BA, Ward JW, Chung RT. Tools needed to support same-day diagnosis and treatment of current hepatitis C virus infection. J Infect Dis 2024; 229(Suppl 3):S362-9. doi: 10.1093/infdis/jiad177 [Crossref] [ Google Scholar]
- Abdallah M, Waked I, El-Kassas M. Global hepatitis C virus elimination—where are we?. Curr Hepatol Rep 2024; 23(3):373-7. doi: 10.1007/s11901-024-00655-6 [Crossref] [ Google Scholar]
- Liu CH, Kao JH. Acute hepatitis C virus infection: clinical update and remaining challenges. Clin Mol Hepatol 2023; 29(3):623-42. doi: 10.3350/cmh.2022.0349 [Crossref] [ Google Scholar]
- D’Souza R, Foster GR. Diagnosis and treatment of hepatitis C. J R Soc Med 2004; 97(5):223-5. doi: 10.1177/014107680409700504 [Crossref] [ Google Scholar]
- Law JL, Drummer HE. Bridging the gap: a new tool to down select HCV vaccine candidates. Hepatology 2024; 80(5):1006-8. doi: 10.1097/hep.0000000000000948 [Crossref] [ Google Scholar]
- Rzymski P, Jibril AT, Rahmah L, Abarikwu SO, Hashem F, Lawati AA. Is there still hope for the prophylactic hepatitis C vaccine? A review of different approaches. J Med Virol 2024; 96(9):e29900. doi: 10.1002/jmv.29900 [Crossref] [ Google Scholar]
- Haga Y, Coates S, Ray R. Hepatitis C virus chronicity and oncogenic potential: vaccine development progress. Mol Aspects Med 2024; 99:101305. doi: 10.1016/j.mam.2024.101305 [Crossref] [ Google Scholar]
- Gupta R, Arora K, Mehrotra Arora N, Kundu P. Significance of VLPs in Vlp-circRNA vaccines: a vaccine candidate or delivery vehicle?. RNA Biol 2024; 21(1):17-28. doi: 10.1080/15476286.2024.2399307 [Crossref] [ Google Scholar]
- Hur MH, Lee JH. Toward hepatitis C virus elimination using artificial intelligence. Clin Mol Hepatol 2024; 30(2):147-9. doi: 10.3350/cmh.2024.0135 [Crossref] [ Google Scholar]
- Elbahrawy A, Atalla H, Alboraie M, Alwassief A, Madian A, El Fayoumie M. Recent advances in protective vaccines against hepatitis viruses: a narrative review. Viruses 2023; 15(1):214. doi: 10.3390/v15010214 [Crossref] [ Google Scholar]
- Walker SJ, Shrestha LB, Lloyd AR, Dawson O, Sheehan Y, Sheehan J. Barriers and advocacy needs for hepatitis C services in prisons: Informing the prisons hepatitis C advocacy toolkit. Int J Drug Policy 2024; 126:104386. doi: 10.1016/j.drugpo.2024.104386 [Crossref] [ Google Scholar]