Avicenna Journal of Clinical Microbiology and Infection. 11(4):171-176.
doi: 10.34172/ajcmi.3561
Original Article
Prevalence of Salmonella in Poultry Slaughterhouses of Kerman, Iran
Zahra Mirafzali 1  , Mohammad Hossein Marhamatizadeh 1
, Mohammad Hossein Marhamatizadeh 1  , Ashraf Kariminik 2, 3, *
, Ashraf Kariminik 2, 3, *  , Gholam Hossein Habibi 4
, Gholam Hossein Habibi 4 
Author information:
1Department of Food Hygiene, Faculty of Veterinary Medicine, Kazerun Branch, Islamic Azad University, Kazerun, Iran
2Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran
3Food and Agricultural Safety Research Center, Kerman Branch, Islamic Azad University, Kerman, Iran
4Department of Clinical Sciences, Faculty of Veterinary Medicine, Kazerun Branch, Islamic Azad University, Kazerun, Iran
Abstract
Background: Salmonella is a prevalent infectious agent that infects several animals. Chicken is a main meal for humans, and the infectivity of the animal by bacteria threatens both human health and economic conditions. Improving our knowledge regarding the prevalence of Salmonella in chicken can help us organize new strategies to increase the quality of food in Iran. This project aimed to explore the prevalence of Salmonella infection among chickens from poultry slaughterhouses in Kerman, Iran.
Methods: In this cross-sectional study, 100 samples of chicken meat from poultry slaughterhouses supplied to shopping centers in Kerman were collected for investigation. To confirm the Salmonella infection, tissues were homogenized under sterile conditions and then either cultured in differentiated media, or their bacterial DNA was extracted and tested by real-time polymerase chain reaction (RT-PCR). The infected chicken underwent a PCR test to determine the Salmonella species. The isolates of Salmonella were subjected to antibiotic susceptibility testing by the disc-diffusion method against imipenem (10 μg), and the presence of the blaNDM gene was detected by PCR.
Results: The findings revealed that 50 out of 100 samples were infected by Salmonella, which was confirmed by both microbial culture and RT-PCR. The PCR test demonstrated that three samples were Salmonella Enteritidis, and two samples were Salmonella Typhimurium. Finally, 17 (34%) Salmonella isolates were resistant to imipenem, and the frequency of the blaNDM gene was 38 (76%) out of 50 samples.
Conclusion: The isolation of Salmonella from the chicken’s meat may indicate a chicken’s systemic infection and failure to control the most important microbe for public health. Thus, the control measures have to be revised, and a national Salmonella control program should be put in place urgently.
Keywords: Chicken, Salmonella typhimurium, Salmonella enteritidis
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Mirafzali Z, Marhamatizadeh MH, Kariminik A, Habibi GH. Prevalence of salmonella in poultry slaughterhouses of Kerman, Iran. Avicenna J Clin Microbiol Infect. 2024; 11(4):171-176. doi:10.34172/ajcmi.3561
Introduction
Salmonella is a major pathogen impacting the poultry industry, leading to economic repercussions and public health issues(1,2). As one of the most common pathogens associated with poultry, Salmonella poses risks not only to consumer health but also to the poultry industry, affecting food safety and economic stability (3). The complexity of the poultry supply chain, from farm to table, creates multiple opportunities for contamination, necessitating thorough evaluation and monitoring of Salmonella prevalence in chicken meat (4). The relationship between Salmonella and chickens is intricate, with numerous molecular processes playing a role in the onset of illness and inflammation (5,6). Understanding the dynamics of Salmonella prevalence in poultry is essential for developing effective interventions to mitigate the risks associated with this pathogen. Previous studies have indicated varying prevalence rates, with some regions reporting levels as high as 50% in retail chicken samples, underscoring the need for ongoing surveillance and intervention strategies (7). However, the exact prevalence of the bacteria and their pathogenic species in the Iranian chicken meat, in Kerman province, is yet to be clarified. Reports indicate that the prevalence of various serotypes of Salmonella species isolated from chickens is notably high in several countries (8,9). However, some serotypes, such as Salmonella Typhimuriumand Salmonella Enteritidis, have repeatedly been reported from other countries (10,11). Metallo-beta-lactamases (MBLs) are a type of enzyme that plays a significant role in antibiotic resistance, particularly in Gram-negative bacteria, including Salmonella. MBLs are capable of hydrolyzing beta-lactam antibiotics, rendering them ineffective. Although Salmonella species are primarily associated with foodborne illnesses, antibiotic resistance in these bacteria is becoming an increasing public health concern. The presence of MBLs in Salmonella can complicate the treatment of infections, especially when they carry genes encoding MBLs that confer resistance to beta-lactam antibiotics. These genes, such as blaNDM, can be acquired through horizontal gene transfer from other resistant bacteria (12).
This research paper aims to evaluate the prevalence of Salmonella infection in Iranian chicken meat products, examining the pathogenic species, Salmonella EnteritidisandTyphimurium, distribution. By analyzing data from multiple studies and conducting new assessments, this study seeks to provide a comprehensive understanding of Salmonella contamination in chicken meat, contributing to improved food safety measures and public health strategies.
Materials and Methods
Ethics Committee Approval and Consent of the Owners of Cottage Processor Outlets
Prior to the completion of the questionnaires and the procurement of chickens for the study, consent was obtained from the owners of the Cottage Processor outlets. Additionally, the research protocol received approval from the Ethics Committee of Islamic Azad University, Kazerun Branch, ensuring that all ethical guidelines were adhered to before the study began.
Retail Outlets for Broiler Chickens in Kerman, Iran
The retail outlets involved in this study primarily consisted of cottage poultry processors, commonly referred to as “chicken and fish shopping centers”. In Iran, cottage poultry processors are outside city businesses that slaughter and process chickens on demand from chicken and fish shopping centers in fresh whole birds or cut-up chicken parts. There is a cottage poultry processor in Kerman, which provides services for the chicken and fish shopping center. Chickens are slaughtered, plastic or galvanized cones are used for holding the birds during and after the severing of the jugular vein, large pots or vessels are filled with hot water for scalding prior to feathering, and machines or drums are utilized for the feathering process. Iran’s Health Ministry is the responsible organization for maintaining the health of the chickens through their supervision and analysis. This cross-sectional study included a total of fifty poultry meat samples, which were collected from fifty chicken and fish shopping centers in Kerman, Sirjan, and Bardsir, three cities in Kerman province, Iran. Accordingly, the samples were randomly collected from fresh chicken meats (25 samples of whole, cold-packed chicken carcasses and 25 samples of cut chicken breast), which were slaughtered for less than 24 hours.
Salmonella Detection
Salmonella infection was evaluated using microbial culture methods and real-time polymerase chain reaction (RT-PCR). To facilitate this process, three types of chicken meat were collected and transported to the molecular laboratory in sterile media. All samples were analyzed within 4‒6 hours post-collection. For pre-enrichment, 25 g of each sample were homogenized with 225 mL of 2% buffered peptone water for 2 minutes at maximum speed using a stomacher. The homogenized samples were then placed into 10 mL of selective enrichment media, specifically tetrathionate broth (Merck, Germany), and incubated at 37 °C for 24 hours. The following day, two loopfuls of the broth were streaked onto bismuth sulfite agar and xylose lysine deoxycholate agar (Merck, Germany), which were then incubated at 37 °C for 24‒48 hours to isolate visible colonies of Salmonella species. Pink colonies with a black center on xylose lysine deoxycholate agar and brown, gray, or black colonies exhibiting a metallic sheen on bismuth sulfite agar were initially identified as presumptive Salmonella isolates. The identification process for these bacterial isolates included biochemical tests such as catalase, oxidase, indole production, citrate utilization, triple sugar iron, lysine iron agar, and methyl red-Voges Proskauer tests (10). Subsequently, the bacteria were preserved in brain heart infusion broth (Merck, Germany) supplemented with glycerol and stored at -70 °C for future studies. The Salmonella-positive isolates underwent additional culturing and molecular confirmation to ensure accurate identification. For RT-PCR amplification, the genomic DNA extraction kit (Karmania Pars Gene, Iran) was employed to purify DNA templates following the manufacturer’s instructions. Universal primers were utilized to detect the Salmonella genus, with the list of primers provided in Table 1. The RT-PCR was conducted in a 20 μL volume. The temperature protocol consisted of an initial step at 95 °C for 15 minutes, followed by 40 cycles of 95 °C for 20 seconds, annealing at 64 °C for 20 seconds, extension at 72°C for 1 minute, and a final step to generate a melt curve from 50 °C to 95 °C. After the confirmation of the Salmonella genus by RT-PCR, a conventional PCR was used for detecting two serotypes of Salmonella, Salmonella enteritidisand Salmonella Typhimurium, using specific primers (Table 1). The conventional PCR temperature protocol consisted of an initial step at 95 °C for 2 minutes, followed by 35 cycles of 95 °C for 20 seconds, annealing at 64 °C for 20 seconds, and extension at 72 °C for 30 seconds, followed by 72 °C for 5 minutes as the final extension. The PCR products were run on a 1% agarose gel in parallel with a 100 bp ladder, which was pre-treated with a safe stain (Karmania Pars Gene, Iran). PCR products for Salmonella Enteritidis and Salmonella Typhimurium were 316 bp and 107 bp, respectively.
Table 1.
Primer Sequences
|
Genes
|
Primer Sequences (5'-3')
|
Product Size (bp)
|
| Universal Salmonella forward |
ATTACTTGTGCCGAAGAGCC |
145 |
| Universal Salmonella reverse |
GATGCTGTTATCGTCCAGGC |
|
Salmonella Typhimurium forward |
ACGACTGGGATATGAACGGGGAA |
107 |
|
Salmonella Typhimurium reverse |
TCGTTGTACTTGATGCTGCGGAG |
|
Salmonella Enteritidis forward |
AGTGCCATACTTTTAATGAC |
316 |
|
Salmonella Enteritidis reverse |
ACTATGTCGATACGGTGGG |
|
blaNDMforward |
TCTCGACATGCCGGGTTT |
472 |
|
blaNDMreverse |
GAGATTGCCGAGCGACTT |
Determination of Antimicrobial Susceptibility and Frequency of the blaNDM Gene
The antibiotic susceptibility pattern for bacterial isolates was determined using the disk diffusion method according to the Clinical and Laboratory Standards Institute recommendation. The tested antibiotic disk was imipenem (10 μg) from Padtanteb Company, Iran. All the isolates were screened for the presence of theblaNDMgene by the PCR assay. The conventional PCR for the detection of the blaNDMgene was performed using a commercial master mix from Karmania Pars Gene Company, Iran, and specific primers (Table 1). The temperature protocol consisted of an initial step at 95 °C for 2 minutes, followed by 35 cycles of 95 °C for 20 seconds, annealing at 62 °C for 20 seconds, and extension at 72 °C for 30 seconds, followed by 72 °C for 5 minutes. The PCR products were run on a 1% agarose gel in parallel with a 1 kb ladder, which was pre-treated with a safe stain (Karmania Pars Gene, Iran). The PCR product for the blaNDMgene was 472 bp (12).
Statistical Analysis
SPSS software (version 21) was used to calculate the raw data. The Kolmogorov-Smirnov test revealed that the raw data had no normal distribution. Accordingly, the chi-square test was used to analyze the data, which are presented as percentages.
Results
Out of the 20 samples tested, 50 (100%) were found to be contaminated with Salmonella species. This contamination was initially identified through biochemical tests and further confirmed using molecular detection methods.
The bacteria culture proved that the isolated bacteria were Salmonella, which was confirmed by the RT-PCR test.
The Chi-square test showed that the Salmonella infection is significantly high among the Iranian chickens (P< 0.001).
As illustrated in Figure 1, three out of fifty samples were positive for Salmonella Enteritidis. The positive samples of Salmonella Enteritidis were from Kerman, Sirjan, and Bardsir cities, where the first two samples were from whole packaged chicken carcasses and the third sample was from chicken breast samples. Moreover, two out of 50 samples were positive for Salmonella Typhimurium (Figure 2). The positive samples of Salmonella Typhimurium were all from Bardsir and whole chicken carcasses. The antibiogram tests revealed that 17 (34%) Salmonella isolates were resistant to imipenem, and the PCR results demonstrated that the frequencies of the blaNDM gene were 38 (76%) out of 50 samples. Figure 3 displays the PCR product of the blaNDM gene.
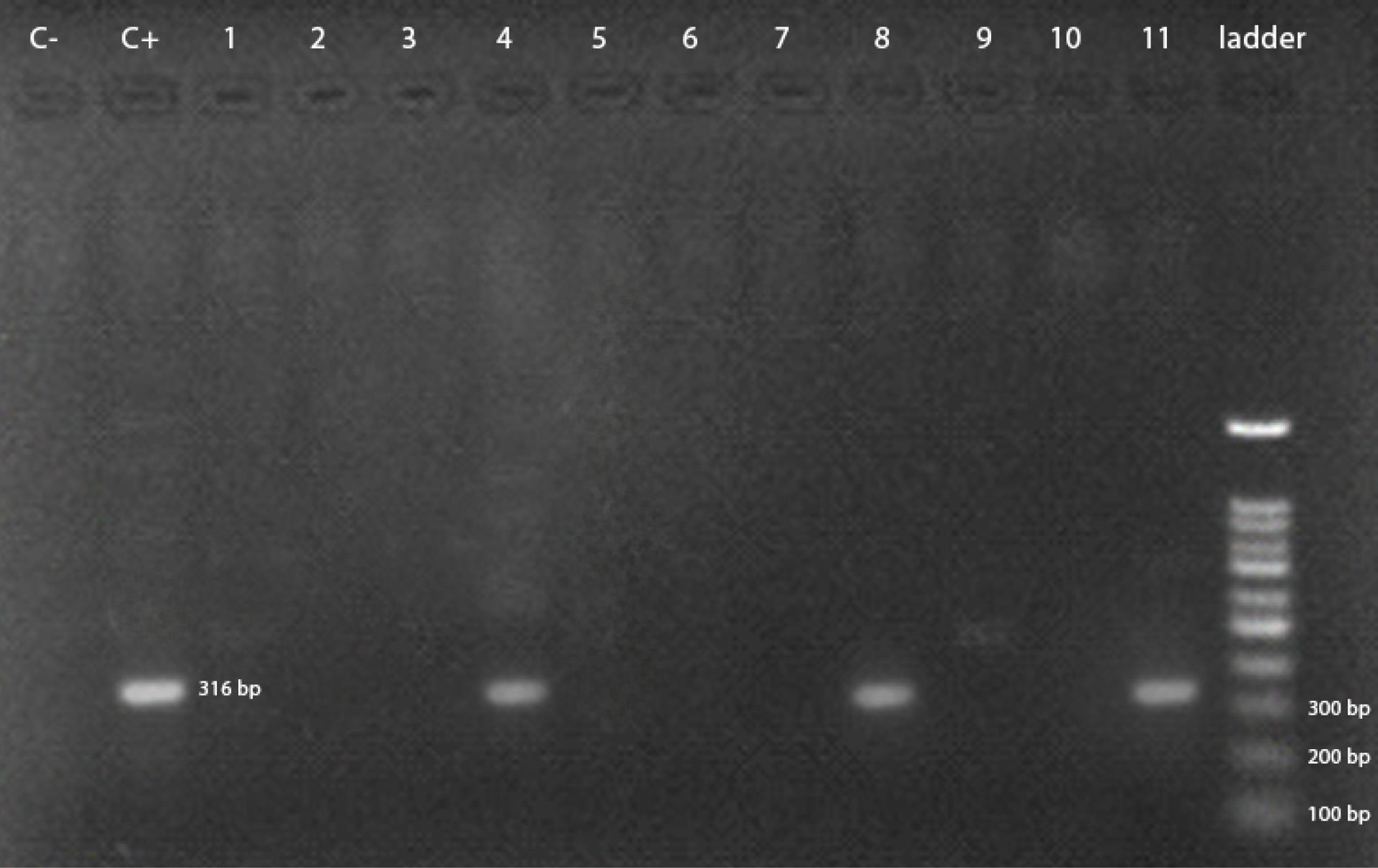
Figure 1.
PCR Product Gel Electrophoresis for the Detection of Salmonella Enteritidis. Note. PCR: Polymerase chain reaction. Three out of 50 samples, which were infected by Salmonella, were Salmonella enteritidis.
.
PCR Product Gel Electrophoresis for the Detection of Salmonella Enteritidis. Note. PCR: Polymerase chain reaction. Three out of 50 samples, which were infected by Salmonella, were Salmonella enteritidis.
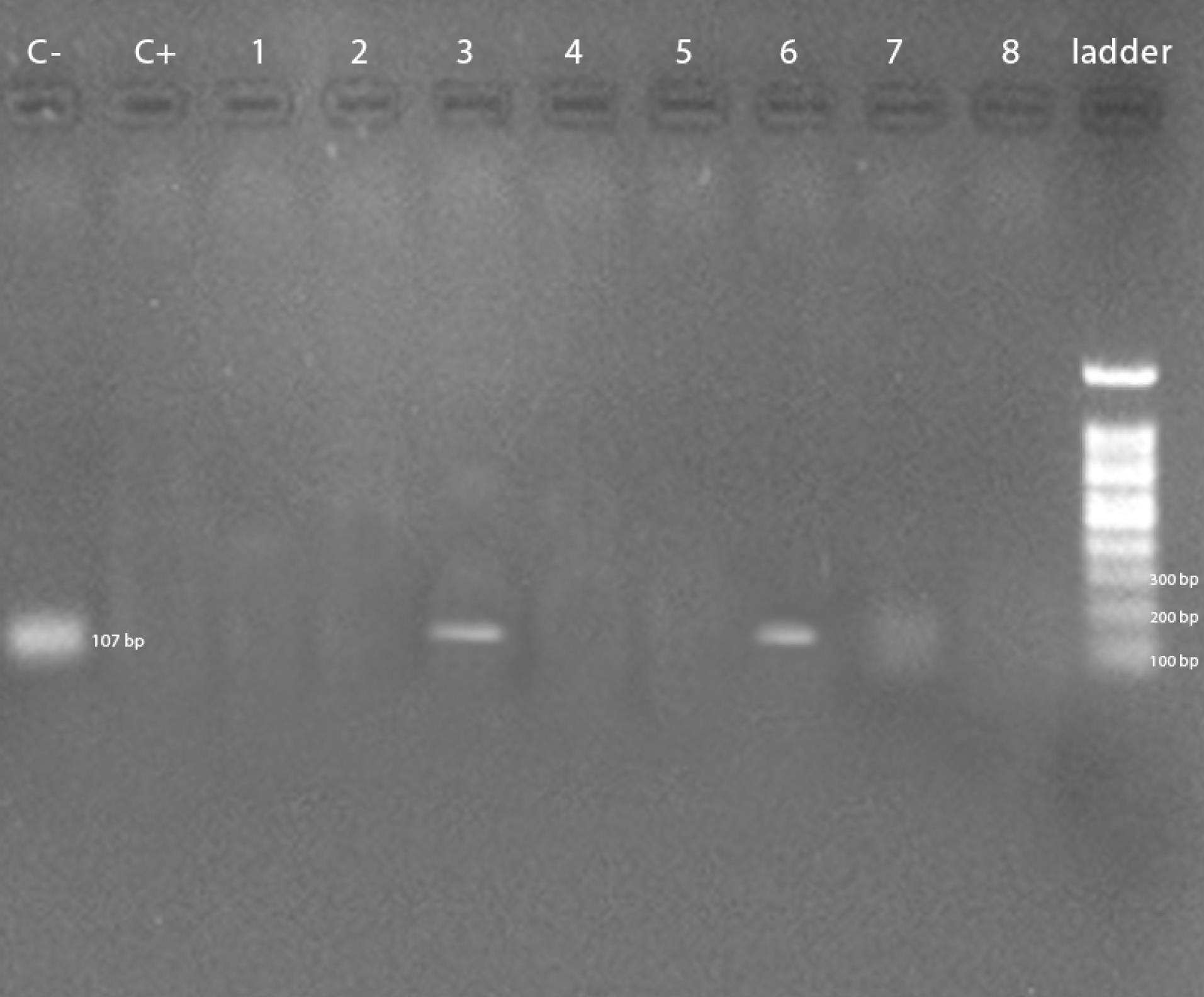
Figure 2.
PCR Product Gel Electrophoresis for the Detection of Salmonella Typhimurium. Note. PCR: Polymerase chain reaction. Two out of 50 samples, which were infected by Salmonella, were Salmonella Typhimurium.
.
PCR Product Gel Electrophoresis for the Detection of Salmonella Typhimurium. Note. PCR: Polymerase chain reaction. Two out of 50 samples, which were infected by Salmonella, were Salmonella Typhimurium.
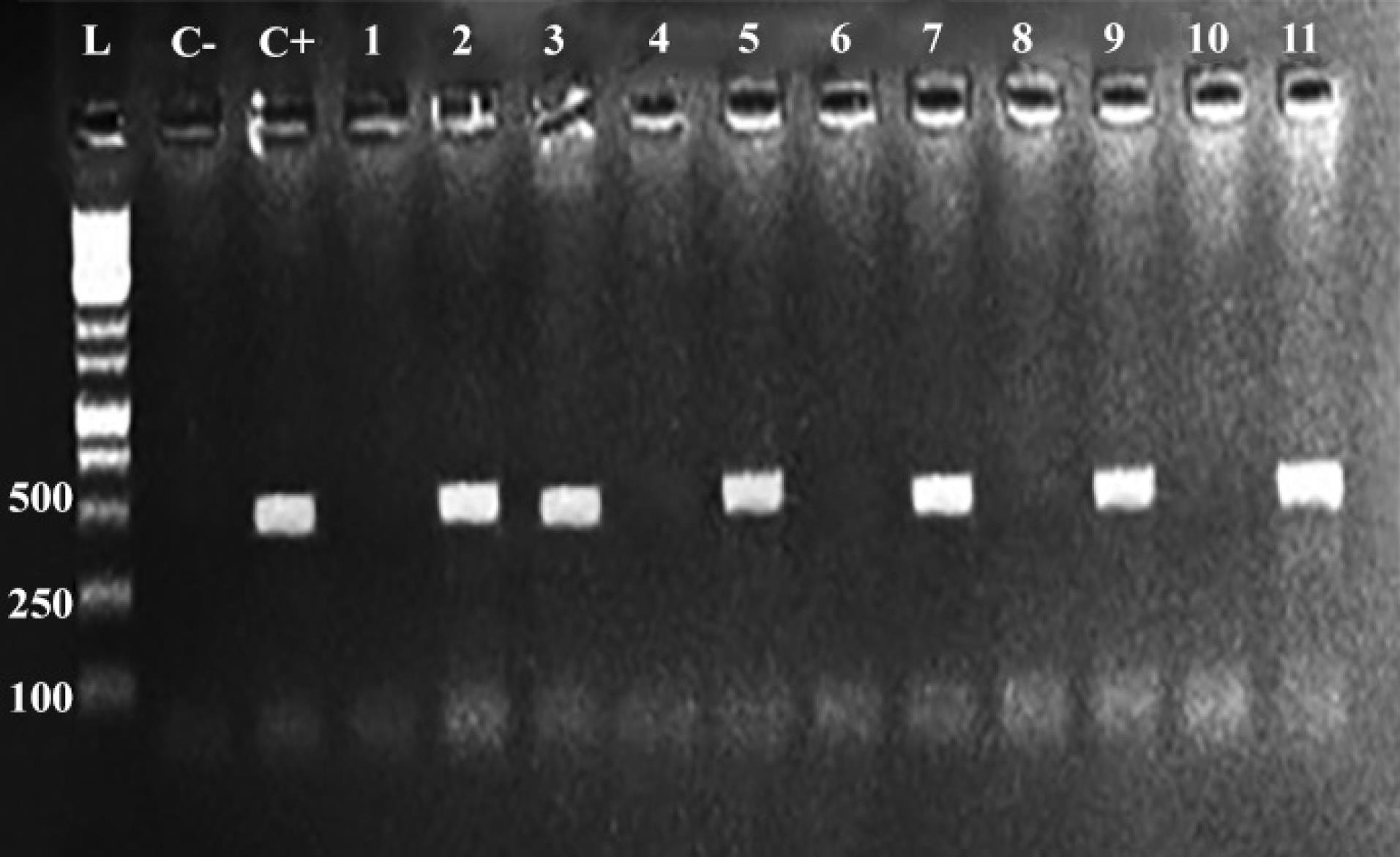
Figure 3.
Agarose Gel Electrophoresis of the PCR-Amplified blaNDM Gene. Note. PCR: Polymerase chain reaction. The blaNDM geneappears at 472 bp. L: Ladder; C-: Negative control; C + : Positive control.
.
Agarose Gel Electrophoresis of the PCR-Amplified blaNDM Gene. Note. PCR: Polymerase chain reaction. The blaNDM geneappears at 472 bp. L: Ladder; C-: Negative control; C + : Positive control.
Discussion
Salmonella infection in chicken meat is a critical public health concern due to its prevalence and the severe foodborne illnesses it can cause (13). Salmonella species, particularly Salmonella enterica serotypes, such as TyphimuriumandEnteritidis, are commonly found in poultry and are significant contributors to global salmonellosis outbreaks (14). The contamination can occur at various stages, including production, processing, distribution, and preparation, making it a complex issue to address (15). The results confirmed that 100% of the poultry meat was infected by Salmonella species. Among them, 5 samples were infected by pathological species of Salmonella, including three Salmonella Enteritidisandtwo Salmonella Typhimurium. Considering that poultry meat needs to be clear of pathological bacteria, it needs to be checked by the Iranian Health Organization using molecular testing. Several studies have reported the prevalence of Salmonella infections in poultry in Iran. According to various studies conducted over the last ten years, the prevalence of Salmonella infections in broiler chicken farms in Iran ranges from 22.5% to 64.2% (16). In a cross-sectional study, 7.9% of broiler breeder farms in Iran were found to be infected with Salmonella, with older flocks and farms with more houses at greater risk (16). These findings highlight the widespread nature of Salmonella infections in poultry, which can pose risks to human health through the food supply and direct contact. However, there are no reports regarding the prevalence of Salmonella infections in chickens in the Kerman province, Iran. Continued surveillance and control measures are needed to mitigate the public health impact of salmonellosis. It may be announced that although the infection of chicken meat is high regarding Salmonella species, the infection is not high via eating chicken meat in Iran. It appears that cooking the chicken leads to killing the bacteria. However, direct exposure to raw chicken meat may increase the risk of transmission of Salmonella species to humans via infected chicken meat. Research indicates that improper cooking and handling of chicken meat are primary factors that lead to Salmonella infections in humans (17). For instance, undercooked poultry can harbor viable Salmonella cells, posing a risk for consumers (17). Studies have shown that proper cooking methods significantly reduce the risk of infection; nonetheless, many consumers do not adhere to recommended cooking temperatures, leading to potential outbreaks. Moreover, the emergence of antibiotic-resistant Salmonella strains complicates treatment options for infected individuals, highlighting the need for stringent control measures throughout the poultry production chain (18). Effective interventions, such as biosecurity measures on farms, improved processing techniques, and public education on safe food handling practices, are essential to mitigate the risks associated with Salmonella in chicken meat (19). Further, tackling Salmonella infections in chicken meat necessitates a comprehensive strategy that includes improved food safety practices, consumer education, and continuous research aimed at developing effective control measures to safeguard public health. The research represents that Salmonella infections in chicken meat can indeed influence epigenetic factors in humans that affect several molecules, including pro-inflammatory factors, although the specific mechanisms and effects require further investigation (20). The isolation of Salmonella from chicken meat suggests not only the presence of systemic infection in chickens but also highlights significant gaps in the current food safety and public health measures. This finding raises serious concerns about the potential risks to consumers, as Salmonella is a leading cause of foodborne illness worldwide. The failure to effectively control this pathogen in poultry production confirms an urgent need for a comprehensive reassessment of existing control measures (21). Furthermore, several investigations proved that Iranian chicken meat is infected by Salmonella serotypes that are resistant to antibiotics. For example, Mir et al reported that 100% of the infected chicken meat in Zahedan, a southeastern province of Iran, was resistant to penicillin (22). The results were also confirmed by other Iranian investigators. Thus, it is essential to evaluate and update current biosecurity protocols and hygiene practices in poultry farms (23,24). This includes improving sanitation, monitoring flock health, and implementing strict measures to prevent cross-contamination during processing. In addition, establishing a coordinated national program focusing on the surveillance, prevention, and control of Salmonella in poultry is crucial. This program should involve collaboration between government agencies, poultry producers, and public health organizations. Additionally, it is essential to establish strong surveillance systems to monitor the prevalence of Salmonella in poultry throughout all stages, from farm to table. Regular testing and reporting will facilitate the early identification of outbreaks and guide necessary interventions. By implementing these suggestions, it is possible to enhance the safety of chicken meat, protect public health, and reduce the burden of Salmonella-related illnesses. Immediate action is essential to address the current challenges and ensure a safer food supply. Overall, 34% of Salmonella isolates were resistant to imipenem, and 76% tested positive for the blaNDM gene, which are concerning findings. Imipenem is an important antibiotic used to treat serious bacterial infections, and resistance to it among Salmonella isolates can pose a significant public health threat. The presence of the blaNDM gene, which confers resistance to carbapenem antibiotics such as imipenem, suggests the potential for the spread of multidrug-resistant strains of Salmonella. These results highlight the issue of antibiotic resistance in chicken meat, which can serve as a source of transmission of resistant bacteria to humans through food consumption. If these antibiotic-resistant strains enter the food chain and cause infections in humans, it can lead to difficult-to-treat or even untreatable infections, compromising the effectiveness of antibiotic therapy and posing a serious risk to public health (25). Given the high prevalence of antibiotic resistance found in this study, Iranian health authorities must take immediate action to address this issue. Strategies such as having surveillance regulations of antibiotic use in livestock, implementing strict regulations on antibiotic usage in poultry farming, promoting responsible antibiotic prescribing practices, and increasing awareness about the dangers of antibiotic resistance are essential to mitigate the impact of antibiotic resistance on public health in Iran. Additionally, further research and monitoring of antibiotic resistance patterns in food-producing animals and their products are necessary to better understand and combat this emerging threat (26).
Conclusion
The study found a significant prevalence of Salmonella infections, with 50% of the samples testing positive for Salmonella. Among these, specific serotypes, Salmonella Enteritidis and Salmonella Typhimurium, were identified. Additionally, a concerning level of antibiotic resistance was observed, with 34% of the Salmonella isolates showing resistance to Imipenem. Furthermore, a high frequency of the blaNDM gene, which is associated with antibiotic resistance, was detected in 76% of the samples. This suggests not only a notable presence of Salmonella in the samples but also raises alarms about the potential for antibiotic-resistant strains, highlighting the need for ongoing monitoring and effective management strategies.
Acknowledgements
The authors would like to express their gratitude to the staff of the Food and Agricultural Safety Research Center at the Islamic Azad University of Kerman and the Department of Food Hygiene at the Islamic Azad University of Kazerun, Iran.
Authors’ Contribution
Conceptualization: Zahra Mirafzali.
Data curation: Zahra Mirafzali.
Investigation: Zahra Mirafzali.
Project administration: Mohammad Hossein Marhamatizadeh.
Supervision: Ashraf Kariminik and Mohammad Hossein Marhamatizadeh.
Validation: Gholam Hossein Habibi.
Writing–original draft: Ashraf Kariminik.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
The project protocol was approved by the local ethical committee (IR.IAU.KAU.REC.1402.073).
Funding
None.
References
- Vajda Á, Ózsvári L, Szakos D, Kasza G. Estimation of the impact of foodborne salmonellosis on consumer well-being in Hungary. Int J Environ Res Public Health 2021; 18(19):10131. doi: 10.3390/ijerph181910131 [Crossref] [ Google Scholar]
- Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, Chemaly M. Salmonella control in poultry flocks and its public health impact. EFSA J 2019; 17(2):e05596. doi: 10.2903/j.efsa.2019.5596 [Crossref] [ Google Scholar]
- Ehuwa O, Jaiswal AK, Jaiswal S. Salmonella, food safety and food handling practices. Foods 2021; 10(5):907. doi: 10.3390/foods10050907 [Crossref] [ Google Scholar]
- Withenshaw SM, Cawthraw S, Gosling B, Newton K, Oastler CE, Smith RP. Risk factor analysis for Salmonella contamination of broiler chicken (Gallus gallus) hatcheries in Great Britain. Prev Vet Med 2021; 196:105492. doi: 10.1016/j.prevetmed.2021.105492 [Crossref] [ Google Scholar]
- Ijaz A, Veldhuizen EJA, Broere F, Rutten V, Jansen CA. The interplay between Salmonella and intestinal innate immune cells in chickens. Pathogens 2021; 10(11):1512. doi: 10.3390/pathogens10111512 [Crossref] [ Google Scholar]
- Sreekantapuram S, Berens C, Barth SA, Methner U, Berndt A. Interaction of Salmonella Gallinarum and Salmonella Enteritidis with peripheral leucocytes of hens with different laying performance. Vet Res 2021; 52(1):123. doi: 10.1186/s13567-021-00994-y [Crossref] [ Google Scholar]
- Sun T, Liu Y, Qin X, Aspridou Z, Zheng J, Wang X. The prevalence and epidemiology of Salmonella in retail raw poultry meat in China: a systematic review and meta-analysis. Foods 2021; 10(11):2757. doi: 10.3390/foods10112757 [Crossref] [ Google Scholar]
- Foley SL, Johnson TJ, Ricke SC, Nayak R, Danzeisen J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol Mol Biol Rev 2013; 77(4):582-607. doi: 10.1128/mmbr.00015-13 [Crossref] [ Google Scholar]
- Vinueza-Burgos C, Cevallos M, Ron-Garrido L, Bertrand S, De Zutter L. Prevalence and diversity of Salmonella serotypes in Ecuadorian broilers at slaughter age. PLoS One 2016; 11(7):e0159567. doi: 10.1371/journal.pone.0159567 [Crossref] [ Google Scholar]
- Álvarez-Fernández E, Alonso-Calleja C, García-Fernández C, Capita R. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: comparison between 1993 and 2006. Int J Food Microbiol 2012; 153(3):281-7. doi: 10.1016/j.ijfoodmicro.2011.11.011 [Crossref] [ Google Scholar]
- Antunes P, Mourão J, Campos J, Peixe L. Salmonellosis: the role of poultry meat. Clin Microbiol Infect 2016; 22(2):110-21. doi: 10.1016/j.cmi.2015.12.004 [Crossref] [ Google Scholar]
- Ghazaei C. Phenotypic and molecular detection of metallo-β-lactamase genes of Salmonella enterica strains isolated from poultry meat. J Epigenetics 2019; 1(1):14-8. doi: 10.22111/jep.2019.26402.1004 [Crossref] [ Google Scholar]
- Wessels K, Rip D, Gouws P. Salmonella in chicken meat: consumption, outbreaks, characteristics, current control methods and the potential of bacteriophage use. Foods 2021; 10(8):1742. doi: 10.3390/foods10081742 [Crossref] [ Google Scholar]
- Sukumaran AT, Nannapaneni R, Kiess A, Sharma CS. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int J Food Microbiol 2015; 207:8-15. doi: 10.1016/j.ijfoodmicro.2015.04.025 [Crossref] [ Google Scholar]
- Sukumaran AT, Nannapaneni R, Kiess A, Sharma CS. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult Sci 2016; 95(3):668-75. doi: 10.3382/ps/pev332 [Crossref] [ Google Scholar]
- Ansari F, Bokaie S, Peighambari SM, Fallah MH, Tehrani F, Rajab A. Survey of Salmonella infections in broiler farms in Iran during 2013-2014: a cross-sectional study. Iran J Microbiol 2020; 12(5):404-10. doi: 10.18502/ijm.v12i5.4600 [Crossref] [ Google Scholar]
- Koh Y, Bae Y, Lee YS, Kang DH, Kim SH. Prevalence and characteristics of Salmonella spp isolated from raw chicken meat in the Republic of Korea. J Microbiol Biotechnol 2022; 32(10):1307-14. doi: 10.4014/jmb.2207.07031 [Crossref] [ Google Scholar]
- Castro-Vargas RE, Herrera-Sánchez MP, Rodríguez-Hernández R, Rondón-Barragán IS. Antibiotic resistance in Salmonella spp isolated from poultry: a global overview. Vet World 2020; 13(10):2070-84. doi: 10.14202/vetworld.2020.2070-2084 [Crossref] [ Google Scholar]
- Youssef DM, Wieland B, Knight GM, Lines J, Naylor NR. The effectiveness of biosecurity interventions in reducing the transmission of bacteria from livestock to humans at the farm level: a systematic literature review. Zoonoses Public Health 2021; 68(6):549-62. doi: 10.1111/zph.12807 [Crossref] [ Google Scholar]
- Dieye Y, Hull DM, Wane AA, Harden L, Fall C, Sambe-Ba B. Genomics of human and chicken Salmonella isolates in Senegal: broilers as a source of antimicrobial resistance and potentially invasive nontyphoidal salmonellosis infections. PLoS One 2022; 17(3):e0266025. doi: 10.1371/journal.pone.0266025 [Crossref] [ Google Scholar]
- Logue CM, De Cesare A, Tast-Lahti E, Chemaly M, Payen C, LeJeune J. Salmonella spp in poultry production-a review of the role of interventions along the production continuum. Adv Food Nutr Res 2024; 108:289-341. doi: 10.1016/bs.afnr.2023.11.001 [Crossref] [ Google Scholar]
- Mir R, Salari S, Najimi M, Rashki A. Determination of frequency, multiple antibiotic resistance index and resistotype of Salmonella spp in chicken meat collected from southeast of Iran. Vet Med Sci 2022; 8(1):229-36. doi: 10.1002/vms3.647 [Crossref] [ Google Scholar]
- Nazari Moghadam M, Rahimi E, Shakerian A, Momtaz H. Prevalence of Salmonella Typhimurium and Salmonella Enteritidis isolated from poultry meat: virulence and antimicrobial-resistant genes. BMC Microbiol 2023; 23(1):168. doi: 10.1186/s12866-023-02908-8 [Crossref] [ Google Scholar]
- Boraei-Nezhad G, Saadati D, Jahantigh M, Saadat-Jou S. Prevalence of Salmonella infection in village chickens and determination of the tetracycline resistance genes in the Salmonella isolates in the Sistan region, Iran. Braz J Microbiol 2023; 54(3):2375-82. doi: 10.1007/s42770-023-01033-y [Crossref] [ Google Scholar]
- Wang Z, He J, Li Q, Tang Y, Wang J, Pan Z. First detection of NDM-5-positive Salmonella enterica serovar Typhimurium isolated from retail pork in China. Microb Drug Resist 2020; 26(5):434-7. doi: 10.1089/mdr.2019.0323 [Crossref] [ Google Scholar]
- Mohammadzadeh M, Montaseri M, Hosseinzadeh S, Majlesi M, Berizi E, Zare M. Antibiotic residues in poultry tissues in Iran: a systematic review and meta-analysis. Environ Res 2022; 204(Pt B):112038. doi: 10.1016/j.envres.2021.112038 [Crossref] [ Google Scholar]