Avicenna Journal of Clinical Microbiology and Infection. 11(2):51-54.
doi: 10.34172/ajcmi.3553
Original Article
Anthrax Epidemıc Management in an Eastern Turkey Region
Ayhan Akbulut 1  , Büşra Tanır 2, *
, Büşra Tanır 2, *  , Ayşe Sağmak Tartar 3
, Ayşe Sağmak Tartar 3  , Şafak Özer Balin 3
, Şafak Özer Balin 3  , Mitat Şahin 4
, Mitat Şahin 4
Author information:
1Professor Doctor, Fırat University Hospital, Infectious Diseases and Clinical Microbiology, Elazığ, Turkey
2Specialist, Elaziğ Fethi Sekin City Hospital, Infectious Diseases and Clinical Microbiology, Elazığ, Turkey
3Associate Professor, Fırat University Hospital, Infectious Diseases and Clinical Microbiology, Elazığ, Turkey
4Professor Doctor, Caucasian University Faculty of Veterinary Medicine, Kars, Turkey
Abstract
Background: Anthrax is a zoonotic disease caused by Bacillus anthracis. It has been known for centuries and remains hyperendemic in certain parts of the world, with occasional epidemic outbreaks. This study aimed to investigate an anthrax outbreak affecting a family and their close contacts in the Akçakiraz town of Elazığ province in eastern Turkey.
Methods: Twenty-two individuals who had contact with a sick animal in Akçakiraz were included in the study. Weekly anamnesis, physical examinations, and follow-ups were conducted over three weeks. Swab samples were collected from the wound sites of hospitalized patients for culture and Gram-staining. Blood and wound samples were sent for polymerase chain reaction (PCR) analysis to detect B. anthracis. A sample was taken from the liver tissue of the infected animal. Statistical analysis was performed using SPSS 25.
Results: Of the 22 individuals, 9 (40.9%) were hospitalized and treated, while 13 (59.1%) were monitored on an outpatient basis. B. anthracis spores were identified in Gram-stained samples from the liver of diseased animal. PCR positivity for B. anthracis was detected in the wound samples of two patients (22.2%), although the PCR results from the blood samples of these patients were negative. No bacterial growth was observed in wound cultures, and gram-staining did not reveal any notable features. All patients were successfully treated. Contacts, family members, and residents were informed and educated about the disease, and necessary public health measures were implemented through coordination with the Provincial Hygiene Board.
Conclusion: Increasing awareness of zoonotic diseases, especially anthrax, is essential. It is crucial to implement societal prevention measures, including controlling animal production, educating at-risk populations in endemic areas, vaccinating animals, preventing contact with sick or deceased animals, and preventing environmental contamination by decontaminating affected areas.
Keywords: Anthrax, Epidemic, Bacillus anthracis
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Akbulut A, Tanır B, Tartar AS, Balin ŞÖ, Şahin M. Anthrax epidemıc management in an eastern turkey region. Avicenna J Clin Microbiol Infect. 2024; 11(2):51-54. doi:10.34172/ajcmi.3553
Introduction
Anthrax is an infection caused by Bacillus anthracis, a motile, catalase-positive, anaerobic, and Gram-positive bacillus (1). This bacterium forms colonies that are white-gray, have a matte appearance, are nonhemolytic, and display a “medusa head” morphology when grown on blood agar (2). In vivo, within a host body, B. anthracis exists in a vegetative state, but it forms subterminal spores in the presence of free oxygen. These spores are highly resistant to desiccation and disinfectants (2,3).
Bacillus anthracis is equipped with a poly-gamma-D-glutamic acid capsule and contains a somatic antigen of polysaccharide structure. It also produces edema and lethal toxins, which are proteins. The capsule and toxins contribute to the bacterium’s virulence and are encoded on plasmids; the pXO1 plasmid encodes the toxin, while the pXO2 plasmid encodes the capsule. Strains that lose the pXO2 plasmid do not form capsules and are non-virulent (2,3).
Anthrax can be transmitted through direct contact with, ingestion, or inhalation of spores from infected animals or contaminated animal products such as meat, skin, and hair. Flies that have fed on the blood of infected animals can also transmit the spores. Anthrax cases can be classified as natural occurrences or as bioterrorism-related incidents. Infection can result in cutaneous, gastrointestinal, or inhalation anthrax, septicemia, and anthrax associated with intravenous drug use (1,2).
High-risk groups for anthrax include individuals residing in endemic areas who are involved in animal husbandry, such as shepherds, butchers, veterinarians, leather workers, shoemakers, fur workers, and those in industries such as drum making, wool processing, carpet weaving, and laboratory work. Additionally, people who consume traditionally prepared raw meat and those who use intravenous drugs are also at increased risk, a concern that has been growing in recent years (1,3-5).
This study seeks to examine the epidemic response to an anthrax outbreak in a town in the Eastern Anatolia region of Turkey in November 2019, where livestock farming plays a significant role in the local economy.
Materials and Methods
Study Population
The study included 22 individuals from the town of Akçakiraz in Elazığ province, eastern Turkey, who had either slaughtered a sick animal, consumed its meat, or came into contact with the animal’s skin, hair, or meat. The participants were selected based on their exposure to the infected animal.
Sample Collection and Laboratory Analysis
A sample taken from the liver tissue of the infected animal showed paracentrally located spores upon Gram-staining, and B. anthracis was successfully cultured (Figure 1). For the human subjects, weekly anamnesis was collected for three weeks, along with physical examinations and follow-ups.
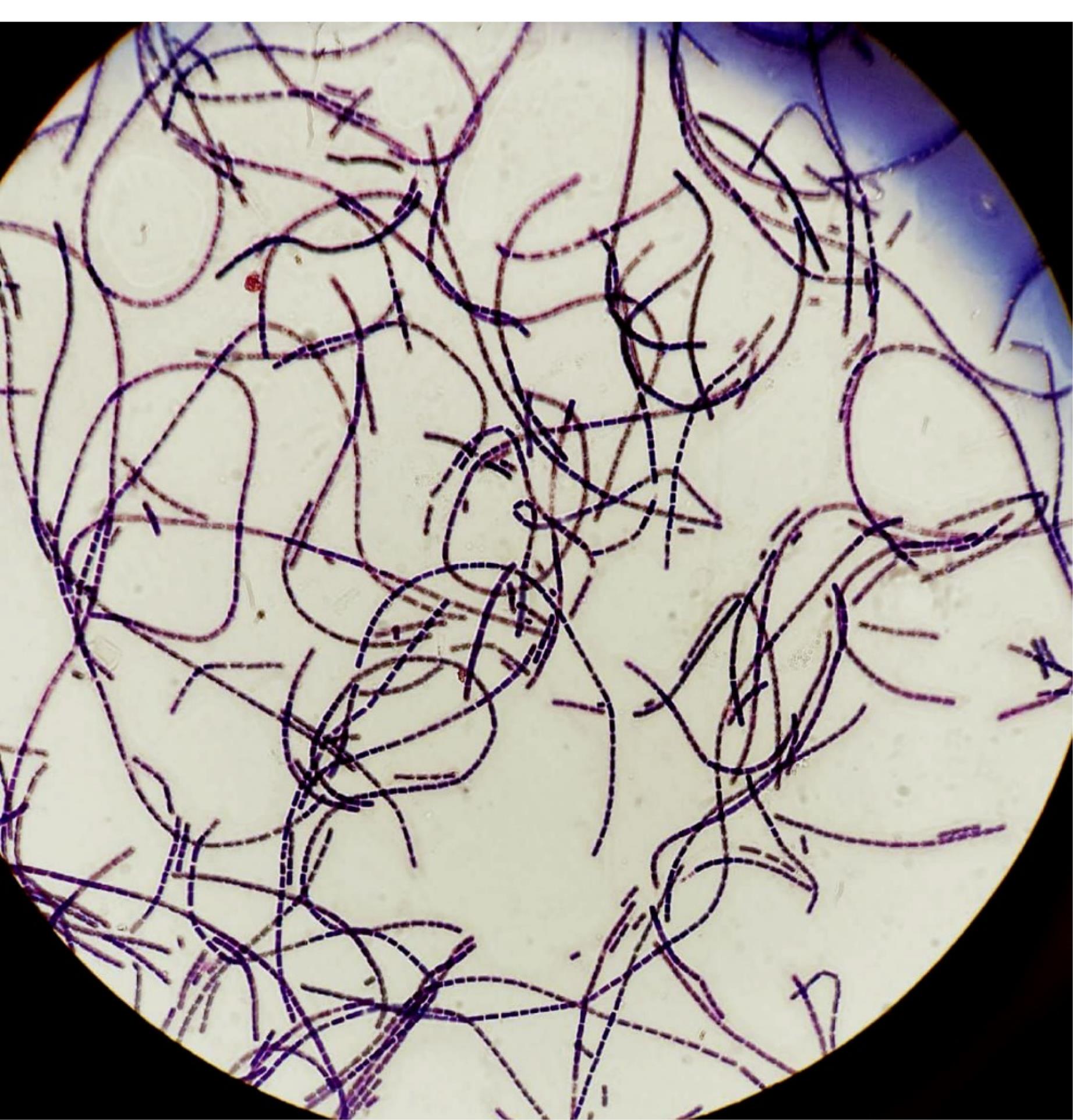
Figure 1.
Paracentrally Located Spores Detected in the Gram-Staining of the Sample Taken From the Liver Tissue of the Infected Animal and Bacillus anthracis Grown in the Culture
.
Paracentrally Located Spores Detected in the Gram-Staining of the Sample Taken From the Liver Tissue of the Infected Animal and Bacillus anthracis Grown in the Culture
The swab samples were collected from the wound sites of hospitalized patients for culture and Gram-staining. Additionally, blood and wound samples were sent for polymerase chain reaction (PCR) analysis to detect B. anthracis. Individual follow-up forms were maintained for each patient.
Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics software, version 25 (SPSS Inc., Chicago, IL, USA). The normality of quantitative data distribution was assessed using the Shapiro-Wilk test. Data that were normally distributed were expressed as means ± standard deviations, while non-normally distributed data were presented as medians (minimum/maximum). Categorical data were summarized as frequencies and percentages. The Mann–Whitney U test was used to compare independent variables due to the sample size in the groups. A P value of less than 0.05 was considered statistically significant for all analyses.
Results
Table 1 presents the basic epidemiological characteristics of the 22 individuals who had contact with infected animals, their exposure history, and their involvement with livestock.
Table 1.
Basic Epidemiological Characteristics of 22 People Monitored
|
|
No. (%)
|
| Adult |
19 (86.4) |
| Child |
3 (13.6) |
| Average age |
33.5 ( ± 13.4) |
| Female |
9 (40.9) |
| Male |
13 (59.1) |
| Contact type with the sick animal hair + skin + meat contact |
13 (59.1) |
| Only eating meat |
7 (31.8) |
| Meat contact |
2 (9.1) |
| With livestock history |
13 (59.1) |
| No livestock history |
9 (40.9) |
Of the 22 individuals monitored, 13 (59.1%) did not develop any post-exposure symptoms, while 9 (40.9%) exhibited symptoms and were diagnosed with cutaneous anthrax. Among the nine individuals diagnosed with cutaneous anthrax, 5 (55.6%) were male and 4 (44.4%) were female, with no statistically significant difference in diagnosis rates between genders (P = 0.245). Additionally, one (33.3%) of the three children developed skin anthrax later.
Symptoms appeared three days after exposure in 6 (66.6%) of the nine symptomatic patients and on days 1, 2, and 6 in the remaining three patients. According to the wound localization, six patients (66.6%) presented with lesions on the hand, two (22.2%) had lesions on the hand, forearm, and arm, and one (11.1%) had a lesion on the nose. All patients exhibited typical cutaneous anthrax symptoms, including edema around the lesion and necrotic eschar tissue. Additionally, 3 (33.3%) patients experienced diarrhea, and 3 (33.3%) developed a fever during follow-up.
The swab samples for culture, PCR, and Gram-staining for B. anthracis were taken from five (55.6%) patients. PCR positivity for B. anthracis was detected in the wound samples of two patients (22.2%), although the PCR results from blood samples of these patients were negative. No bacterial growth was detected in wound cultures, and gram-staining revealed no notable features. Laboratory test results of the nine patients during hospitalization and before discharge are provided in Table 2.
Table 2.
Laboratory Test Results of Patients Diagnosed With Cutaneous Anthrax
|
Parameter
|
During Hospitalization
|
Before Discharge
|
| WBC (/mm3) |
7062 (± 1473.395) |
7585 (± 976.860) |
| CRP (mg/L) |
19.5 (± 22.411) |
1.5 (± 1.603) |
| ESR (mm/h) |
23.714 (± 17.978) |
14.25 (± 10.306) |
| Procalcitonin (ng/mL) |
0.125 (± 0.166) |
0 (± 0.000) |
| AST |
27.111 (± 10.588) |
20.125 (± 7.12) |
| ALT |
20 (± 11.434) |
21.625 (± 12.258) |
Note. WBC: White blood cell; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
A posteroanterior chest X-ray was performed on five patients (55.6%), revealing bilateral hilar lymphadenopathy in two patients with positive PCR tests from wound specimens who presented with wounds on both arms and forearms and significant edema (Figure 2). A comparison with previous chest radiographs from the hospital system confirmed newly developed lymphadenopathies. Additionally, axillary lymphadenopathy was detected in 4 (44.4%) patients.
Among the patients diagnosed with cutaneous anthrax, eight were treated with procaine penicillin G (n = 4, 44.4%) and ciprofloxacin (n = 4, 44.4%), and one was treated with amoxicillin-clavulanic acid (11.1%).
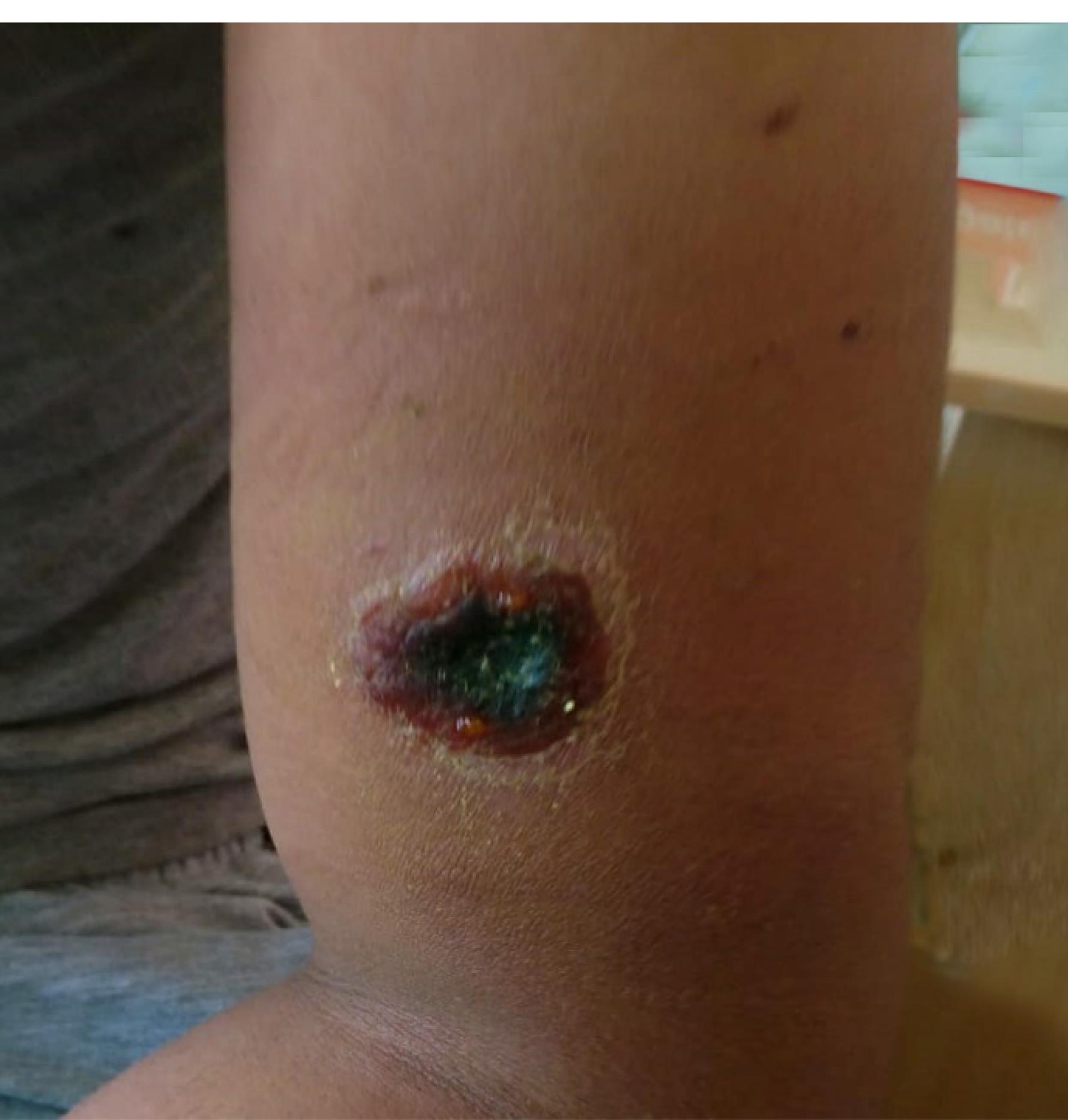
Figure 2.
Arm and Forearm With Significant Edema
.
Arm and Forearm With Significant Edema
Discussion
Anthrax is one of the oldest known zoonoses worldwide, with a history dating back to 5000 BC. B. anthracis was first observed under a microscope in 1838 by Onesime Delafond, and penicillin treatment for anthrax was introduced in 1944 (1). B. anthracis spores contaminate the environment through the slaughter of diseased animals, disposal of carcasses in open areas, and destruction by carnivores. Blood from infected animals can contaminate pastures, leading to the spread of spores by rainwater and wind, causing them to penetrate the soil and disperse widely. Additionally, feeding animals with grass from infected areas in winter can perpetuate the disease cycle (6). Although B. anthracis is primarily found in soil and infects herbivores, it rarely infects humans. However, in cases of human anthrax, infected animals are usually the source, with a parallel increase in animal and human cases. In endemic regions, anthrax is observed throughout the year, with a higher incidence in summer and autumn due to increased grazing activities and transhumance (7).
In Turkey, anthrax is hyperendemic and can cause periodic epidemics (1,4). In our study, the anthrax outbreak occurred in Akçakiraz, a livestock farming center in Elazığ province, during the autumn. The region’s frequent earthquakes and unique soil structure, located on the North Anatolian fault line, contribute to the persistence of anthrax spores in the environment (8). Historical cases, such as the anthrax outbreaks during World War I in the United States and England due to poorly disinfected imported horsehair brushes, demonstrate the persistence of this zoonosis (9).
Anthrax affects all ages and genders, but it is most common in the 20–60 age group actively involved in agriculture and animal husbandry. Children and adults in rural areas are also at risk due to family involvement in animal care and processing of sick animals (10). In our study, three of the 22 individuals exposed to infected animals were children, and one (33.3%) developed cutaneous anthrax.
Anthrax presents in three primary clinical forms based on the site of entry (cutaneous, gastrointestinal, and inhalation). Injection-associated anthrax, recently observed in intravenous drug users, represents another clinical form (1,3-5,11). Cutaneous anthrax, the most common form (95%), is characterized by localized painless necrotic lesions that can spread via lymphatic routes, causing lymph node enlargement and lymphangitis. Painful lymph nodes can occur if a secondary bacterial infection develops (11,12). In our study, all patients developed cutaneous anthrax, with no cases of inhalation or gastrointestinal anthrax. Enlargement of superficial lymph nodes was detected in three (33.3%) patients, and two (22.2%) had pericardiac-perihilar lymph nodes due to significant edema.
The rate of bacillus detection in samples from anthrax lesions typically does not exceed 60–65%, and it significantly declines after antibiotic administration (13). Denk et al (14) detected B. anthracis in only 10.7% of patients using gram-staining and culture methods, with no bacillus production in those who had used antibiotics before hospital admission. Similarly, in our study, samples from five patients did not yield B. anthracis growth, though PCR positivity was detected in two samples, indicating prior antibiotic use.
For anthrax cases without a bioterrorism component, penicillin remains the treatment of choice (13). For patients allergic to penicillin, doxycycline and ciprofloxacin are recommended alternatives. For uncomplicated cutaneous anthrax, oral doxycycline or ciprofloxacin is appropriate, while systemic anthrax requires intravenous therapy combined with another suitable antibiotic (2). In our study, patients were treated with procaine penicillin G, ciprofloxacin, and amoxicillin-clavulanic acid. Ciprofloxacin and amoxicillin-clavulanate were prescribed to three (44.4%) patients by their family physicians, and amoxicillin-clavulanate was prescribed to one (11.1%) by a workplace physician.
Following the anthrax outbreak, public health measures were implemented according to the Regulation on Protection and Struggle against Anthrax Disease published in the Official Gazette of Turkey on November 23, 2011 (15). This involved quarantining affected areas, prohibiting the slaughter and consumption of meat from suspected animals, and safely disposing of contaminated animal products and vegetation. The affected areas were thoroughly disinfected to prevent further contamination.
Conclusion
Therefore, anthrax disease, especially in the eastern and southeastern Anatolia regions, is perceived as endemic in Turkey. Accordingly, it is necessary to raise awareness of the zoonotic disease as well as societal prevention to keep animal production under control, educate the people in the risk group living in endemic areas, deal with livestock, administer vaccines to animals, prevent contact with sick or deceased animals and their attachments, and prevent environmental contamination by identifying and decontaminating the pastures and areas.
Acknowledgements
We would like to thank Professor Mehmet Doğanay, Head of the Department of Infectious Diseases at Lokman Hekim University, for his vast knowledge and experience.
Authors’ Contribution
Conceptualization: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Data curation: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Formal analysis: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Investigation: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Methodology: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Project administration: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Resources: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Software: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Supervision: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Validation: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Visualization: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Writing–original draft: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Writing–review & editing: Ayhan Akbulut, Büşra Tanır, Ayşe Sağmak Tartar, Şafak Özer Balin, Mitat Şahin.
Competing Interests
There was no conflict of interests among the authors in this study.
Ethical Approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Firat University Faculty of Medicine (decision dated May 27, 2021, session number 2021/7-18).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Doganay M, Demiraslan H. Human anthrax as a re-emerging disease. Recent Pat Antiinfect Drug Discov 2015; 10(1):10-29. doi: 10.2174/1574891x10666150408162354 [Crossref] [ Google Scholar]
- Turnbull PC. Anthrax in Humans and Animals. Geneva: World Health Organization; 2008.
- Doganay M. Anthrax. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. China: Elsevier; 2017. p. 1123-8.
- Merİç M, Wİllke A. Anthrax in Gebze, Turkey. Turk J Infect 2008; 22(1):1-9. [ Google Scholar]
- Doganay M, Metan G. Human anthrax in Turkey from 1990 to 2007. Vector Borne Zoonotic Dis 2009; 9(2):131-40. doi: 10.1089/vbz.2008.0032 [Crossref] [ Google Scholar]
- Sahin M, Celebi O, Buyuk F. Anthrax in domestic animals and struggle studies on anthrax in Kars region. 2nd International Congress of Veterinary Microbiology; 16-19 October 2018; Antalya/ Turkey. P. 208.
- Kadanalı A, Toptanoğlu S, Aktaş D, Şahin M, Akbulut A, Kılıç S. Current anthrax status report in Turkey 2020. Turkish Journal of Hygiene and Experimental Biolog 2020; 77(Supplement-2):1-20. [ Google Scholar]
- Kutmanova A, Doganay M, Zholdoshev S. Human anthrax in Kyrgyz Republic: epidemiology and clinical features. J Infect Public Health 2020; 13(8):1161-5. doi: 10.1016/j.jiph.2020.02.043 [Crossref] [ Google Scholar]
- Szablewski CM, Hendricks K, Bower WA, Shadomy SV, Hupert N. Anthrax cases associated with animal-hair shaving brushes. Emerg Infect Dis 2017; 23(5):806-8. doi: 10.3201/eid2305.161554 [Crossref] [ Google Scholar]
- T.C. Ministry of Health, General Directorate of Public Health, Turkey Zoonotic Diseases Action Plan 2019-2023.
- Oğütlü A. Anthrax. J Exp Clin Med 2012; 29(3S):S155-62. doi: 10.5835/jecm.omu.29.s3.011 [Crossref] [ Google Scholar]
- Doğanay M, Perçin D. Bacillus anthracis and other Bacillus species. In: Infectious Diseases and Microbiology. Istanbul: Nobel Medicine Bookstores; 2017. p. 1834-41.
- Lucey D. Bacillus anthracis (Anthrax). In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier-Churchill Livingstone; 2005. p. 2485-93.
- Denk A, Tartar AS, Ozden M, Demir B, Akbulut A. Cutaneous anthrax: evaluation of 28 cases in the Eastern Anatolian region of Turkey. Cutan Ocul Toxicol 2016; 35(3):177-80. doi: 10.3109/15569527.2015.1067818 [Crossref] [ Google Scholar]
- Regulation on Protection and Struggle Against Anthrax Disease. Official Gazette. November 23, 2011. Available from: http://www.resmigazete.gov.tr/eskiler/2011/12/20111223-5.htm. Accessed August 31, 2018.