Avicenna Journal of Clinical Microbiology and Infection. 11(3):119-124.
doi: 10.34172/ajcmi.3548
Original Article
Effects of Propranolol and Lactobacillus rhamnosus on Bone Tissue and Blood Parameters in Ovariectomized Rats
Zahra Abasian 1, Mahnoosh Fatemi 1, *  , Fereshte Ghandehari 2
, Fereshte Ghandehari 2 
Author information:
1Department of Biology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
2Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
Abstract
Background: Osteoporosis is a chronic metabolic disease that leads to decreased bone mass due to an imbalance in bone resorption/formation. The aim of this research was to evaluate the effect of Lactobacillus rhamnosus and propranolol on disorders caused by this disease.
Methods: Forty female rats (250±15 g) were divided into control and ovariectomy groups, as well as ovariectomy treated with probiotics, propranolol, and probiotics with propranolol groups. Blood parameters, concentration of parathyroid hormone, alkaline phosphatase (ALP) activity, and bone calcium (Ca) and phosphorus (P) levels were measured after the treatment period. The histopathological changes in bone were compared among the experimental groups.
Results: No significant changes were observed in the blood parameters among the groups. Despite the significant increase in the parathyroid hormone and ALP levels in ovariectomized rats, in the treatment groups (especially treatment with both probiotics and propranolol), the level of these two factors decreased significantly. Ca and P concentrations also showed a significant increase in the treatment group with probiotics and propranolol compared to the ovariectomy group. Histopathological studies demonstrated an increase in the thickness of trabeculae, the number of bone cells, and the repair of the diaphysis in rats treated with probiotics and propranolol.
Conclusion: It seems that L. rhamnosus and propranolol, by inhibiting the stimulation of the sympathetic system and increasing the reabsorption of minerals from the intestine, led to the inhibition of osteoclastogenesis and the increase of the bone mineral content, followed by the improvement of disorders caused by osteoporosis.
Keywords: Lactobacillus rhamnosus, Propranolol, Osteoporosis, Rats
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Abasian Z, Fatemi M, Ghandehari F. Effects of propranolol and Lactobacillus rhamnosus on bone tissue and blood parameters in ovariectomized rats. Avicenna J Clin Microbiol Infect. 2024; 11(3):119-124. doi:10.34172/ajcmi.3548
Introduction
Osteoporosis is a chronic metabolic disease related to the imbalance of bone resorption/formation, which can be recognized by decreased bone mass and bone strength, increased risk of bone fracture, and changes in its microarchitecture (1). Osteoporosis is of two primary and secondary types. The activity of osteoclasts increases in the primary type, while their apoptosis represents a decrease. Secondary osteoporosis occurs as a result of decreased estrogen levels following ovariectomy and menopause. This type of osteoporosis is the most common type of osteoporosis, which is associated with a high risk of bone fracture (2). The prevalence of this disease in Iranian women is reported to be more than 34.5% (3). Currently, the clinical treatments of this disease are divided into three groups, including (a) bone-building drugs such as parathyroid hormone and analog peptides such as Teripat, (b) bone resorption inhibitors such as calcitonin, estrogen, and bisphosphonates, and (c) bone mineralizing drugs such as vitamin D and calcium (Ca) and magnesium supplements (4).
Unfortunately, despite such treatment methods, long-term use of these drugs leads to disorders in non-bone sites, including cardiovascular diseases, osteonecrosis, and increased risk of breast and endometrial cancer (1,5). Therefore, trying to prevent this disease seems to be a more economical and safer solution. Considering that research has shown that osteoporosis is common in a group of diseases such as celiac disease, bowel syndrome, diabetes, and chronic liver diseases (6), researchers focusing on the gut-bone axis have suggested that the regulation of intestinal flora is directly related to bone homeostasis. In other words, the improvement of the intestinal microbiota contributes to the correct metabolism of bone and inhibition of osteoporosis spread (7).
Intestinal flora consists of a group of non-pathological microorganisms called probiotics. Probiotics include a large family of Bifidobacteria, Lactobacilli, and yeasts. Researchers have reported that the increase in the expression of pro-inflammatory cytokines due to the reduction of estrogen levels is inhibited by consuming sufficient amounts of these microorganisms. Thus, probiotics inhibit the activity of inflammatory osteoclasts and prevent the loss of bone mass (2,8,9). In addition to the relationship between intestinal flora and bone metabolism, the effect of the sympathetic system on bone remodeling has been proven. The stimulation of this system increases bone resorption and inhibits its formation (10). The presence of beta-adrenergic receptors on osteoblasts and osteoclast-like cells has been confirmed, and it has been determined that the treatment of these cells with beta-adrenergic agonists leads to inhibiting the alkaline phosphatase (ALP) activity and increasing the activity of osteoclasts and bone resorption (11). Accordingly, it seems that the antagonists of these receptors can be therapeutic targets for improving bone defects. The present study investigated the possible effects of propranolol and Lactobacillus rhamnosus (as a probiotic) on the improvement of osteoporosis symptoms in ovariectomized rats.
Materials and Methods
Cultivation of Lactobacillus rhamnosus
The bacterial strain with ID PTCC1637 was purchased from the Microbial Bank of Iran Scientific and Industrial Research Organization. After the incubation of bacteria in an MRS broth medium, a suspension was prepared from the sample of liquid microbial culture, and the absorption of the suspension was read at a wavelength of 630 nm. This turbidity is equivalent to 1.5 × 108 CFU/mL of bacteria (half McFarland standard) (12).
Animal Grouping
Forty adult female rats (250 ± 15 g) were obtained from the Animal House of Azad University, Falavarjan Branch, and kept in standard conditions (temperature 23 ± 3 °C and humidity 45 ± 5%). The rats were classified into 5 groups. The rats in the control group underwent surgery without ovariectomy. Three weeks after surgery, rats received physiological serum (1 mL) intragastrically for nine weeks (3 times a week). The rats of the ovariectomy group were ovariectomy, and three weeks after the surgery, they received the same physiological serum as the control group. L. rhamnosus-treated rats were treated with L. rhamnosus (1.5 × 108 CFU/mL) intragastrically (3 times a week for nine weeks) three weeks after ovariectomy. Rats in the fourth group were treated with propranolol (0.15 mg/kg) (13), similar to the previous group. The rats of the fifth group were treated with L. rhamnosus and propranolol three weeks after the ovariectomy, similar to the previous two groups.
Measurement of Hematological Parameters
After the end of the test period, blood was taken from the hearts of the anesthetized animals and transferred to tubes containing anticoagulants. The cell number and blood indices were measured by the AS510 Hematology Analyzer (Italy).
Measurement of Alkaline Phosphatase Activity
According to the protocol of the kit (Pars Azmun, Iran), reagents one and two were prepared and mixed with the blood serum of the rats of each group. The absorption of the mixture was measured at a wavelength of 405 nm using an enzyme-linked immunosorbent assay (ELISA) reader.
Calcitonin Hormone Measurement
Briefly, according to the protocol ab289835-Rat Calcitonin ELISA Kit, 100 µL of each serum sample was added to the well and incubated for 90 minutes at 37 °C. Then, the contents of the wells were drained, and 100 µL of the active solution of the biotinylated detection antibody was immediately added to each well. After washing, horseradish peroxidase conjugate solution was added to each well and incubated for 30 minutes. Then, 90 µL of the substrate reagent was added to each well and incubated for 15 minutes. A stop solution was added to each well, and the absorbance was read at 450 nm.
Measurement of Parathyroid Hormone
According to the kit protocol (Monokit Company), the sample, calibrator, and control were added to the wells. Next, the biotin-conjugated solution and parathyroid hormone (PTH)-conjugated enzyme solution were added to them. After washing, the substrate solution was added to the wells. Subsequently, the stop solution was added, and the absorption was measured at a wavelength of 450 nm.
Measurement of Bone Calcium and Phosphorus Density
For this purpose, 1 mg of the dried bone sample was placed in perchloric acid and concentrated nitric acid in a ratio of 1:4. After 48 hours, the samples were gently heated to remove excess acid. Finally, each sample was diluted with 1 mL of deionized water, and the Ca and phosphorus (P) levels in the samples were measured by the atomic absorption spectrophotometer (14).
Histological Examination of Bone Tissues
A part of the leg bone of the experimental groups was separated and transferred to 10% formalin. After decalcification, dehydration, molding, and cutting of bone tissue sections, the samples were stained with hematoxylin and eosin and evaluated by a light microscope (14).
Statistical Analysis
The data were prepared as means ± standard deviations (SD) and subjected to statistical analysis of variance using SPSS software. The significance level was considered as P≤ 0.05.
Results
Comparison of Blood Parameters Between Groups
According to the results (Table 1), there was no significant change in hematological parameters between the experimental groups in comparison to the control group. Compared to the control group, ALP activity demonstrated a significant increase in all groups. This increase was at the highest level in the ovariectomy group and the lowest level in the group of rats treated with propranolol and probiotics. The serum calcitonin concentration in the experimental groups showed no significant change compared to the control group. The parathyroid hormone concentration in ovariectomized- and propranolol-treated rats increased significantly (P ≤ 0.001 and P ≤ 0.05, respectively) in comparison to the control group. The level of this hormone in the two groups treated with probiotics and probiotics and propranolol revealed no significant difference compared to the control group.
Table 1.
The Level of Factors in the Blood of the Experimental Groups
|
Blood Parameters
|
Control
|
Ovariectomy
|
A
|
B
|
C
|
| WBC (103/µL) |
5.10 ± 0.32 |
5.30 ± 1.6 |
6.60 ± 1.7 |
7.07 ± 0.78 |
7.70 ± 2.70 |
| RBC (106/µL) |
7.52 ± 0.87 |
7.26 ± 0.75 |
7.48 ± 0.66 |
7.25 ± 0.49 |
7.21 ± 0.27 |
| HGB (g/dL) |
14.70 ± 1.24 |
14.12 ± 1.26 |
15.00 ± 0.48 |
14.55 ± 0.77 |
14.48 ± 1.04 |
| HCT (%) |
43.83 ± 4.74 |
41.62 ± 3.02 |
43.01 ± 3.35 |
41.8 ± 2.50 |
41.92 ± 2.08 |
| MCV (fL) |
58.40 ± 0.52 |
57.25 ± 2.02 |
57.53 ± 1.49 |
57.62 ± 0.89 |
58.08 ± 1.55 |
| MCH (pg) |
19.6 ± 1.25 |
19.47 ± 1.30 |
20.17 ± 1.18 |
20.07 ± 0.50 |
20.05 ± 0.83 |
| MCHC (g/dL) |
33.6 ± 1.77 |
34.00 ± 1.41 |
34.95 ± 1.57 |
34.82 ± 0.39 |
34.52 ± 1.12 |
| PLT (µL) |
9.41 ± 0.46 |
9.99 ± 0.64 |
8.03 ± 0.26 |
6.29 ± 0.35 |
7.65 ± 0.36 |
| ALP (mg/mL) |
124.60 ± 10.64 |
276.83 ± 48.59*** |
180.17 ± 13.10** |
141.67 ± 2.87* |
173.83 ± 15.21* |
| Parathormone (Au/mL) |
1.74 ± 0.49 |
6.11 ± 0.90*** |
3.05 ± 0.36* |
2.40 ± 0.97 |
2.05 ± 0.34 |
| Calcitonin (ng/mL) |
1.39 ± 0.72 |
1.12 ± 0.41 |
1.48 ± 0.50 |
2.03 ± 0.55 |
1.22 ± 0.27 |
| Calcitonin (ng/mL) |
1.39 ± 0.72 |
1.12 ± 0.41 |
1.48 ± 0.50 |
2.03 ± 0.55 |
1.22 ± 0.27 |
Note. WBC: White blood count; RBC: Red blood cell; HGB: Hemoglobin; HCT: Hematocrit; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MCHC: Mean cell hemoglobin concentration; PLT: Platelet; ALP: Alkaline phosphatase; SD: Standard deviation. Data are represented as means ± SD. The significance level is defined as *P≤ 0.05, **P≤ 0.01, ***P≤ 0.001. A: propranolol treated group B: L. rhamnosus treated group C: L. rhamnosus and propranolol treated group.
Comparison of Phosphorus and Calcium Content Between Groups
Bone Ca content was significantly lower in the ovariectomized and propranolol-treated rats (P≤ 0.001and P≤ 0.05, respectively) than the control group. However, the Ca level in the two treatment groups with L. rhamnosus alone and L. rhamnosus with propranolol demonstrated no significant difference from the control group (Figure 1). According to Figure 2, P content in all groups decreased compared to the control group. However, after comparing the content of this element in the treatment groups compared to the ovariectomy group, a significant increase in P bone density was observed in rats treated with L. rhamnosus and propranolol (P≤ 0.01) and those treated with L. rhamnosus alone (P≤ 0.05).
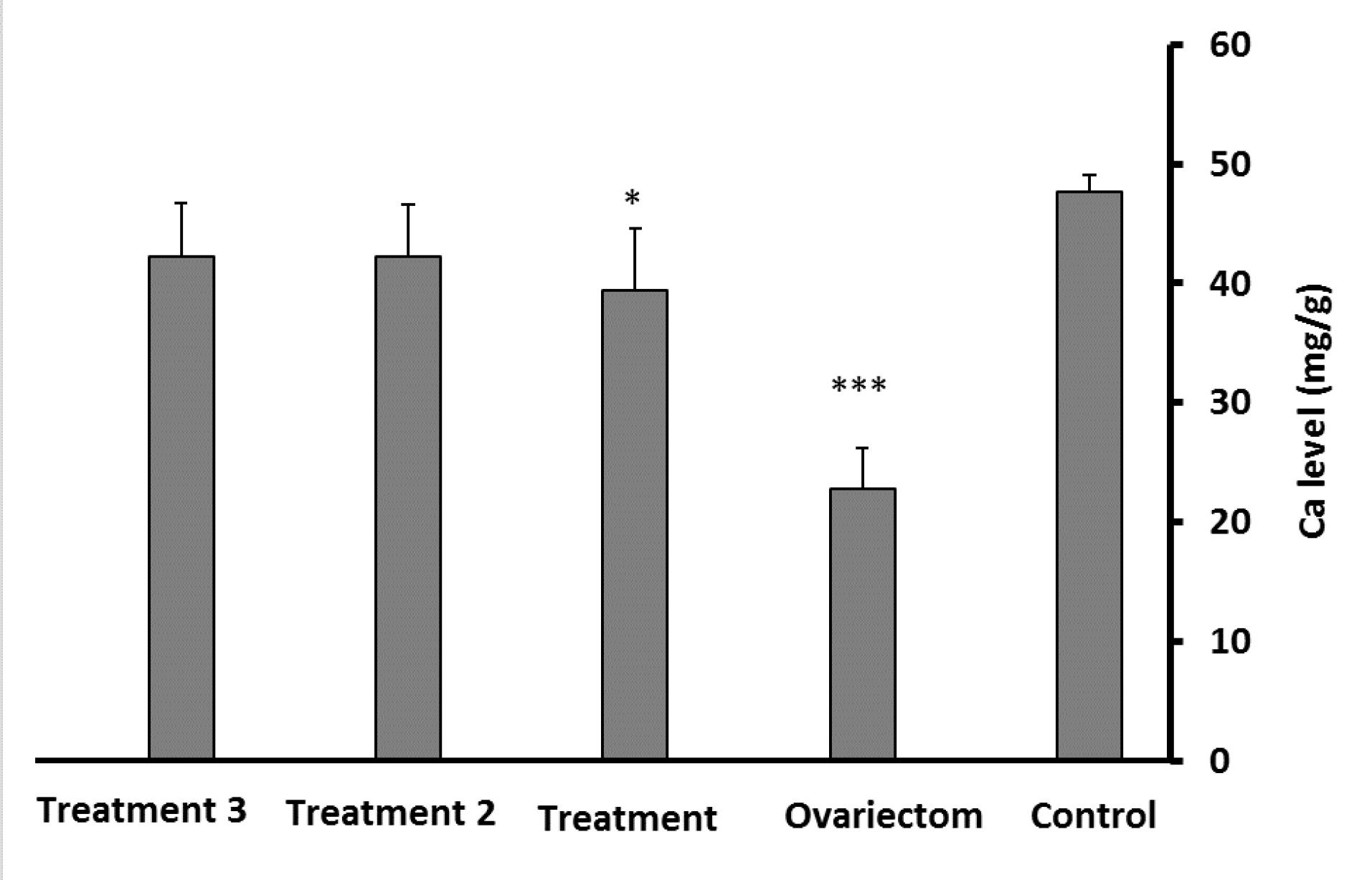
Figure 1.
Comparison of Calcium Levels in the Experimental Groups Compared to the Control Group. Note. L. rhamnosus: Lactobacillus rhamnosus. Data are expressed as means ± standard deviations. The significance level is considered at *P ≤ 0.05 and***P ≤ 0.001.A: Propranolol-treated group, B:L. rhamnosus-treated group, and C: L. rhamnosus- and propranolol-treated group
.
Comparison of Calcium Levels in the Experimental Groups Compared to the Control Group. Note. L. rhamnosus: Lactobacillus rhamnosus. Data are expressed as means ± standard deviations. The significance level is considered at *P ≤ 0.05 and***P ≤ 0.001.A: Propranolol-treated group, B:L. rhamnosus-treated group, and C: L. rhamnosus- and propranolol-treated group
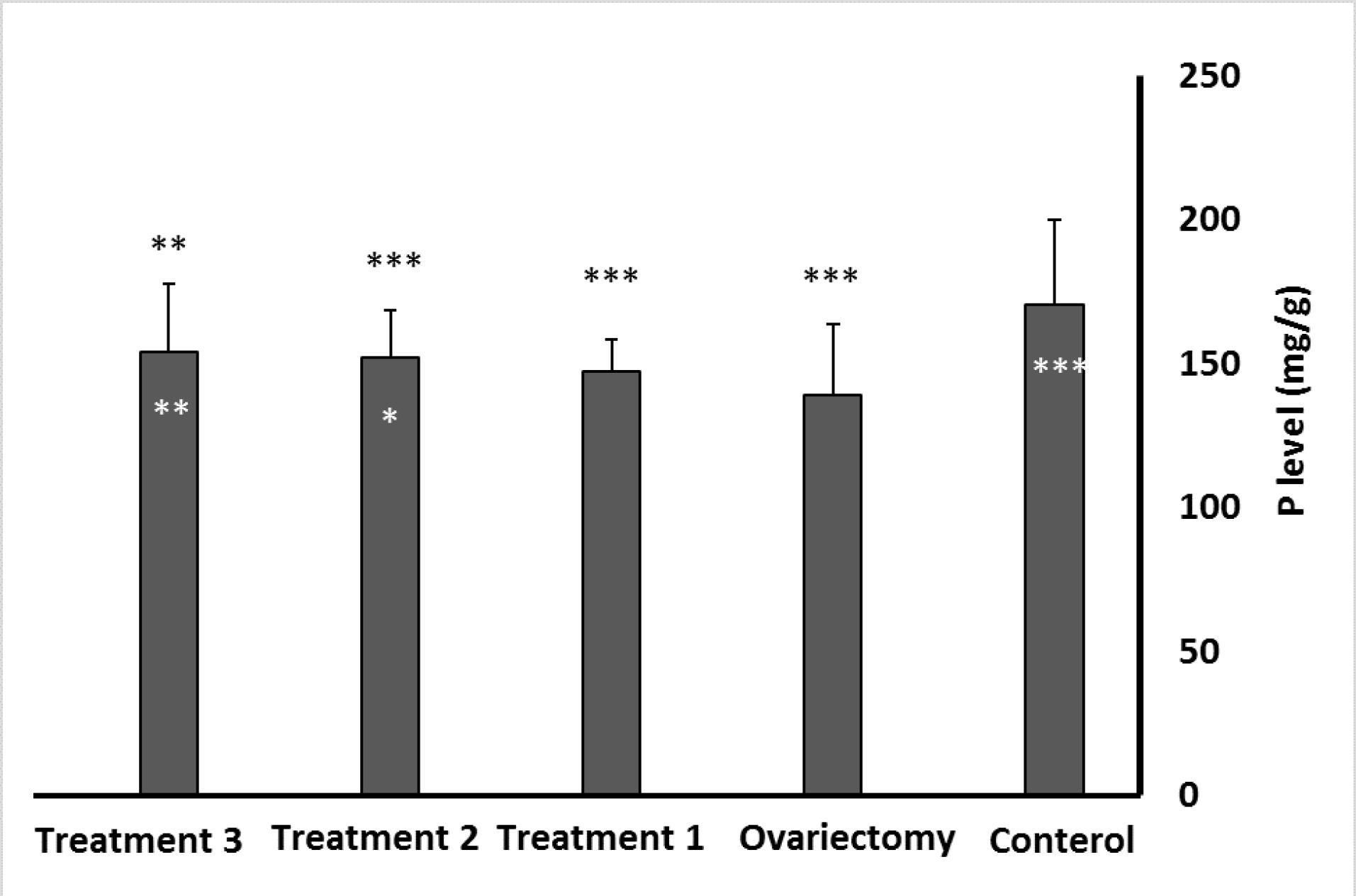
Figure 2.
Comparison of Phosphorus Levels in the Experimental Groups Compared to the Control Group. Note. Data are expressed as means ± standard deviations. The significance level is considered t *P ≤ 0.05, **P ≤ 0.01, and***P ≤ 0.001. The stars above the error bar indicate the comparison of the groups with the control group, and the stars below represent the comparison of the ovariectomy group with other groups
.
Comparison of Phosphorus Levels in the Experimental Groups Compared to the Control Group. Note. Data are expressed as means ± standard deviations. The significance level is considered t *P ≤ 0.05, **P ≤ 0.01, and***P ≤ 0.001. The stars above the error bar indicate the comparison of the groups with the control group, and the stars below represent the comparison of the ovariectomy group with other groups
Comparison of Histopathological Results Between Groups
Figures 3A, 3C, and 3E show a part of the metaphysis in the control, ovariectomy group, and ovariectomy group treated with L. rhamnosus and propranolol, respectively. In the control group, bone trabeculae with normal thickness and texture are found around the fat cavities. However, irregularity and destruction of trabeculae and reduction of their thickness are detected in ovariectomized rats. In the L. rhamnosus- and propranolol-treated group, the structure of the metaphyseal tissue is somewhat similar to that of the metaphyseal tissue in the control group. Figures 3B, 3D, and 3F display parts of the bone diaphysis in the control group, ovariectomy, and the treated group with L. rhamnosus and propranolol, respectively. In Figure 3B, the thickness of the tissue is normal, and many lacunae contain osteocytes. However, the destruction of parts of the diaphysis and lacunae without cells is evident in Figure 3D. The number of cell-containing lacunae and the thickness of bone tissue are somewhat similar to those in the control group (Figure 3F). It should be noted that in this study, ten fields of view were randomly selected from each slide, and the subject of study was determined accordingly. The repeated tissue images were not inserted since the tissue appearance of the propranolol treatment group was almost similar to that of the ovariectomy group, and the tissue figures of the L. rhamnosus group were somewhat similar to those of the L. rhamnosus with the propranolol group.
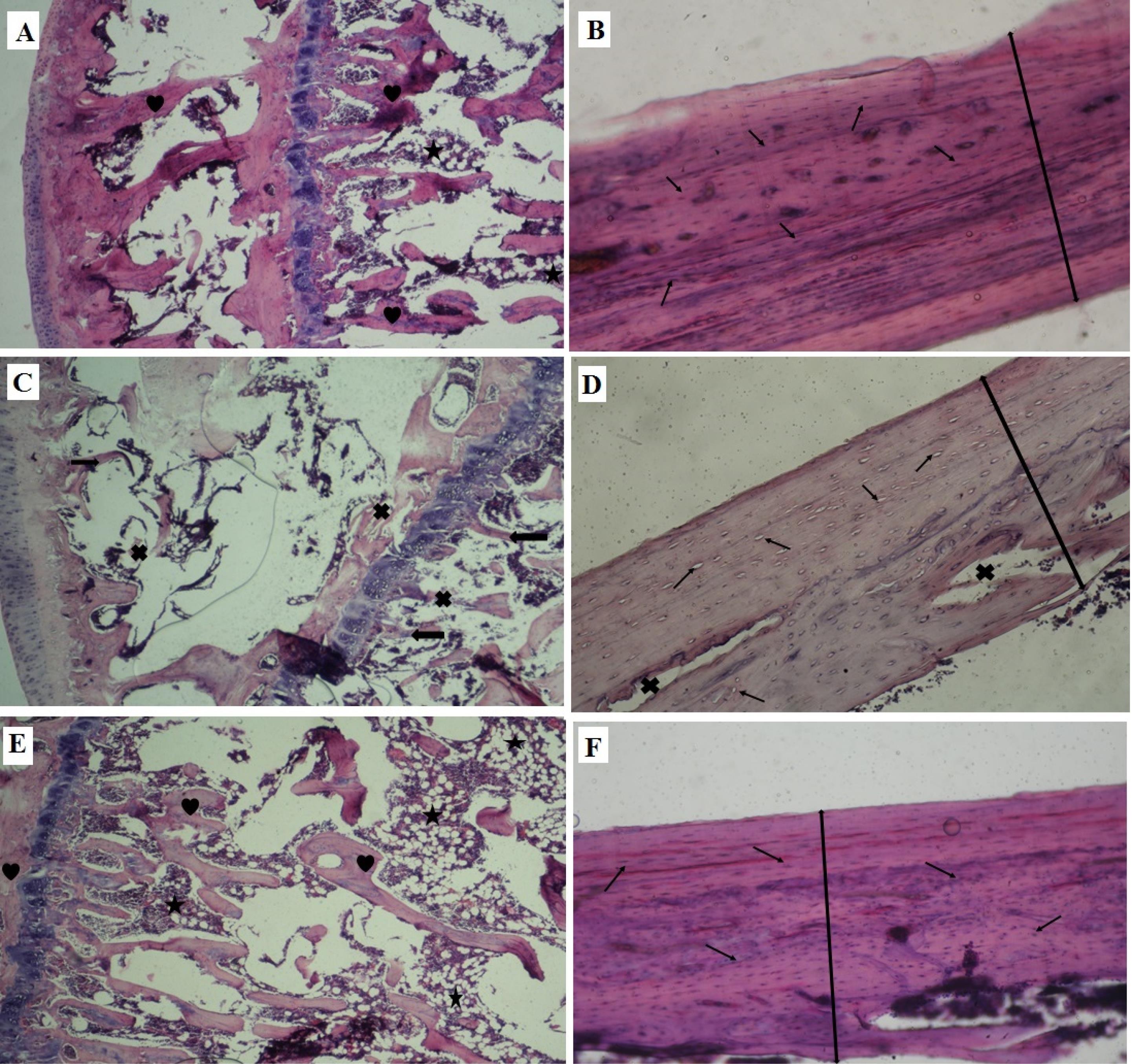
Figure 3.
A, C, and E (at × 100 Magnification), as Well as B, D, and F (at × 400 Magnification) Displaying Parts of the Metaphysis and the Bone Diaphysis in the Control Group, Ovariectomy Group, and Group Treated With L. rhamnosus and Propranolol, Respectively. Note. L. rhamnosus: Lactobacillus rhamnosus. Heart: Normal trabeculae; Cross: Destruction of trabeculae; Star: Adipose tissue; Arrows: Thin trabeculae; Thin arrow: Lacuna and cells inside it
.
A, C, and E (at × 100 Magnification), as Well as B, D, and F (at × 400 Magnification) Displaying Parts of the Metaphysis and the Bone Diaphysis in the Control Group, Ovariectomy Group, and Group Treated With L. rhamnosus and Propranolol, Respectively. Note. L. rhamnosus: Lactobacillus rhamnosus. Heart: Normal trabeculae; Cross: Destruction of trabeculae; Star: Adipose tissue; Arrows: Thin trabeculae; Thin arrow: Lacuna and cells inside it
Discussion
In this study, osteoporosis was induced by ovariectomy in rats. Three weeks after surgery, a decrease in the thickness of trabeculae, a decrease in osteocytes, and the destruction of bone tissue were observed in these animals. Estrogens and androgens increase intestinal permeability to inorganic substances by loosening gap junctions. These substances contribute to bone matrix stability (15). Therefore, the decrease in the sex hormone levels in the present research was probably the key factor in the abnormality of the bone tissue related to the decrease in the absorption of inorganic substances in this tissue. The reduction of Ca and P density in the bone of ovariectomy rats confirmed these results. In addition, in these rats, ALP activity was found at the highest level compared to other groups. ALP plays an important role in bone calcification, so that it leads to the mineralization of bone matrix and acceleration of bone turnover by inhibiting the activity of the pyrophosphate enzyme and supplying inorganic phosphate to bone (16,17). Considering that many ALP isozymes are derived from the bone and liver, measuring the level of this factor can be an indicator for the diagnosis of bone disorders. A group of studies have reported an increase in serum ALP in osteomalacia, Paget’s disease, and secondary osteoporosis (16,18).
In the present study, a significant increase in the PTH level was observed in ovariectomized rats, while the calcitonin level showed no significant change. PTH increases the activity of osteoclasts, subsequently increasing bone resorption and the transfer of inorganic substances (especially Ca and P) into the blood (19). In ovariectomized rats, the increase in PTH was probably due to the decrease in the absorption of mineral elements necessary for bone formation due to the decrease in sex hormones. After the treatment of rats with L. rhamnosus, PTH concentration and ALP activity decreased, while bone P and Ca density indicated an increase. Histological images also demonstrated relative improvements in the bone structure. Gholami et al found an increase in bone mineral density in the femurs and vertebrae of rats treated with a mixture of three probiotics, L. casei, L. reuteri, and L. acidophil, compared to the control group (7). In one study, male rats were treated with L. reuteri for four weeks and represented a significant increase in the femur and vertebrae thickness, as well as an increase in bone mineral density compared to control group rats (20).
The treatment of rats with L. rhamnosus probably led to a decrease in the secretion of PTH by increasing the reabsorption of inorganic salts from the intestine, resulting in ultimately bone remodeling by increasing the Ca and P absorption.
By producing lactic acid and other organic acids, lactobacilli decrease the pH of the intestine and thus lead to the conversion of insoluble inorganic salts into solution and increase their intestinal absorption (6).
In addition to the role of intestinal microorganisms in bone metabolism, epidemiological studies have reported that there is a direct relationship between high blood pressure and bone loss, and beta blockers are effective in improving bone function (21). Considering the confirmation of the presence of beta-adrenergic receptors on the surface of bone cells, especially osteoclasts (10), in the present study, a group of ovariectomized rats were treated with propranolol as a classical beta-blocker. According to the obtained data (Table 1), a relative improvement in the level of examined factors was detected in these rats. Nonetheless, in the histopathological results, there were no signs of bone structure improvement. Wu et al observed the beginning of implant osseointegration in rabbits after treatment with propranolol and suggested that osteogenic differentiation of osteoblasts by propranolol led to increased bone regeneration (10). Okada et al also found a decrease in periodontal bone resorption after treatment with propranolol without any adverse effects on cardiac function (22). Conversely, Gunes et al observed no significant changes in ALP activity, Ca, P, and creatine levels after propranolol therapy (21). It seems that the effect of propranolol on bone function depends on different factors, including the species of the animal and the drug dosage. Rodrigues et al concluded that propranolol in low doses has no effect on hemodynamic parameters and only leads to osteoclastogenesis by improving inflammatory markers (23). A group of researchers proved that propranolol in a low dose (0.1–5 mg) alone has no effect on improving bone function (10,23). Therefore, in the present study, a group of rats were treated with propranolol and L. rhamnosus, and the results revealed that the levels of factors in these rats were significantly improved compared to those in rats treated with propranolol or probiotics alone. Histological images also showed an increase in the thickness of the metaphyseal trabeculae, the number of osteocytes, and the diaphysis diameter. In line with these results, in two separate studies, it was observed that propranolol with ginger (24) and rifampicin (25) is much more effective than propranolol alone in reducing ALP and ALT activity in treated animals. Treyball et al also reported an increase in bone mineralization and improved bone function following treatment with propranolol and PTH (26).
Conclusion
The results of this research confirmed that the treatment of ovariectomized rats with L. rhamnosus, especially with L. rhamnosus and propranolol, led to bone mineralization following the reduction of ALP activity and PTH level. This probiotic could increase the intestinal absorption of soluble inorganic salts, and propranolol could reduce the secretion of inflammatory cytokines, subsequently resulting in maintaining bone density and inhibiting osteoclastogenesis. To confirm this claim, many additional tests, including the evaluation and comparison of the density of inorganic salts in the feces and the serum level of various cytokines (e.g., interleukin 6, interleukin-1β, and tumor necrosis factor-α), are necessary between the experimental groups.
Acknowledgements
We would like to thank the Faculty of Basic Sciences, Islamic Azad University, Falavarjan Branch, for cooperating with us and supplying the experimental equipment.
Authors’ Contribution
Conceptualization: Zahra Abasian, Mahnoosh Fatemi.
Data curation: Mahnoosh Fatemi.
Formal analysis: Mahnoosh Fatemi.
Investigation: Zahra Abasian, Mahnoosh Fatemi.
Methodology: Mahnoosh Fatemi.
Project administration: Mahnoosh Fatemi.
Resources: Mahnoosh Fatemi, Zahra Abasian.
Software: Fereshte Ghandehari.
Supervision: Mahnoosh Fatemi.
Validation: Fereshte Ghandehari.
Visualization: Mahnoosh Fatemi,Fereshte Ghandehari.
Writing–original draft: Zahra Abasian.
Writing–review & editing: Mahnoosh Fatemi, Fereshte Ghandehari.
Competing Interests
The authors declare that there is no conflict of interests.
Ethical Approval
The ethical guidelines on the use of laboratory animals for research were observed, as approved by the Institutional Review Board of the Islamic Azad University at Flavarjan Branch, Flavarjan, Isfahan (Certificate No. IR.IAU.FALA.REC.1396.022).
Funding
The authors received no financial support for this study.
References
- Chen Z, Cai Z, Zhuang P, Li F, Cui W, Li Z. Living probiotic biomaterials for osteoporosis therapy. Biomed Technol 2023; 1:52-64. doi: 10.1016/j.bmt.2022.11.007 [Crossref] [ Google Scholar]
-
Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR. The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr 2017;5(4). doi: 10.1128/microbiolspec.BAD-0015-2016.
- Doosti Irani A, Poorolajal J, Khalilian A, Esmailnasab N, Cheraghi Z. Prevalence of osteoporosis in Iran: a meta-analysis. J Res Med Sci 2013; 18(9):759-66. [ Google Scholar]
- Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet 2019; 393(10169):364-76. doi: 10.1016/s0140-6736(18)32112-3 [Crossref] [ Google Scholar]
- Foessl I, Dimai HP, Obermayer-Pietsch B. Long-term and sequential treatment for osteoporosis. Nat Rev Endocrinol 2023; 19(9):520-33. doi: 10.1038/s41574-023-00866-9 [Crossref] [ Google Scholar]
- Nath A, Molnár MA, Csighy A, Kőszegi K, Galambos I, Huszár KP. Biological activities of lactose-based prebiotics and symbiosis with probiotics on controlling osteoporosis, blood-lipid and glucose levels. Medicina (Kaunas) 2018; 54(6):98. doi: 10.3390/medicina54060098 [Crossref] [ Google Scholar]
- Gholami A, Dabbaghmanesh MH, Ghasemi Y, Koohpeyma F, Talezadeh P, Montazeri-Najafabady N. The ameliorative role of specific probiotic combinations on bone loss in the ovariectomized rat model. BMC Complement Med Ther 2022; 22(1):241. doi: 10.1186/s12906-022-03713-y [Crossref] [ Google Scholar]
- Han HS, Kim JG, Choi YH, Lee KM, Kwon TH, Kim SH. Effect of Lactobacillus fermentum as a probiotic agent on bone health in postmenopausal women. J Bone Metab 2022; 29(4):225-33. doi: 10.11005/jbm.2022.29.4.225 [Crossref] [ Google Scholar]
- Madel MB, Halper J, Ibáñez L, Claire L, Rouleau M, Boutin A. Specific targeting of inflammatory osteoclastogenesis by the probiotic yeast S boulardii CNCM I-745 reduces bone loss in osteoporosis. Elife 2023; 12:e82037. doi: 10.7554/eLife.82037 [Crossref] [ Google Scholar]
- Wu Y, Zhang Q, Zhao B, Wang X. Effect and mechanism of propranolol on promoting osteogenic differentiation and early implant osseointegration. Int J Mol Med 2021; 48(4):191. doi: 10.3892/ijmm.2021.5024 [Crossref] [ Google Scholar]
- Wu H, Song Y, Li J, Lei X, Zhang S, Gao Y. Blockade of adrenergic β-receptor activation through local delivery of propranolol from a 3D collagen/polyvinyl alcohol/hydroxyapatite scaffold promotes bone repair in vivo. Cell Prolif 2020; 53(1):e12725. doi: 10.1111/cpr.12725 [Crossref] [ Google Scholar]
- Fatemi M, Ghandehari F, Ghazanfarpour E, Fatemi Y. The effect of Lactobacillus fermentum against lead-induced oxidative damages in rat kidneys. Iran J Toxicol 2023; 17(1):53-62. doi: 10.32598/ijt.17.1.596.2 [Crossref] [ Google Scholar]
- Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V. Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol 2008; 217(3):819-27. doi: 10.1002/jcp.21564 [Crossref] [ Google Scholar]
- Azari Morchegani B, Fatemi M, Amiri G. Nanohydroxyapatite synthesized from kombucha SCOBY and its effect on ovariectomized-induced osteoporosis in rats. Nanomed J 2024; 11(2):145-54. doi: 10.22038/nmj.2024.75861.1845 [Crossref] [ Google Scholar]
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health?. Curr Osteoporos Rep 2020; 18(3):273-84. doi: 10.1007/s11914-020-00591-6 [Crossref] [ Google Scholar]
- Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res 2015; 27(4):413-8. doi: 10.1007/s40520-014-0296-x [Crossref] [ Google Scholar]
- Vimalraj S. Alkaline phosphatase: structure, expression and its function in bone mineralization. Gene 2020; 754:144855. doi: 10.1016/j.gene.2020.144855 [Crossref] [ Google Scholar]
- Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 2001; 19(6):331-7. doi: 10.1007/s007740170001 [Crossref] [ Google Scholar]
- Tonk CH, Shoushrah SH, Babczyk P, El Khaldi-Hansen B, Schulze M, Herten M. Therapeutic treatments for osteoporosis-which combination of pills is the best among the bad?. Int J Mol Sci 2022; 23(3):1393. doi: 10.3390/ijms23031393 [Crossref] [ Google Scholar]
- McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 2013; 228(8):1793-8. doi: 10.1002/jcp.24340 [Crossref] [ Google Scholar]
- Gunes N, Gül M, Dundar S, Artas G, Kobat MA, Tekin S. Effects of systemic propranolol application on the new bone formation in periimplant guided bone regeneration. J Oral Maxillofac Res 2021; 12(3):e2. doi: 10.5037/jomr.2021.12302 [Crossref] [ Google Scholar]
- Okada Y, Hamada N, Kim Y, Takahashi Y, Sasaguri K, Ozono S. Blockade of sympathetic b-receptors inhibits Porphyromonasgingivalis-induced alveolar bone loss in an experimental rat periodontitis model. Arch Oral Biol 2010; 55(7):502-8. doi: 10.1016/j.archoralbio.2010.04.002 [Crossref] [ Google Scholar]
- Rodrigues WF, Madeira MF, da Silva TA, Clemente-Napimoga JT, Miguel CB, Dias-da-Silva VJ. Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br J Pharmacol 2012; 165(7):2140-51. doi: 10.1111/j.1476-5381.2011.01686.x [Crossref] [ Google Scholar]
- Abdelsameea AA, El-Menshawy SS, Pasha HF, Emara MW. Effects and interactions of ginger and propranolol in pre-hepatic portal hypertensive rats. Clin Exp Pharmacol 2015; 5(4):179. doi: 10.4172/2161-1459.1000179 [Crossref] [ Google Scholar]
- Abrar H, Ahmed MR, Ali AB, Yasin H, Ibrahim S. Effect of propranolol on hepatic blood flow for reduction of the hepatotoxicity of rifampicin in rabbits. Med Forum Mon 2017; 28(7):105-9. [ Google Scholar]
- Treyball A, Bergeron AC, Brooks DJ, Langlais AL, Hashmi H, Nagano K. Propranolol promotes bone formation and limits resorption through novel mechanisms during anabolic parathyroid hormone treatment in female C57BL/6J mice. J Bone Miner Res 2022; 37(5):954-71. doi: 10.1002/jbmr.4523 [Crossref] [ Google Scholar]