Avicenna Journal of Clinical Microbiology and Infection. 11(3):107-112.
doi: 10.34172/ajcmi.3532
Original Article
The Application of Spirulina platensis-Based Green Synthesized Silver Nanoparticles Demonstrating Potent Anti-Shigella flexneri Effects
Sahar Karami 1  , Zahra Heidary 1, Sepideh Khaleghi 2, Sarvenaz Falsafi 3, Mohammad karim Rahimi 4, Shadi Hajrasouliha 5, *
, Zahra Heidary 1, Sepideh Khaleghi 2, Sarvenaz Falsafi 3, Mohammad karim Rahimi 4, Shadi Hajrasouliha 5, * 
Author information:
1Department of Biology, School of Basic Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Biotechnology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
3Department of Microbiology, Faculty of Advenced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
4Department of Microbiology, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
5Herbal Pharmacology Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
Abstract
Background: The emergence of antibiotic-resistant strains of Shigella flexneri, an important cause of Shigellosis, has led to extensive research to find alternative treatment approaches. Therefore, the current study investigated the antibacterial effects of the green synthesized silver nanoparticles (AgNPs) using Spirulina platensis on S. flexneri and the expression of pathogenic genes ipaB, ipaD, ipaH, and qnrS.
Methods: After synthesizing AgNPs using S. platensis, its antibacterial effects on S. flexneri were studied using the microdilution method with 96-well plates. In addition, to determine the minimum bactericidal concentration (MBC), 10 µL of the contents of the minimum inhibitory concentration (MIC) well and the like were swapped on the nutrient agar medium. After RNA extraction, complementary DNA (cDNA) synthesis, and primer design, the expression levels of ipaB, ipaD, ipaH, and qnrS genes were evaluated using the real-time polymerase chain reaction (PCR) technique. The data were analyzed by GraphPad Prism 8.
Results: The MIC of the green synthesized AgNPs was measured as 0.0625 μg/mL, and its MBC was 0.125 μg/mL. The results of RT-PCR analysis indicated a significant decrease in the expression levels of pathogenic genes ipaB, ipaD, ipaH, and qnrS in AgNP-treated S. flexneri.
Conclusion: The green synthesized AgNPs using S. platensis had strong antibacterial effects on S. flexneri, and the action mechanism was attributed to the downregulations of ipaB, ipaD, ipaH, and qnrS genes. In vivo and clinical studies are needed in this respect.
Keywords: Gene Expression, Nanoparticles, Shigella, Silver
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Karami S, Heidary Z, Khaleghi S, Falsafi S,Rahimi MK, Hajrasouliha S. The application of Spirulina platensis based green synthesized silver nanoparticles demonstrated potent anti Shigella flexneri effects by specifically targeting pathogenic gene expressions. Avicenna J Clin Microbiol Infect. 2024; 11(3):107-112. doi:10.34172/ajcmi.3532
Introduction
Infectious diseases are among the most important and common diseases in the world, causing many problems to the health systems of most countries, especially developing countries (1). One of these kinds of diseases is shigellosis, which is caused by Shigella sp. bacteria, especially Shigella flexneri (2), and is an important cause of bacterial gastroenteritis and dysentery (3). About 12.5% of deaths caused by diarrheal diseases are due to Shigella, and its mortality rate is higher in children under 5 years of age (4). Clinical manifestations include diarrhea, dysentery, high fever, abdominal cramps, myalgia, and rectal tenesmus or spasm (5).
The ability of S. flexneri to penetrate epithelial cells is due to the presence of its large invasive plasmid, whose genes are responsible for the coding of invasive proteins (6), including ipaA, ipaB, ipaC,ipaD, and ipaH (7). After the bacteria contact the host cells, IpaB and IpaC inject inosines into the cytoplasm of the cell by creating a pore on the plasma membrane (8). IpaD provides bacteria with the ability to phagocytize (9), and IpaA causes the depolymerization of F-actin by binding to vinculin (10). On the other hand, ipaH protects Shigella from macrophages (11), which can move to the nucleus of the host cell and stimulate the secretion of Shigella proteins (12). One of the acquired genes involved in creating relative resistance to quinolones in Shigella is qnrS, and it protects bacterial DNA by inhibiting the binding of quinolones to DNA gyrase and topoisomerase 4 (13).
The administration of water and electrolytes and prescription of antibiotics such as ampicillin, tetracycline, erythromycin, trimethoprim/sulfamethoxazole, and in severe cases, ciprofloxacin are among the treatment approaches (14). However, the emergence of antibiotic-resistant strains of S. flexneri has reduced the effectiveness of treatments (15,16). Therefore, there is a need for new approaches to the treatment of shigellosis.
Nanoparticles (NPs) have many applications in medicine due to their unique physicochemical and biological properties (17). The synthesis of NPs using chemical approaches is associated with side effects and environmental harms, which limit their application (18). To overcome these problems, NP green synthesis methods using plant extracts have been introduced, which are cost-effective and environmentally friendly (18). One of the widely used NPs is silver (Ag) NP, whose anticancer and antimicrobial properties have been investigated in many studies (19-21). AgNPs have antibacterial properties against gram-positive and gram-negative bacteria, and this compound has shown antimicrobial effects against antibiotic-resistant bacteria (22). For example, AgNPs have shown antibacterial effects on Escherichia coli, Salmonella Typhimurium, Staphylococcus aureus, and Bacillus subtilis, and it seems that the smaller size of this NP is associated with increased antimicrobial activity (23).
Therefore, the current research aimed to investigate the antibacterial effects of green synthesized AgNPs on S. flexneri and evaluate the pathogenic ipaA, ipaD, ipaH, and qnrS gene expressions.
Materials and Methods
Silver Nanoparticle Synthesis
Ethanol extract of Spirulina algae (Spirulife, Esfahan, Iran) was used for the synthesis of AgNPs. For this purpose, 20 g of dry Spirulina powder was dissolved in 200 mL of 96% ethanol and placed in a shaker at 140 rpm for 35 minutes. Then, the solution was filtered with Whatman paper, and the obtained extract was centrifuged at 13 000 rpm for 20 minutes. In addition, 340 mg of AgNO3 (Merck, Germany) was mixed in 100 mL of distilled water and 100 mL of Spirulina extract and placed in a shaker for 24 hours. After observing the color change of the solution and ensuring the complete reduction of Ag ions to AgNPs, the sediment was washed three times using a centrifuge at 13 000 rpm for 20 minutes. Finally, the final sediment was collected after drying at 40 °C for 120 minutes.
Shigella flexneri Culture
Shigella flexneri (ATCC 12022) was obtained from the Microbiology Department of the Pasteur Institute of Iran and cultured in nutrient broth at 32 °C for 24 hours. Then, the bacteria were separated by centrifugation at 4000 rpm, and the McFarland method was used to determine the microbial population. The initial turbidity of the microbial suspension was determined using a 0.5 McFarland solution. The physiological serum was utilized to prepare a microbial population equal to 1.5 × 106 bacteria/mL.
Minimum Inhibitory and Bactericidal Concentrations
The microdilution method based on the CLSI 2017 standard was employed to measure the minimum inhibition concentration (MIC) of AgNO3-NPs (19). Briefly, successive dilutions of AgNO3-NPs in the concentration range of 0.063–32 mg/mL were poured into the wells of 96-well plates, and then 1 mL of the nutrient broth was added to it, along with 1 mL of microbial inoculum (1.5 × 106 bacteria). The plates were incubated for 24 hours at 37 °C. The well containing nutrient broth culture medium with bacteria and the well containing culture medium without bacteria were considered positive and negative controls, respectively.
To determine the minimum bactericidal concentration (MBC), 10 µL from the last well showing no bacterial growth was taken and cultured in the Mueller-Hinton agar medium. The plates were incubated for 24 hours at 37 °C.
Gene Expression
RNA was extracted using the RNX-PLUS method. Briefly, bacteria were trypsinized and separated by centrifuge 48 hours after treatment with AgNPs, and 500 μL of the RNX-PLUS solution was added to the samples. Then, 200 µL of chloroform was added and incubated at 4 °C for 5 minutes and centrifuged at 12 000 rpm for 15 minutes. Complementary DNA synthesis was performed using a kit (BioFact, South Korea) according to the manufacturer’s instructions.
The primer design was implemented using the NCBI (National Center for Biotechnology Information) database and Primer 3 software. The sequences of the primers of ipaD, ipaB, ipaH, and qnrS genes are presented in Table 1.
Table 1.
The Sequences of Primers Used for Measuring the Expression Levels of ipaD, ipaB, ipaH, and qnrS Genes by the RT-PCR Technique
|
Genes
|
Sequence [5'-3']
|
GC%
|
TM (°C)
|
|
ipaB
|
Forward: ACGACTGCTGCAACTAGGAC
Reverse: GGAACAAGCCCTGAATCCGA |
55 |
60 |
|
ipaD
|
Forward: ACGGAGTTTCCGTCGTTACC
Reverse: GAAGCCGAGCTTGATGGAGA |
55 |
60 |
|
ipaH
|
Forward: ACGACTGCTGCAACTAGGAC
Reverse: TGAGATGCTGGAGAATGAGTACC |
50 |
59.6 |
|
qnrS
|
Forward: TCACACATATCGGCACCACA
Reverse: TCGCAAGTTGGCATTGTTGG |
55 |
59.97 |
Note. RT-PCR: Real-time polymerase chain reaction; GC: Guanine-cytosine; TM: Melting temperature.
The expression levels of the studied genes were determined by the RT-PCR technique using the Cyber Green method (Q Rotor-Gene, Qiagen). The 16s rRNA gene was used as the control. The reaction mixture included 7 µL of master mix, 0.5 µL of forward and reverse primers, 5 µL of deionized water, and 1 µL of complementary DNA.
The time-temperature schedule of the RT-PCR machine is provided in Table 2.
Table 2.
The Time-Temperature Schedule of the RT-PCR
|
Steps
|
Temperature (°C)
|
Time
|
| Denaturation and enzyme activation |
95 |
10 minutes |
| Step 1: Denaturation |
95 |
15 seconds |
| Step 2: Annealing |
59 |
25 seconds |
| Step 3: Extension and fluorescence acquiring |
72 |
30 seconds |
| Melting curve analysis |
65-95 |
1°C each step |
Note. RT-PCR: Real-time polymerase chain reaction.
Statistical Analysis
The 2-ΔΔCt method was utilized to analyze the expression levels of ipaD, ipaB, ipaH, and qnrS genes. Further, the gene expressions between the groups were analyzed by an unpaired Pearson t-test at probability levels of P < 0.05.
Results
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
The microdilution method was employed to determine the AgNPs MIC against S. flexneri, and the results revealed that the growth of bacteria decreased with increasing the concentration of AgNPs, and no bacterial growth was observed at the concentration of 0.0625 μg/mL. Therefore, this concentration was considered the MIC of AgNPs. Next, 10 µL of the wells containing 0.0312 μg/mL AgNPs and the like were removed and cultured in the nutrient agar medium, and after 48 hours, it was observed that the bacteria did not grow in the medium containing 0.125 μg/mL AgNPs and the like. Hence, the MBC of AgNPs against S. flexneri was considered at 0.125 μg/mL.
Gene Expression Analysis
ipaB and ipaH
Both ipaB (P = 0.006) and ipaH (P = 0.004) gene expressions in AgNP-treated S. flexneriwere decreased significantly compared to the control (Figure 1). The expression level of ipaH in control was measured 1.18 ± 0.3. However, in AgNP-treated S. flexneri, this expression level was measured 0.33 ± 0.08, indicating the downregulation of ipaH. The same result was found for the ipaB gene, and the expression level was decreased ~3 times compared to untreated S. flexneri(control).
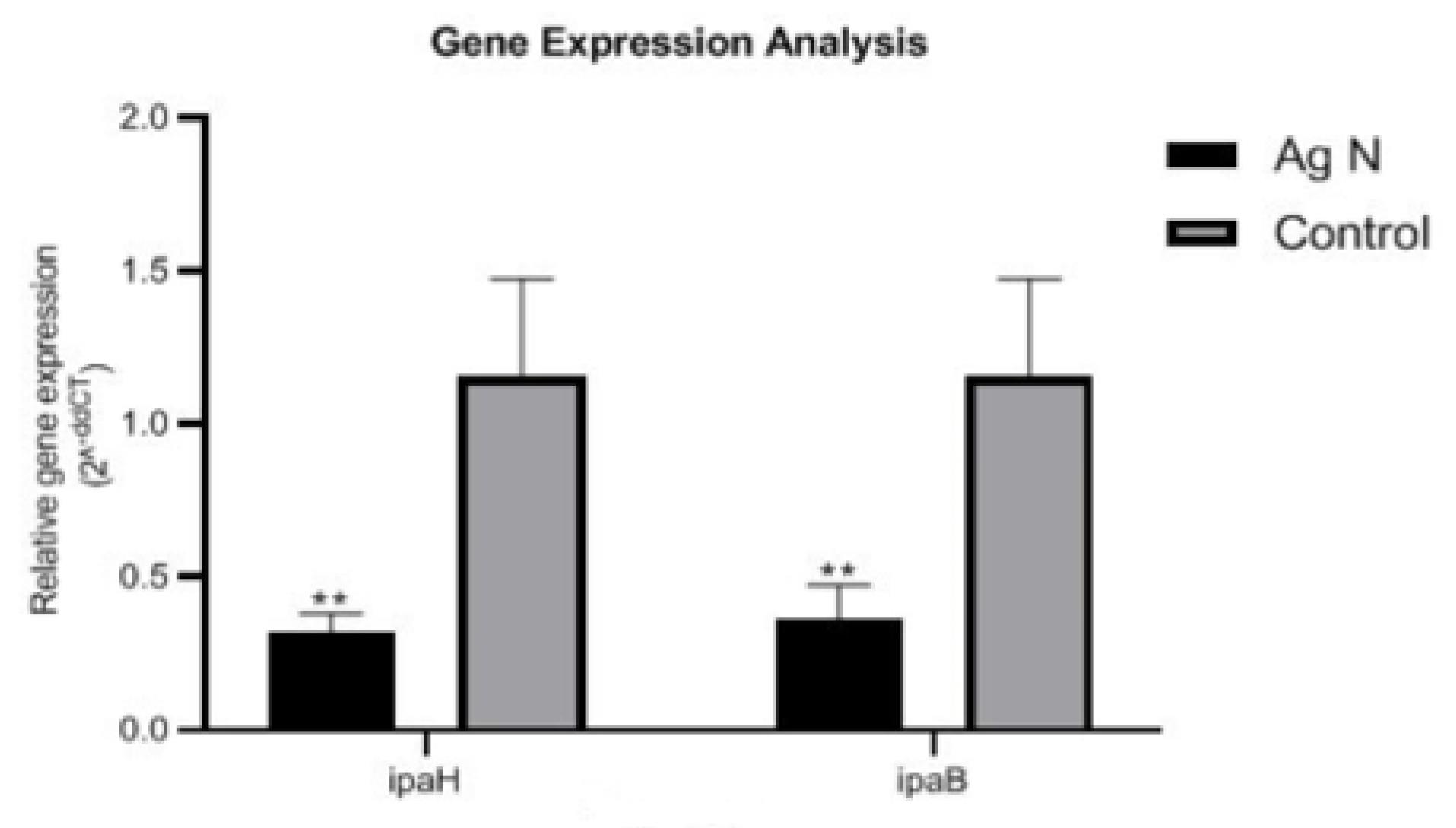
Figure 1.
ipaB and ipaH Gene Expressions in Shigella flexneri Treated With or Without (Control) Silver Nanoparticles. The symbol ** displays significant differences at the probability level of P < 0.01
.
ipaB and ipaH Gene Expressions in Shigella flexneri Treated With or Without (Control) Silver Nanoparticles. The symbol ** displays significant differences at the probability level of P < 0.01
ipaD and qnrS
Significant differences in terms of ipaD (P = 0.005) and qnrS (P = 0005) gene expression were observed in S. flexneri treated with the MIC of AgNPs compared to untreated bacteria (Figure 2). The expressions of both genes decreased in AgNP-treated S. flexneri, highlighting the effect of AgNPs on reducing the expression of S. flexneri pathogenic genes.
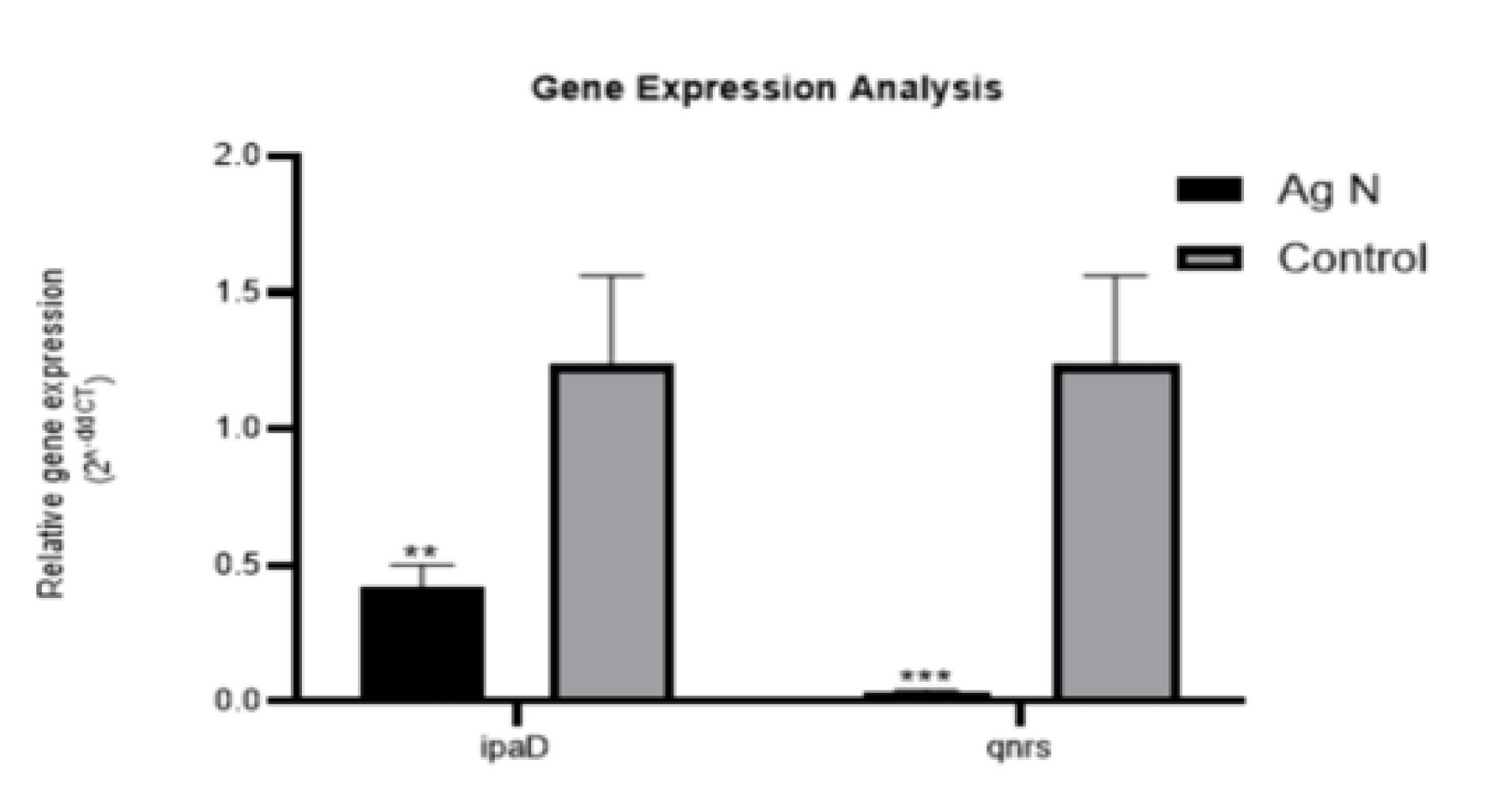
Figure 2.
ipaD and qnrS Gene Expressions in Shigella flexneri Treated With or Without (Control) Silver Nanoparticles. The symbol ** shows significant differences at the probability level of P < 0.01. Symbols ** and*** represent significant differences at the probability level of P< 0.01 and P < 0.001, respectively
.
ipaD and qnrS Gene Expressions in Shigella flexneri Treated With or Without (Control) Silver Nanoparticles. The symbol ** shows significant differences at the probability level of P < 0.01. Symbols ** and*** represent significant differences at the probability level of P< 0.01 and P < 0.001, respectively
Discussion
The results of the present study demonstrated that green synthesized AgNPs had antibacterial effects against S. flexneri, and the mechanism of antibacterial effects was attributed to the downregulation of pathogenic genes ipaB, ipaD, ipaH, and qnrS.
Spirulina platensis was used to synthesize green AgNPs. The green synthesis of NPs can reduce its side effects on organisms and the environment (24). This algal shows good anti-inflammatory and antioxidant properties due to having various beneficial compounds such as vitamins and amino acids (25) and is widely utilized in the synthesis of most metal NPs (26,27). For example, Gunasundari et al employed ultrasonic‐assisted S. platensis to synthesize metal NPs, including Zn, Fe, and Ag, and reported antimicrobial effects on Gram‐positive and Gram-negative bacteria as well as Aspergillus niger (28). Likewise, Mahdieh et al used this alga for the synthesis of crystallized AgNPs (29). Therefore, S. platensis has great potential in the green synthesis of metal NPs due to its convenience to handle, low toxicity, and reduction of harmful effects on the environment (30), and the results of the present study confirm that this alga can be utilized in the green synthesis of AgNPs.
The synthesized green AgNPs revealed antibacterial effects on S. flexneri, which showed the potential of its application in the treatment of diseases caused by this pathogen. Its MIC was calculated as 0.0625 μg/mL, which is lower than the results of other studies investigating the anti-Shigella effects of AgNPs. This difference can be attributed to the NP synthesis method and bacterial species. For example, Angamuthu et al estimated the MIC of M. indica AgNPs on the multi-drug-resistant strain S. flexneri to be 20 μg/mL (31), which is much higher than that of the present study. This difference can be related to different strains and the method for the synthesis of AgNPs. In the study of Bagherzade et al, the MIC of green AgNPs synthesized by the aqueous extract of the saffron plant on pathogenic bacteria was reported to be 250 μg/mL, representing a very high value (32). It seems that synthesis factors, bacterial strains, and toxicity criteria are important factors in this difference. In another study, Muthukrishnan et al reported the highest inhibitory concentration of pathogenic bacteria by AgNPs synthesized with Ceropegia thwaitesii at 100 μg/mL (33). They used the disk diffusion method to investigate the antimicrobial effects of AgNPs, while in the present study, the microdilution method was employed, which can explain the reason for this difference in the antibacterial concentration of this NP.
In the present study, it was observed that green synthesized AgNPs using spirulina caused changes in the expression of pathogenic genes such as ipaB, ipaD, ipaH, and qnrS in S. flexneri bacteria and reduced their expressions. Therefore, in this research, it was found that the anti-Shigella mechanism of AgNPs is the effect on the expression of pathogenic genes. These genes play an important role in the penetration of bacteria into the epithelial cells and protect the DNA against destructive factors (12). Accordingly, the synthesized green AgNPs increase the sensitivity of Shigella flexneri to protective agents by reducing the expressions of ipaB, ipaD, ipaH, and qnrS genes, thus exerting anti-Shigella effects.
Conclusion
The green synthesized AgNPs using S. platensis had strong antibacterial effects on S. flexneri, and the action mechanism was attributed to the downregulations of ipaB, ipaD, ipaH, and qnrS genes. In vivo and clinical studies are necessary in this regard.
Antibacterial Mechanism of Nanosilver
AgNPs are recognized for their potent antibacterial properties, which are attributed to several interrelated mechanisms. Understanding these mechanisms is crucial for their application in medical and consumer products.
Interaction With Bacterial Cell Membranes
AgNPs primarily exert their antibacterial effects through direct interaction with bacterial cell membranes. The positively charged AgNPs adhere to the negatively charged components of the bacterial cell wall, facilitating their penetration into the cell. This interaction disrupts the integrity of the cell membrane, leading to increased permeability and eventual leakage of cellular contents, which can result in cell death (34,35).
Electrostatic Attraction
The electrostatic interaction between AgNPs and the bacterial membrane enhances adhesion, promoting physical changes that compromise membrane integrity (35).
Size-Dependent Effects
Smaller NPs have a larger surface area relative to their volume, allowing for more effective contact with bacterial cells and facilitating deeper penetration into the cytoplasm (35).
Release of Silver Ions
Another significant mechanism is the release of Ag ions (Ag + ), which can occur when AgNPs dissolve upon contact with bacteria. These ions are highly reactive and can interact with various cellular components:
Protein Interactions
Ag + ions bind to thiol groups in proteins, disrupting their function by forming stable complexes that inhibit enzymatic activity (36,37). This interaction can lead to oxidative damage and cellular dysfunction.
Reactive Oxygen Species Generation
The presence of AgNPs and Ag + can induce oxidative stress within bacterial cells by promoting the formation of reactive oxygen species, damaging cellular components such as lipids, proteins, and DNA (34,37). This oxidative damage is a critical pathway leading to bacterial cell death.
Induction of Oxidative Stress
The generation of ROS is a key factor in the antibacterial action of AgNPs. Upon entering bacterial cells, AgNPs can catalyze reactions that produce free radicals:
Membrane Damage
ROS can oxidize fatty acids in the membrane, leading to increased permeability and structural failure (36,37).
DNA Damage
Inside the cell, ROS can interact with DNA, causing mutations and impairing replication processes (36).
Biofilm Disruption
AgNPs also play a role in preventing biofilm formation, which is a significant challenge in treating bacterial infections. By adhering to surfaces and disrupting the initial stages of biofilm development, AgNPs can reduce bacterial colonization on medical devices and tissues (35). This property is particularly beneficial in combating infections associated with implants and catheters.
The antibacterial mechanisms of silver nanoparticles (nanoAg) are multifaceted, involving direct membrane disruption, ion release, oxidative stress induction, and biofilm prevention. These properties make AgNPs a valuable tool in the fight against antibiotic-resistant bacteria and a promising candidate for various biomedical applications. Continued research into optimizing their use while minimizing potential toxicity is essential for advancing their application in healthcare settings.
Authors’ Contribution
Conceptualization: Shadi Hajrasouliha.
Data curation: Sahar Karami and Zahra Heidary.
Formal analysis: Sepideh Khaleghi.
Funding acquisition: Sahar Karami and Zahra Heidary.
Investigation: Sahar Karami and Zahra Heidary.
Methodology: Sarvenaz Falsafi.
Project administration: Shadi Hajrasoulih.
Resources: Mohammad Karim Rahimi.
Software: Sepideh Khaleghi.
Supervision: Shadi Hajrasoulih.
Validation: Sarvenaz Falsafi.
Visualization: Mohammad Karim Rahimi.
Writing-original draft: Shadi Hajrasouliha.
Writing-review and editing: Shadi Hajrasouliha.
Competing Interests
The authors declare no competing interests.
Consent to Participate
Not applicable.
Consent to Publish
All authors agree to publish the article.
Data Availability Statement
Not applicable.
Ethical Approval
Not applicable.
Funding
This research was conducted with funding provided by the authors.
References
- Barreto ML, Teixeira MG, Carmo EH. Infectious diseases epidemiology. J Epidemiol Community Health 2006; 60(3):192-5. doi: 10.1136/jech.2003.011593 [Crossref] [ Google Scholar]
- Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AK. Shigellosis. Lancet 2018; 391(10122):801-12. doi: 10.1016/s0140-6736(17)33296-8 [Crossref] [ Google Scholar]
- Barrett J, Fhogartaigh CN. Bacterial gastroenteritis. Medicine 2017; 45(11):683-9. doi: 10.1016/j.mpmed.2017.08.002 [Crossref] [ Google Scholar]
- Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16(11):877-97. doi: 10.1016/s1474-4422(17)30299-5 [Crossref] [ Google Scholar]
- von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med 2006; 3(9):e353. doi: 10.1371/journal.pmed.0030353 [Crossref] [ Google Scholar]
- Watarai M, Funato S, Sasakawa C. Interaction of Ipa proteins of Shigella flexneri with alpha5beta1 integrin promotes entry of the bacteria into mammalian cells. J Exp Med 1996; 183(3):991-9. doi: 10.1084/jem.183.3.991 [Crossref] [ Google Scholar]
- Buysse JM, Stover CK, Oaks EV, Venkatesan M, Kopecko DJ. Molecular cloning of invasion plasmid antigen (Ipa) genes from Shigella flexneri: analysis of Ipa gene products and genetic mapping. J Bacteriol 1987; 169(6):2561-9. doi: 10.1128/jb.169.6.2561-2569.1987 [Crossref] [ Google Scholar]
- Kotloff KL, Pasetti MF, Barry EM, Nataro JP, Wasserman SS, Sztein MB. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis 2004; 190(10):1745-54. doi: 10.1086/424680 [Crossref] [ Google Scholar]
- Dickenson NE, Zhang L, Epler CR, Adam PR, Picking WL, Picking WD. Conformational changes in IpaD from Shigella flexneri upon binding bile salts provide insight into the second step of type III secretion. Biochemistry 2011; 50(2):172-80. doi: 10.1021/bi101365f [Crossref] [ Google Scholar]
- Bourdet-Sicard R, Rüdiger M, Jockusch BM, Gounon P, Sansonetti PJ, Nhieu GT. Binding of the Shigella protein IpaA to vinculin induces F-actin depolymerization. EMBO J 1999; 18(21):5853-62. doi: 10.1093/emboj/18.21.5853 [Crossref] [ Google Scholar]
- Fernandez-Prada CM, Hoover DL, Tall BD, Hartman AB, Kopelowitz J, Venkatesan MM. Shigella flexneri IpaH(78) facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect Immun 2000; 68(6):3608-19. doi: 10.1128/iai.68.6.3608-3619.2000 [Crossref] [ Google Scholar]
- Sethuvel DP, Perumalla S, Anandan S, Michael JS, Ragupathi NK, Gajendran R. Antimicrobial resistance, virulence & plasmid profiles among clinical isolates of Shigella serogroups. Indian J Med Res 2019; 149(2):247-56. doi: 10.4103/ijmr.IJMR_2077_17 [Crossref] [ Google Scholar]
- Pu XY, Pan JC, Wang HQ, Zhang W, Huang ZC, Gu YM. Characterization of fluoroquinolone-resistant Shigella flexneri in Hangzhou area of China. J Antimicrob Chemother 2009; 63(5):917-20. doi: 10.1093/jac/dkp087 [Crossref] [ Google Scholar]
- Williams PC, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr Int Child Health 2018; 38(Suppl 1):S50-65. doi: 10.1080/20469047.2017.1409454 [Crossref] [ Google Scholar]
- Shen H, Chen J, Xu Y, Lai Z, Zhang J, Yang H. An outbreak of shigellosis in a Children Welfare Institute caused by a multiple-antibiotic-resistant strain of Shigella flexneri 2a. J Infect Public Health 2017; 10(6):814-8. doi: 10.1016/j.jiph.2017.01.003 [Crossref] [ Google Scholar]
- Puzari M, Sharma M, Chetia P. Emergence of antibiotic resistant Shigella species: a matter of concern. J Infect Public Health 2018; 11(4):451-4. doi: 10.1016/j.jiph.2017.09.025 [Crossref] [ Google Scholar]
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 2008; 83(5):761-9. doi: 10.1038/sj.clpt.6100400 [Crossref] [ Google Scholar]
- Kharissova OV, Dias HV, Kharisov BI, Pérez BO, Pérez VM. The greener synthesis of nanoparticles. Trends Biotechnol 2013; 31(4):240-8. doi: 10.1016/j.tibtech.2013.01.003 [Crossref] [ Google Scholar]
- Abass Sofi M, Sunitha S, Ashaq Sofi M, Khadheer Pasha SK, Choi D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J King Saud Univ Sci 2022; 34(2):101791. doi: 10.1016/j.jksus.2021.101791 [Crossref] [ Google Scholar]
- Hembram KC, Kumar R, Kandha L, Parhi PK, Kundu CN, Bindhani BK. Therapeutic prospective of plant-induced silver nanoparticles: application as antimicrobial and anticancer agent. Artif Cells Nanomed Biotechnol 2018; 46(Suppl 3):S38-51. doi: 10.1080/21691401.2018.1489262 [Crossref] [ Google Scholar]
- Ghramh HA, Ibrahim EH, Kilany M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci Nutr 2020; 8(1):445-55. doi: 10.1002/fsn3.1328 [Crossref] [ Google Scholar]
- Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol 2012; 112(5):841-52. doi: 10.1111/j.1365-2672.2012.05253.x [Crossref] [ Google Scholar]
- Cheon JY, Kim SJ, Rhee YH, Kwon OH, Park WH. Shape-dependent antimicrobial activities of silver nanoparticles. Int J Nanomedicine 2019; 14:2773-80. doi: 10.2147/ijn.S196472 [Crossref] [ Google Scholar]
- Gour A, Jain NK. Advances in green synthesis of nanoparticles. Artif Cells Nanomed Biotechnol 2019; 47(1):844-51. doi: 10.1080/21691401.2019.1577878 [Crossref] [ Google Scholar]
- Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol 2016; 90(8):1817-40. doi: 10.1007/s00204-016-1744-5 [Crossref] [ Google Scholar]
- Kalabegishvili T, Kirkesali E, Rcheulishvili A. Synthesis of Gold Nanoparticles by Blue-Green Algae Spirulina platensis. Frank Lab. of Neutron Physics; 2012.
- Muthusamy G, Thangasamy S, Raja M, Chinnappan S, Kandasamy S. Biosynthesis of silver nanoparticles from Spirulina microalgae and its antibacterial activity. Environ Sci Pollut Res Int 2017; 24(23):19459-64. doi: 10.1007/s11356-017-9772-0 [Crossref] [ Google Scholar]
- Gunasundari E, Senthil Kumar P, Christopher FC, Arumugam T, Saravanan A. Green synthesis of metal nanoparticles loaded ultrasonic-assisted Spirulina platensis using algal extract and their antimicrobial activity. IET Nanobiotechnol 2017; 11(6):754-8. doi: 10.1049/iet-nbt.2016.0223 [Crossref] [ Google Scholar]
- Mahdieh M, Zolanvari A, Azimee AS, Mahdieh M. Green biosynthesis of silver nanoparticles by Spirulina platensis. Scientia Iranica 2012; 19(3):926-9. doi: 10.1016/j.scient.2012.01.010 [Crossref] [ Google Scholar]
- Mukherjee A, Sarkar D, Sasmal S. A review of green synthesis of metal nanoparticles using algae. Front Microbiol 2021; 12:693899. doi: 10.3389/fmicb.2021.693899 [Crossref] [ Google Scholar]
- Angamuthu S, Thangaswamy S, Raju A, Husain FM, Ahmed B, Al-Shabib NA. Biogenic preparation and characterization of silver nanoparticles from seed kernel of Mangifera indica and their antibacterial potential against Shigella spp. Molecules 2023; 28(6):2468. doi: 10.3390/molecules28062468 [Crossref] [ Google Scholar]
- Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop Biomed 2017; 7(3):227-33. doi: 10.1016/j.apjtb.2016.12.014 [Crossref] [ Google Scholar]
- Muthukrishnan S, Bhakya S, Senthil Kumar T, Rao MV. Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Ceropegiathwaitesii–an endemic species. Ind Crops Prod 2015; 63:119-24. doi: 10.1016/j.indcrop.2014.10.022 [Crossref] [ Google Scholar]
- Yan X, He B, Liu L, Qu G, Shi J, Hu L. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: proteomics approach. Metallomics 2018; 10(4):557-64. doi: 10.1039/c7mt00328e [Crossref] [ Google Scholar]
- Qing Y, Cheng L, Li R, Liu G, Zhang Y, Tang X. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine 2018; 13:3311-27. doi: 10.2147/ijn.S165125 [Crossref] [ Google Scholar]
- Mikhailova EO. Silver nanoparticles: mechanism of action and probable bio-application. J Funct Biomater 2020; 11(4):84. doi: 10.3390/jfb11040084 [Crossref] [ Google Scholar]
- Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 2016; 7:1831. doi: 10.3389/fmicb.2016.01831 [Crossref] [ Google Scholar]