Avicenna Journal of Clinical Microbiology and Infection. 11(1):42-50.
doi: 10.34172/ajcmi.3529
Original Article
Bioactive Compounds and Nanoparticles Conjugated with Chitosan Composites for Clinical Purposes
Adedayo Olajide Ajayi 1, *  , Olugbenga Ebenezer Ige 2, Patience Yakubu 3
, Olugbenga Ebenezer Ige 2, Patience Yakubu 3
Author information:
1Department of Microbiology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria; Centre for Infectious Diseases Control and Drug, Development (CIDCDD), Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
2Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria; Office of the Vice-Chancellor, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
3Department of Microbiology, School of Applied Science and Technology, Auchi Polytechnic, Auchi, Edo State, Nigeria
Abstract
Background: Various forms of microorganisms have developed multiple resistances against the effects of applied antibacterial agents controlling them. Hence, there is a need to formulate potent antimicrobial agents from natural sources to eliminate them from the environment. The aim of this study was to investigate the potency of chitosan conjugated with some medicinal plants against recalcitrant microorganisms and overcome the problem of multiple antibiotic resistance.
Methods: In this study, the synergistic effects of chitosan derived from snail shells were explored in conjunction with eight plant sources, including Ocimum gratissimum, Croton zambesicus, Phyllanthus niruri, Aloe barbadensis, Moringa oleifera, Andrographis paniculate, Aloe barbadensis, and Curcuma longa. The investigation focused on the antimicrobial properties of these combinations, aiming at enhancing the efficacy of pharmaceutical products against antibiotic-resistant strains. Four samples were analyzed in this regard. Sample A consisted of 0.5 g of sulphur nanoparticles (SNPs) conjugated with chitosan, and Sample B contained 1 g of SNPs conjugated with chitosan from shrimp shells dissolved in 1% acetic acid. In addition, Sample C involved chitosan from shrimp shells dissolved in 1% acetic acid, and Sample D utilized chitosan from snail shells dissolved in 1% acetic acid. All demonstrated varied antimicrobial potentials.
Results: Notably, Sample B, which utilized chitosan from shrimp shells as nanocarriers for SNPs, exhibited significant antimicrobial activity, with zones of inhibition measuring up to 35 mm and 37 mm against multidrug-resistant strains of Staphylococcus sp. (coagulase-negative), Klebsiella oxytoca, and Escherichia coli, respectively. Additionally, promising antimicrobial effects were observed against organisms such as Pseudomonas aeruginosa, Klebsiella ornithinolytica, and E. coli, with zone sizes reaching 20 mm, 23 mm, and 25 mm, respectively. Iron oxide NPs (Fe3O4) and silica-coated iron oxide NPs displayed lower activity levels, except at the 100 mg/mL concentration, where they represented efficacy against certain multidrug-resistant strains such as AKR 18 - Enterobacter agglomerans, OKI 10 - Acinetobacter haemolyticus, and T30 – K. oxytoca.
Conclusion: The findings of this study offer valuable insights into the management of infectious disease and the treatment of challenging pathogens in healthcare systems, providing a potential roadmap for combating antibiotic resistance and improving therapeutic strategies.
Keywords: Chitosan, Diseases, Nanocarriers, Nanoparticles
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Ajayi AO, Ige OE, Yakubu P. Bioactive compounds and nanoparticles conjugated with chitosan composites for clinical purposes. Avicenna J Clin Microbiol Infect. 2024; 11(1):42-50. doi:10.34172/ajcmi.3529
Introduction
Nanoparticles (NPs) have become of interest to scientists based on some breakthroughs attained with regard to their use in disease detection and control, water purification, and other industrial applications. The Nobel Prize-winning American physicist, Richard Feynman, introduced the idea of nanotechnology. After fifteen years, Norio Taniguchi, a Japanese scientist, was the first to use and define the term “nanotechnology” in 1974 as “nanotechnology mainly consists of the processing of separation, consolidation, and deformation of materials by one atom or one molecule” (1).
In recent scientific research, chitosan has been conjugated with NPs as nanocarriers for some clinical and industrial purposes. Chitosan has recently become the most researched natural polymer (2), with commercial applications in the biomedical, food, chemical, and pharmaceutical industries. Chitosan is a linear polysaccharide that is composed of and linked with some glucosamine. It is highly useful in everyday life for some domestic and various industrial purposes. It can be made by treating the chitin shells of crustaceans with alkaline substances such as sodium hydroxide. Chitosan is a safe and friendly substance for humans. It is useful in the adsorption, cosmetics, pharmaceutical, flocculant, anticancer, and antimicrobial industries. Chitosan can be synthesized from chitin extracted from crayfish. Methods such as deproteinization, demineralization, and deacetylation, respectively, can be used in the synthesis of chitosan from crayfish. It has good antimicrobial activity and, thus, can form an antibacterial synergistic composite with some plant sources. This is due to its unique characteristics as a naturally occurring, non-toxic, biodegradable, and biocompatible polymer with direct antibacterial action (3).
Chitosan’s biological activities and commercial uses can be applied in the biomedical, food, and chemical industries (4). It has been shown to have antimicrobial action against various bacteria with a broad antibacterial spectrum, including gram-positive and gram-negative bacterial strains. Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus, Bacillus megaterium, Listeria monocytogenes, Lactobacillus brevis, Escherichia coli, Pseudomonas aeruginosa, Pseudomonas fluorescens, and Salmonella typhimurium were all found to be susceptible to chitosan (5). The multidrug-resistant nature of the different strains of microorganisms can be caused by various factors, including the formation of biofilms due to some environmental conditions as well as some clinical factors (6).
Rather than a single compound, chitosan [poly- (β-1/4)-2-amino-2-deoxy-D-glucopyranose] (7) is a group of partially or fully de-acetylated chitin molecules, a major component of the shells of crustaceans. Researchers are interested in chitosan due to some of its exceptional properties, such as biodegradability, biocompatibility, nontoxicity or low toxicity, non-antigenicity or low immunogenicity, and low-cost, as well as numerous antimicrobial, antitumor, antioxidant, antidiabetic, and immunoenhancing pharmacological properties (8). Chitosan has found wide applications in medicine, pharmacy, and food sciences (9).
Materials and Methods
Sample sources
The medicinal plant samples used for this study were collected from the wild and related sources in the Akungba-Akoko community and identified by experts in the Department of Plant Sciences and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria.
Sampling method
Collection and Preparation of Medicinal Plant Sources
There are hosts of medicinal plants, but some are commonly useful for resistant and recalcitrant microbes. Eight plant sources were used in this study, including Ocimum gratissimum, Croton zambesicus, Phyllanthus niruri, Aloe barbadensis, Moringa oleifera, Andrographis paniculate, Aloe barbadensis,and Curcuma longa.
The applied medicinal plants were obtained from the wild and Akungba-Akoko community, Ondo State, Nigeria. The leaves and roots of the plants were used, spread on clean plastic sheets, and allowed to air dry for 3 weeks in the laboratory. After complete drying, the individual parts were milled using the Methods Optimization in Accelerated Solvent Extraction in Technical Note (10) and stored in a plastic jar.
Extraction
Extraction processes were performed according to standard methods by the Association of Official Agricultural Chemists (11). About 5.00 g of the powderedsamples were weighed and transferred to conical flasks and treated with 5 different solvents (petroleum ether, chloroform, butanol, ethanol, N-hexane, and water) until the powders were fully soaked. The flasks were shaken every hour for the first six hours, and then they were kept aside and shaken after 24 hours (12). The flasks were allowed to sit for three days, and then the extracts were filtered. The extracts were collected and evaporated to dryness using a rotary evaporator. A total of 30 extracts were obtained and carefully kept in airtight sample bottles prior to analysis.
Collection and Identification of Microorganisms
Thirty-five bacterial isolates recovered from the clinical specimens of patients in designated health facilities in Ondo State and related research sources were stored using nutrient agar medium at the University Health Centre as well as the Centre for Infectious Disease Research and Drug Development, Adekunle Ajasin University, Akungba-Akoko, Ondo State. The preliminary confirmatory test was earlier performed to confirm the identity of the organisms using the MicrobactTM 24E (Oxford, UK) identification kit for this purpose (13).
Antibiotic Susceptibility Test
This test was conducted on bacterial isolates using a modified Kirby-Bauer’s disc diffusion method on Muller-Hinton agar (MHA; CM337 – Oxoid, UK) (14). The isolates were subcultured into nutrient broth and incubated at 37 °C overnight (24 hours). The broth cultures were diluted with normal saline to the 0.5 McFarland turbidity standard. Appropriate inoculums were used accordingly. The MHA was incubated at 37 °C for 18 hours. The diameter of the zone of inhibition was measured and interpreted according to the Clinical Laboratory Standard Institute guidelines (15). Calibrated measuring rulers or devices have been adopted for this purpose.
Antimicrobial Screening of Applied Medicinal Plant Part Extracts
The antimicrobial screening of the medicinal plant part extracts against some predetermined antibiotic-resistant clinical isolates was performed using the modified agar well diffusion method (14). A stock concentration of 100 mg/mL was constituted by dissolving 1 g of the extract in a 10 mL mixture of 30% dimethyl sulfoxide and sterile distilled water (1:3). The two-fold serial dilution of the stock was conducted to obtain varying concentrations of the extracts (50 mg/mL, 25 mg/mL, and 12.5 mg/mL). An aliquot of 1 mL of the standardized inoculum (0.5 McFarland turbidity standard) was mixed with 19 mL of molten MHA and left to solidify in a sterile Petri dish.
Using a sterile cork borer (6 mm in diameter), the wells were carefully made on the agar plate (at least 20 mm apart). In addition, 50 µL of each concentration of the extract and the controls were introduced into each well and left on the table to settle. The MHA plate was incubated at 37 °C for 20 hours, and the diameter of the zone of inhibition was measured. A potent antibiotic (Ofloxacin) and dimethyl sulfoxide were used as positive and negative controls, respectively.
A cork borer with a size of 6 mm was utilized, while for the control, water and ofloxacin served as negative and positive controls, respectively.
Preparation of Chitosan Samples
The synthesis of chitosan was based on the methods of previous studies (16,17). The sample (crayfish powder) can be demineralized by soaking in a 10% hydrogen chloride solution for 24 hours at 60 °C. Deproteinized by soaking in a 10% sodium hydroxide (NaOH) solution for 24 hours at 60 °C and allowed to cool for 1 hour. The solution was then filtered and washed with demineralized water. After filtering the solution, the residue was washed with demineralized water, and the process was followed by deacetylation by adding 50% NaOH for 8 hours at 60 °C and washed with demineralized water to neutral pH. Commercial chitosan can be obtained from foreign countries such as Germany and the UK. Modified versions of the methods by Maulin et al (16) and Paul et al (17) were employed for the synthesis and extraction of chitosan from snail shells.
Sample Preparation
Maulin’s method (16) was used for the synthesis and extraction of chitosan from two crustaceans, namely, crayfish and prawn.
Iron oxide (Fe3O4) NP used in this study is spherical and is a mineral also known as magnetite or magnetic oxide that is dark in color, ranging from a brownish hue to grey or black. The size of this particle, determined by the dynamic light scattering technique, helps measure random changes in the intensity scattered from the suspension/solution. This size can range from 0.3 to 10 000 nm. Fe3O4 NPs and silica-coated iron oxide NPs (Fe3O4 @Si) with plant extracts such as Ocimum gratissimumwere tested as alternate antimicrobial agents against these resistant clinical strains.
Results
The multidrug-resistant bacterial isolates used in this study were identified by standard microbiological methods and included Candida albicans,Budricia aquatic, Staphylococcus sp.(coagulase –ve), Klebsiella ornithinolytica, Escherichia coli, Staphylococcus aureus, Acinetobacter haemolyticus, Klebsiella ornithinolytica,Burkholderia cepacia, Pseudomonas aeruginosa, Escherichia hermannii, Staphylococcus aureus, Pseudomonas fluorescens, Enterobacter gergoviae, Pseudomonas aeruginosa, Klebsiella terrigena, Klebsiella terrigena, Citrobacter gilleric, Klebsiella oxytoca,and Salmonella typhi(Table 1).Similarly, the preparation flow chart of chitosan used for this research is shown in Figure 1.
Table 1.
Chitosan Bioactive Activity with Nanoparticles on Clinical Isolates
|
Isolates
|
Zone of Inhibition in mm Unit
|
|
A
|
B
|
C
|
D
|
|
Candida albicans
|
- |
- |
- |
- |
|
Escherichia coli
|
14 (R.) |
20 |
- |
12 |
|
Budricia aquatic
|
- |
14 |
12 |
11 |
|
Staphylococcus sp.
|
32 |
35 |
13 |
- |
|
Escherichia coli
|
29 |
27 |
12 |
11 |
|
Escherichia coli
|
27 |
37 |
11 |
- |
|
Klebsiella ornithinolytica
|
22 |
31 |
11 |
- |
|
Escherichia coli
|
25 |
15 |
- |
13 |
|
Escherichia coli
|
30 |
31 |
- |
- |
|
Escherichia coli
|
25 |
22 |
11 |
- |
|
Staphylococcus aureus
|
- |
- |
- |
- |
|
Acinetobacter haemolyticus
|
23 |
26 |
- |
12 |
|
Klebsiella ornithinolytica
|
29 |
32 |
- |
11 |
|
Burkholderia cepacia
|
31 |
18 |
11 |
11 |
|
Pseudomonas aeruginosa
|
- |
12 |
12 |
11 |
|
Escherichia hermannii
|
25 |
30 |
- |
- |
|
Staphylococcus aureus
|
27 |
21 |
- |
- |
|
Pseudomonas fluorescens
|
35 |
26 |
11 |
- |
|
Pseudomonas fluorescens
|
35 |
20 |
10 |
12 |
|
Enterobacter gergoviae
|
- |
32 |
- |
21 |
|
Pseudomonas aeruginosa
|
- |
20 (R.) |
12 |
D |
|
Klebsiella terrigena
|
26 |
27 |
19 |
12 |
|
Klebsiella terrigena
|
- |
17 (B.S) |
- |
- |
|
Citrobacter gilleric
|
27 |
25 |
- |
- |
|
Escherichia coli
|
17 (B.S) |
15 (B.S) |
- |
- |
|
Klebsiella oxytoca
|
27 |
26 |
- |
- |
|
Klebsiella oxytoca
|
20 |
19 |
- |
- |
|
Escherichia coli
|
20 |
28 |
- |
- |
|
Klebsiella oxytoca
|
25 |
35 |
12 |
11 |
|
Escherichia coli
|
31 |
12 (B.S) |
- |
- |
|
Escherichia coli
|
30 |
25 |
13 |
- |
|
Salmonella typhi
|
30 |
- |
12 |
- |
Legend:
Sample A: 0.5 g of SNPs together with chitosan.
Sample B: 1 g of SNPs together with chitosan.
The chitosan from the shrimp shell was dissolved in 1% acetic acid.
Sample C: Chitosan from shrimp shell dissolved in 1% acetic acid.
Sample D: Chitosan from snail shell dissolved in 1% acetic acid.
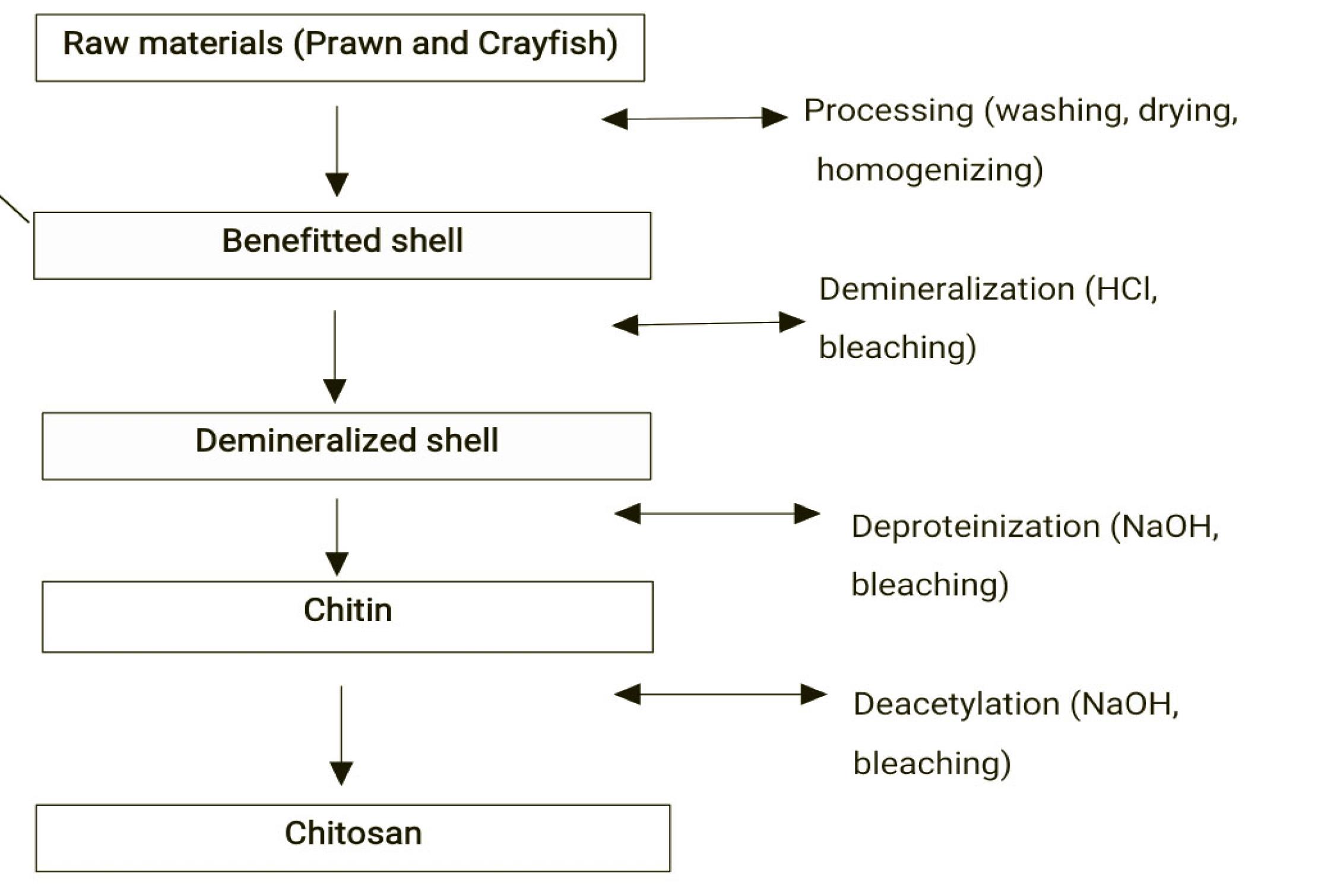
Figure 1.
Flowchart for Chitosan Preparation.Sample A: 0.5 g of sulphur NPs (SNPs) together with chitosan. Sample B: 1 g of SNPs together with chitosan. The chitosan from the shrimp shell was dissolved in 1% acetic acid. Sample C: Chitosan from shrimp shell dissolved in 1% acetic acid. Sample D: Chitosan from snail shell dissolved in 1% acetic acid. Other tested NPs.
.
Flowchart for Chitosan Preparation.Sample A: 0.5 g of sulphur NPs (SNPs) together with chitosan. Sample B: 1 g of SNPs together with chitosan. The chitosan from the shrimp shell was dissolved in 1% acetic acid. Sample C: Chitosan from shrimp shell dissolved in 1% acetic acid. Sample D: Chitosan from snail shell dissolved in 1% acetic acid. Other tested NPs.
The size of the applied Fe3O4 NP was 10.31 nm. Fe3O4 has a cubic inverse spinel group structure, which consists of a close-packed array of oxide ions where all the Fe22+ ions occupy half of the octahedral sites and Fe3 is split evenly across the remaining octahedral sites and the tetrahedral sites. Various sample sources were used in this study, including Sample A, 0.5 g of SNPs together with chitosan, and Sample B: 1 g of SNPs together with chitosan from shrimp shell dissolved in 1% acetic acid. The other sources were Sample C: Chitosan from shrimp shell dissolved in 1% acetic acid and Sample D: Chitosan from snail shell dissolved in 1% acetic acid. They all showed diversified antimicrobial potentials in the test isolates (1).
Table 1 presents NPs synergized with chitosan as nanocarriers in Sample B with 1 g of SNPs together with chitosan from shrimp shell dissolved with 1% acetic acid, demonstrating high antimicrobial activity up to 35 mm and 37 mm zones of inhibition in Staphylococcus sp. (coagulase –ve), Klebsiella oxytoca, and E. coli, respectively.
Table 2 also provides the results related to the effectiveness of chitosan in synergy with some medicinal plants. It was observed that organisms such as P. aeruginosa, K. ornithinolytica, and E. coli with relatively high zone inhibition up to 20 mm and 23 mm susceptibility demonstrated the antimicrobial potential of the use of this biopolymer with selected medicinal plants. The observations made at this level can serve as a useful guide for the control of infectious diseases and the treatment of some recalcitrant etiologic agents in health management systems.
Table 2.
Chitosan Conjugated With Selected Medicinal Plants
|
Isolates
|
(Zone of Inhibition in mm)
|
|
A
|
B
|
C
|
D
|
E
|
F
|
G
|
H
|
I
|
J
|
|
Candida albicans
|
|
|
|
|
|
|
|
|
|
12 |
|
Budricia aquatic
|
|
|
|
|
|
|
|
|
|
|
|
Staphylococcus sp. (coagulase – ve )
|
|
|
|
|
|
10 |
10 |
11 |
|
20 BS |
|
Escherichia coli
|
|
|
|
|
|
11 BS |
|
|
|
|
|
Escherichia coli
|
|
|
|
|
|
10 BS |
|
12 |
|
15 BS |
|
Klebsiella ornithinolytica
|
11 |
|
|
|
|
|
|
|
|
|
|
Escherichia coli
|
|
|
|
|
|
|
|
|
|
|
|
Escherichia coli
|
|
|
|
|
|
10 |
10 |
|
11 |
15 |
|
Escherichia coli
|
|
|
|
|
|
9 |
11BS |
|
9BS |
13 |
|
Staphylococcus aureus
|
12 |
11BS |
|
|
|
|
|
|
|
|
|
Acinetobacter haemolyticus
|
|
|
|
|
|
12 |
10 |
18BS |
11 |
|
|
Klebsiella ornithinolytica
|
|
|
|
|
|
10BS |
|
20BS |
|
10 |
|
Burkholderia cepacia
|
|
|
|
|
|
|
|
|
|
14 |
|
Pseudomonas aeruginosa
|
|
|
|
|
|
13BS |
10BS |
|
|
11BS |
|
Escherichia hermannii
|
11 |
|
10BS |
|
|
|
|
|
|
|
|
Staphylococcus aureus
|
|
10 |
12 |
19 |
10 |
13 |
9 |
|
10 |
18 |
|
Pseudo fluorescens
|
11 |
|
12 |
|
|
|
|
|
10 |
14BS |
|
Pseudomonas fluorescens
|
12 |
|
15BS |
|
|
|
|
|
|
15 |
|
Enterobacter gergoviae
|
|
|
|
|
|
|
|
|
|
|
|
Pseudomonas aeruginosa
|
|
|
|
|
|
|
|
|
|
23BS |
|
Klebsiella terrigena
|
|
|
|
|
|
|
|
|
|
|
|
Klebsiella terrigena
|
|
|
|
|
|
|
11 |
|
10 |
15 |
|
Citrobacter gilleric
|
11BS |
10 |
11 |
|
10 |
|
|
|
|
|
|
Escherichia coli
|
|
|
11 |
|
|
|
|
|
10BS |
|
|
Klebsiella oxytoca
|
|
|
|
|
|
10BS |
|
|
|
15 |
|
Klebsiella oxytoca
|
|
|
|
|
|
|
|
|
|
|
|
Escherichia coli
|
|
|
|
|
|
|
|
15BS |
|
|
|
Klebsiella oxytoca
|
17BS |
|
13BS |
|
|
|
|
|
|
|
|
Escherichia coli
|
|
|
|
|
|
|
10 |
|
|
11 |
|
Escherichia coli
|
|
|
|
|
|
11 |
|
|
|
25 |
|
Salmonella typhi
|
|
|
|
|
|
|
|
15BS |
|
11 |
Specimen Number
Ocimum gratissimum(large) ethanol- A BOA
Ocimum gratissimum(small) ethanol- B SOB
Croton zambesicusethanol- C CZC
Phyllanthus niruriethanol -D PND
Moringa oleifera ethanol -E MOE
Ocimum gratissimum(large) N-hexane – F BOF
Ocimum gratissimum (small) N-hexane – G SOG
Croton zambesicus N-hexane -H CZH
Phyllanthus niruri N-hexane – I PNI
Andrographis paniculataethanol -J APJ
BS - Bacteriostatic
The antimicrobial potential of chitosan synthesized from snail and shrimp sources, respectively, was conjugated with Aloe barbadensisand Curcuma longa (Table 3). The use of A. barbadensis during this study shows low antimicrobial activity. Nevertheless, a combination of A. barbadensis and chitosan (from shrimp sources) revealed some antimicrobial potential, while chitosan (from shrimp sources) conjugated with C. longa had the highest antimicrobial potential against most multidrug-resistant clinical isolates tested in the present study (Table 3). Typical plant sources used for this purpose are shown in Figures 2–8, while Figure 10 displays applied antimicrobial susceptibility test cultures. Table 4 summarizes the results of the average minimum inhibitory and minimum bactericidal concentrations for selected isolates tested in this study.
Table 3.
Chitosan Conjugated with Aloe barbadensisand Curcuma longa
|
Isolates
|
(Zone of Inhibition in mm)
|
|
A
|
B
|
C
|
|
Owo 15 - Pseudomonas aeruginosa
|
11 mm |
- |
15 mm |
|
IK 24 - Escherichia coli
|
- |
- |
- |
|
T 32 - Escherichia coli
|
- |
- |
- |
|
Owo 6 - Klebsiella terrigena
|
- |
- |
12 mm |
|
Owo 5 - Pseudomonas aeruginosa
|
- |
- |
- |
|
T11 - Citrobacter gilleric
|
- |
12 mm |
14 mm |
|
IK 13 - Klebsiella ornithinolytica
|
- |
14 mm |
13 mm |
|
Owo 28 - Escherichia coli
|
- |
- |
- |
|
Owo 16 - Escherichia hermannii
|
- |
- |
13 mm |
|
IK 27 - Budricia aquatic
|
- |
14 mm |
12 mm |
|
T 29 - Escherichia coli
|
- |
- |
- |
|
Owo 9 - Klebsiella terrigena
|
- |
- |
- |
|
T 12 - Budricia aquatica
|
- |
- |
- |
|
OKI 26 - Staphylococcus spp.
|
- |
- |
- |
|
OKI 10 - Acinetobacter haemolyticus
|
- |
- |
- |
|
OKI 18 - Enterobacter agglomerans
|
- |
- |
25 mm |
|
IK 9 - Escherichia coli
|
- |
- |
- |
|
T 28 - Escherichia coli
|
- |
- |
- |
|
T 23- Klebsiella oxytoca
|
- |
- |
16 mm |
|
AKR 18 - Enterobacter agglomerans
|
- |
- |
14 mm |
|
AKR 17 - Escherichia coli
|
- |
- |
- |
|
AKR 7 - Staphylococcus sp. (coagulase – ve )
|
- |
- |
14 mm |
|
AKR 16 - Escherichia coli
|
- |
- |
12 mm |
|
IK 14 - Escherichia coli
|
- |
- |
12 mm |
|
OKI 05 - Staphylococcus aureus
|
- |
- |
11 mm |
|
T 30 - Klebsiella oxytoca
|
- |
- |
- |
|
Owo 30 - Enterobacter gergoviae
|
- |
- |
- |
|
Owo 13 - Burkholderia cepacia
|
- |
- |
- |
|
Owo 22 - Pseudomonas fluorescens
|
- |
12 mm |
15 mm |
Specimen Number
A = Aloe barbadensis + Chitosan (from snail shell) ABA
B = Aloe barbadensis + Chitosan (from shrimp) ABB
C = Curcuma longa + Chitosan (from shrimp) CLC

Figure 2.
Typical Plant Sources: Phyllanthus niruri
.
Typical Plant Sources: Phyllanthus niruri

Figure 4.
Croton zambesicus
.
Croton zambesicus

Figure 5.
Moringa Oleifera
.
Moringa Oleifera

Figure 6.
Ocimum gratissimum
.
Ocimum gratissimum

Figure 7.
Ocimum gratissimum
.
Ocimum gratissimum

Figure 8.
Andrographis paniculata
.
Andrographis paniculata

Figure 10.
Cultures of Bacterial Strains Showing Zones of Inhibition Against Applied Samples
.
Cultures of Bacterial Strains Showing Zones of Inhibition Against Applied Samples
Table 4.
Average MIC and MBC for Selected Isolates Tested
|
Name
|
Control
|
12.5
|
25
|
50
|
100
|
|
Staphylococcus aureus
|
29 |
- |
11 |
13 |
21 |
|
Bacillus cereus
|
24 |
22 |
22 |
24 |
26 |
|
Escherichia coli
|
15 |
12 |
14 |
14 |
15 |
|
Salmonella typhi
|
22 |
- |
- |
- |
14 |
|
Proteus mirabilis
|
22 |
9 (BS) |
9.5 (BS) |
11 (BS) |
12 (BS) |
|
Pseudomonas aeruginosa
|
15 |
- |
- |
11 (BS) |
11 |
|
Salmonella pullorum
|
18 |
- |
- |
20 |
22 |
Note. MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration; Ofloxacin was used as the standard antibiotics control.
The result of the use of sulphur NPs has been positive and encouraging. Other tested NPs, including Fe3O4 NPs and Fe3O4@Si NPs, revealed relatively low activity, except at 100 mg/mL for some multidrug-resistant strains such as AKR 18 - Enterobacter agglomerans, OKI 10 - Acinetobacter haemolyticus, and T30 - Klebsiella oxytoca. Similarly, NPs combined with plant extracts such as Ocimum gratissimum, tested as alternate antimicrobial agents against these resistant clinical strains, partly, demonstrated relatively high activity, possibly due to some synergistic reactions (Table 5)..Generally, typical medicinal plant sources that their bioactive substances were tested for this research are shown in Figures 2 to 10.
Table 5.
Iron Oxide Nanoparticles Tested Cultures
|
Bacterial Isolates
|
Zone of Inhibition in (MM)
|
|
Lab code
|
A
|
B
|
C
|
D
|
E - Control
|
| AKR 18 - Enterobacter agglomerans |
25 |
- |
- |
- |
25 |
| T18 - Klebsiella oxytoca |
- |
32
|
33
|
- |
30 |
| OKI 10 - Acinetobacter haemolyticus |
22 |
- |
- |
- |
- |
| T30 - Klebsiella oxytoca |
24 |
34
|
- |
- |
32 |
Key: A – Fe3O4 NPS (50%); B – Fe3O4 NPS (100%); C – Fe3O4 NPS + Ocimum gratissimum L.; D – Fe3O4 @Si; E – Control, Ofloxacin; BS – Bacteriostatic.
Note. MIC: Minimum inhibitory concentration. The average MIC for Andrographis paniculata, which is among the most potent antimicrobial agents against multidrug-resistant strains, and the mostly tested plant extract is 25 mg/mL, while the MBC stands at 50 mg/mL.
Discussion
The emergence of multidrug-resistant microbes is globally worrisome. Thus, frantic scientific efforts have been made to conquer this resistant battalion. The findings of this study demonstrated the potential and complementary use of nanomaterials conjugated with chitosan in the control of multidrug-resistant and recalcitrant-etiologic agents (Tables 1 and 2). Nanomaterials are developed to exhibit novel physical, chemical, and biological characteristics that make them suitable for use in various applications, including the degradation of pollutants and wastewater treatment. The findings further highlighted the potency of the nanomaterials used for biocontrol purposes, which corroborates with the results of a study by Rezaei et al(18), confirming the benefits of the use of NPs in the control of diseases and some damages that can be incurred in the process if care is not taken.
Nanomaterials are also used as nanofilters for water purification. Similarly, they are valuable for pollutant sensing, which is another important application of microbial nanotechnology in nanocatalysis. The application of nanomaterials enumerated here corroborates the research and development efforts at the atomic or molecular level to create structures and systems applicable in diverse aspects, as reported by Drexler et al (19) as well as Balzani (20). This nanotechnology approach, with the complementary use of chitosan, covers various fields of the medical, pharmaceutical, clinical, and food industries (5).
Nanomaterials can be grouped into four major types, including inorganic-based, carbon-based, organic-based, and composite-based nanomaterials. However, SNPs prepared by sodium thiosulphate and hydrochloric acid capped with chitosan in this study showed diversified antimicrobial potentials on test isolates. The results of this study also conform to those of the study by Kalia et al (21), demonstrating the potential inhibitor for quorum sensing-controlled virulence factors and biofilm formation in P. aeruginosa,which helps overcome the multidrug-resistant nature of this kind. As intensified in this study, SNPs are more effective than Fe3O4 NPs and Fe3O4@Si NPs that represented relatively low activity, except at 100 mg/mL for some multidrug-resistant strains such as AKR 18 - Enterobacter agglomerans, OKI 10 - Acinetobacter haemolyticus, and T30 - Klebsiella oxytoca. Similarly, NPs combined with plant extracts such as Ocimum gratissimumtested as alternate antimicrobial agents against these resistant clinical strains partly revealed relatively high activity, possibly due to some synergistic reactions (Table 5).
To this end, the synergistic impact of the use of nanomaterials has intensified clinical specificity in this research and diversified applications, as reported by Upadhayay et al(22); they demonstrated the synergistic impact of nanomaterials and plant probiotics in agriculture. This study enhances measures to safeguard health through the adaptation of an appropriate nutritional approach. Further work is still ongoing on green NP synthesis.
The complementary use of chitosan, which is a biopolymer as a nanocarrier, is found valuable for its ability to reduce infectivity when conjugated and synergized with some potent medicinal plants. However, pharmacological standardization and clinical evaluation of medicinal plants are essential to making the natural products discussed in this context standard remedies that could effectively combat some mutated pathogens that have developed resistance against antibiotic abuse. The study of chitosan has proven its use to be valuable for clinical applications. It can also be useful for some industrial purposes, including animal feed supplements (23).
Chitosan-based biomaterials have been used in various biomedical and industrial processes, including drug tissue engineering, wound healing, regenerative medicine, blood anticoagulation, bone, tendon, or blood vessel engineering, dentistry, biotechnology, biosensing, cosmetics, water treatment, agriculture, and vaccine systems (24).
Chitosan composites have been conjugated with bioactive compounds and NPs for clinical purposes. For instance, chitosan-based composites have been employed in the preservation of meat and meat products, postharvest foods, and monitoring freshness/spoilage. In the biomedical industry, chitosan-based composites have been utilized in drug delivery, tissue engineering, and wound healing (24,25).
The antimicrobial properties of chitosan-based composites have undergone extensive investigation. Chitosan has been shown to have significant antimicrobial activity against a wide variety of fungi and bacteria. However, the mechanism of action for its antimicrobial activity is not yet fully understood, and further research is needed to establish a consensus. Chitosan-based (nano) materials have also attracted significant attention in the biomedical field due to their unique biocompatible, non-toxic, and antimicrobial nature. Chitosan-based nanomaterials have been used as antimicrobial wound dressings, brain drug delivery carriers, and in other biomedical applications (26,27).
Chitosan-based composites have been utilized in the preservation of food products by incorporating plant extracts to enhance their antimicrobial and antioxidant properties. This approach has been shown to improve the shelf life and quality of food products while minimizing the use of plastic material (28,29).
In summary, chitosan composites have shown great potential in clinical applications due to their unique properties and versatility. Further research is required to fully understand the mechanisms of action and to expand their applications in various fields.
Conclusion
The complementary use of Chitosan which is a biopolymer as nanocarrier is found valuable for its ability to reduce infectivity when conjugated and synergized with some potent medicinal plants. However, pharmacological standardization and clinical evaluation on medicinal plants are essential to make the natural products discussed in this context to be standard remedies that could effectively combat some mutated pathogens that have developed resistance against antibiotics abuse. Study of chitosan has proven its use to be valuable for clinical applications. It can also be useful for some industrial purposes including animal feed supplements. Use of some nanomaterials in biosensors can be much valuable in diseases detection. This research work therefore provide a valuable database that can be adopted by health management systems.
Acknowledgements
This paper was presented at the LASU-Humboldt Kolleg /International Conference in May 2023, Nigeria. I appreciate the support of the staff members of the Centre for Infectious Disease Control and Drug Development as well as the management of Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria, for providing the enabling environment for this research.
Authors’ Contribution
Conceptualization: Adedayo Olajide Ajayi.
Data curation: Olugbenga Ebenezer Ige.
Formal analysis: Adedayo Olajide Ajayi.
Funding: Olugbenga Ebenezer Ige.
Funding acquisition: Olugbenga Ebenezer Ige.
Investigation: Patience Yakubu.
Methodology: Adedayo Olajide Ajayi.
Project administration: Adedayo Olajide Ajayi.
Resources: Olugbenga Ebenezer Ige.
Software: Yakubu Patience.
Supervision: Olugbenga Ebenezer Ige.
Validation: Olugbenga Ebenezer Ige.
Competing Interests
There is no conflict of interests.
Ethical Approval
AAUA Conference and Research Grant Committee 2023/2024.
Funding
Adekunle Ajasin University. Centre for Infectious Disease Control and Drug Development. CIDCDD Academic research.2023/2024.
References
- Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules 2019; 25(1):112. doi: 10.3390/molecules25010112 [Crossref] [ Google Scholar]
- Bullock G, Blazer V, Tsukuda S, Summerfelt S. Toxicity of acidified chitosan for cultured rainbow trout (Oncorhynchus mykiss). Aquaculture 2000; 185(3-4):273-80. doi: 10.1016/s0044-8486(99)00359-2 [Crossref] [ Google Scholar]
- Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state-of-the-art review. Int J Food Microbiol 2010; 144(1):51-63. doi: 10.1016/j.ijfoodmicro.2010.09.012 [Crossref] [ Google Scholar]
- Harish Prashanth KV, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential—an overview. Trends Food Sci Technol 2007; 18(3):117-31. doi: 10.1016/j.tifs.2006.10.022 [Crossref] [ Google Scholar]
- Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci 2006; 31(7):603-32. doi: 10.1016/j.progpolymsci.2006.06.001 [Crossref] [ Google Scholar]
- Singh D, Sharma D, Agarwal V. Screening of anti-microbial, anti-biofilm activity, and cytotoxicity analysis of a designed polyherbal formulation against shigellosis. J Ayurveda Integr Med 2021; 12(4):601-6. doi: 10.1016/j.jaim.2021.06.007 [Crossref] [ Google Scholar]
- Aruchami M, Gowri N, Sundara-Rajulu G. Chitin deacetylases in invertebrates. In: Muzzarelli RA, Jeuniaux C, Gooday GW, eds. Chitin in Nature and Technology. New York, NY: Plenum Press; 1986. p. 263-5.
- Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev 2010; 62(1):3-11. doi: 10.1016/j.addr.2009.09.004 [Crossref] [ Google Scholar]
- Ngo DH, Vo TS, Ngo DN, Kang KH, Je JY, Pham HN. Biological effects of chitosan and its derivatives. Food Hydrocoll 2015; 51:200-16. doi: 10.1016/j.foodhyd.2015.05.023 [Crossref] [ Google Scholar]
- Thermo Fisher Scientific. Methods Optimization in Accelerated Solvent Extraction in Technical Note. Thermo Fisher Scientific; 2013.
- AOAC. Official Methods of Analysis. 18th ed. Maryland, USA: AOAC International; 2005.
- Azwanida NN. Azwanida NNA review on the extraction methods use in medicinal plants, principle, strength and limitationMed Aromat Plants. 2015 Jul 6; 4(3):196. doi: 10.4172/2167-0412.1000196 [Crossref] [ Google Scholar]
- Ajayi AO, Olajubu FA, Fadipe DO, Babaleye DO. Antibiogram and molecular analysis of clinical bacteria isolates from the three geographical regions of Ondo state, Nigeria. J Clin Exp Immunol 2020; 5(1):12-9. [ Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 2016; 6(2):71-9. doi: 10.1016/j.jpha.2015.11.005 [Crossref] [ Google Scholar]
- CLSI M100 - Clinical and Laboratory Standards Institute antimicrobial susceptibility testing standards. Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition. CLSI M02, Performance Standards for Antimicrobial Disk Susceptibility Tests -14th Edition (2024).
- Maulin S, Heet C, Gaurav D, Rushabh P, Anjali B, Sandeep R. Synthesis and Antimicrobial Properties of Chitosan. 2017.
- Paul ED, Garba ZN, James DO. Synergistic-antagonistic antibacterial potential of chitosan composites with Moringa oleifera leaf powder. J Appl Sci Environ Manage 2019; 23(4):759-62. doi: 10.4314/jasem.v23i4.29 [Crossref] [ Google Scholar]
- Rezaei R, Safaei M, Mozaffari HR, Moradpoor H, Karami S, Golshah A. The role of nanomaterials in the treatment of diseases and their effects on the immune system. Open Access Maced J Med Sci 2019; 7(11):1884-90. doi: 10.3889/oamjms.2019.486 [Crossref] [ Google Scholar]
- Drexler KE, Peterson C, Pergamit G. Unbounding the Future: The Nanotechnology Revolution. William Morrow and Company; 1991.
- Balzani V. Nanoscience and nanotechnology: a personal view of a chemist. Small 2005; 1(3):278-83. doi: 10.1002/smll.200400010 [Crossref] [ Google Scholar]
- Kalia M, Singh D, Sharma D, Narvi SS, Agarwal V. Senna alexandriana Mill as a potential inhibitor for quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Pharmacogn Mag 2020; 16(72):797-802. doi: 10.4103/pm.pm_315_20 [Crossref] [ Google Scholar]
- Upadhayay VK, Chitara MK, Mishra D, Jha MN, Jaiswal A, Kumari G. Synergistic impact of nanomaterials and plant probiotics in agriculture: a tale of two-way strategy for long-term sustainability. Front Microbiol 2023; 14:1133968. doi: 10.3389/fmicb.2023.1133968 [Crossref] [ Google Scholar]
- Pal K, Bharti D, Sarkar P, Anis A, Kim D, Chałas R. Selected applications of chitosan composites. Int J Mol Sci 2021; 22(20):10968. doi: 10.3390/ijms222010968 [Crossref] [ Google Scholar]
- Fatullayeva S, Tagiyev D, Zeynalov N, Mammadova S, Aliyeva E. Recent advances of chitosan-based polymers in biomedical applications and environmental protection. J Polym Res 2022; 29(7):259. doi: 10.1007/s10965-022-03121-3 [Crossref] [ Google Scholar]
- Kumar A, Yadav S, Pramanik J, Sivamaruthi BS, Jayeoye TJ, Prajapati BG. Chitosan-based composites: development and perspective in food preservation and biomedical applications. Polymers (Basel) 2023; 15(15):3150. doi: 10.3390/polym15153150 [Crossref] [ Google Scholar]
- Nasaj M, Farmany A, Shokoohizadeh L, Aziz Jalilian F, Mahjoub R, Roshanaei G. Development of chitosan-assisted Fe3O4@SiO2 magnetic nanostructures functionalized with nisin as a topical combating system against vancomycin-intermediate Staphylococcus aureus (VISA) skin wound infection in mice. J Nanomater 2022; 2022(1):2914210. doi: 10.1155/2022/2914210 [Crossref] [ Google Scholar]
- Nasaj M, Farmany A, Shokoohizadeh L, Aziz Jalilian F, Mahjoub R, Roshanaei G. Vancomycin and nisin-modified magnetic Fe3O4@SiO2 nanostructures coated with chitosan to enhance antibacterial efficiency against methicillin resistant Staphylococcus aureus (MRSA) infection in a murine superficial wound model. BMC Chem 2024; 18(1):43. doi: 10.1186/s13065-024-01129-y [Crossref] [ Google Scholar]
- Souza VGL, Pires JRA, Rodrigues C, Coelhoso IM, Fernando AL. Chitosan composites in packaging industry-current trends and future challenges. Polymers (Basel) 2020; 12(2):417. doi: 10.3390/polym12020417 [Crossref] [ Google Scholar]
- Shukla SK, Mishra AK, Arotiba OA, Mamba BB. Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 2013; 59:46-58. doi: 10.1016/j.ijbiomac.2013.04.043 [Crossref] [ Google Scholar]