Avicenna Journal of Clinical Microbiology and Infection. 11(3):125-129.
doi: 10.34172/ajcmi.3506
Original Article
In Vitro Investigation of the Effects of Certain Antibiotics and Plant Oils on the Clinical Isolates of Streptococcus pneumoniae
Mazen Safi 1, *  , Marwa Khawajkiah 1, Nour Al-Sahar 1, Ayman Al-Mariri 1
, Marwa Khawajkiah 1, Nour Al-Sahar 1, Ayman Al-Mariri 1
Author information:
1Department of Molecular Biology and Biotechnology, Atomic Energy Commission, Damascus, Syria
Abstract
Background: With the serial trend toward antibiotic resistance in Streptococcus pneumoniae, novel drug development is needed to deal with pathogenic microorganisms that have developed widespread microbial resistance to antibiotics.
Methods: Eighty-seven clinical samples were collected from six hospitals in Damascus. A polymerase chain reaction (PCR) was performed to identify the bacterial genus and type. Minimum inhibitory concentrations on Luria-Bertani agar were conducted using several antibiotics and essential oils (EOs).
Results: Twenty-five isolates of S. pneumoniae were found, and amoxicillin and cephalosporins were the most effective antibiotics against 90% of S. pneumoniae bacteria. On the other hand, Thymus syriacus Boiss., Origanum syriacum L., Rosmarinus officinalis L., Cinnamomum zeylanicum L., and Juniperus foetidissima Willd were the most effective EOs.
Conclusion: Only T. syriacus Boiss., O. syriacum L., R. officinalis L., C. zeylanicum L., and J. foetidissima Willd oils had good inhibitory effects against S. pneumoniae.
Keywords: Antibiotics, Essential oils, MIC, Streptococcus pneumoniae
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Safi M, Khawajkiah M, Al-Sahar N, Al-Mariri A. In vitro investigation of the effects of certain antibiotics and plant oils on the clinical isolates of Streptococcus pneumoniae. Avicenna J Clin Microbiol Infect. 2024; 11(3):125-129. doi:10.34172/ajcmi.3506
Introduction
Streptococcus is still the number one cause of lung diseases acquired through contact between people in adults and children (1). It is responsible for more than 1.6 million deaths annually, mostly in children and the elderly (2). There are increasing concerns around the world regarding high levels of resistance to antibiotics as well as a lack of efficacy in antimicrobial therapy, leading to the ineffectiveness of treatment and a decline in the usefulness of previous treatment programs. Streptococcus pneumoniae can be found in the upper part of the throat and causes most cases of community-acquired respiratory infections. Infections with such bacteria cause a huge increase in disease and mortality rates, such as serious meningitis and pneumonia (3-5). In many Asian countries, treatment options for antibacterial infections are normally experimental due to the lack of specialized laboratories to detect bacterial susceptibility tests, as well as its very elevated costs (6). High antibiotic resistance renders existing treatments ineffective and threatens future efforts to treat bacterial infections caused by these bacteria (7-9). Therefore, follow-up studies are a good tool for profiling antibiotic susceptibility and guiding experimental therapy (10).
This study aims to isolate and characterize a few isolates of S. pneumoniae by using reliable serological diagnostic methods as well as the polymerase chain reaction (PCR) by applying specific primers in order to study their sensitivity against some antibiotics and essential oils (EOs). Considering that there are different resistance levels through different geographic regions, awareness about the presence of local resistance types is important before conducting experimental therapy in vivo.
Materials and Methods
Collection of Samples
Eighty-seven clinical samples were collected from six hospitals in Damascus between January and August 2019. These samples were distributed as 39 blood samples, 20 bronchial secretions, 18 pharyngeal swabs, and 10 oral swabs.
Isolation of Streptococcus pneumoniae
Briefly, the samples were cultured on blood agar media with 5% sheep blood and then incubated for 24 hours at 37°C in the presence of 5% CO2.
Biochemical Tests
An isolate was considered to be S. pneumoniae if it was alpha-hemolytic, bile soluble, optochin susceptible, and formed gram-positive cocci in chains. The fermentation of some sugars was tested using motion, catalase, and oxidase tests.
Extraction of DNA
The cetyltrimethylammonium bromide method was used to isolate DNA (11). Briefly, 2 µL of DNA extraction in the Tris-ethylenediamine tetra-acetic acid buffer was read using a Nanodrop machine in order to detect DNA concentration. This buffer was used as a blank. Then, the concentration of 100 ng/mL in each sample was prepared and stored at -20 °C.
Polymerase Chain Reaction
In the preparation of the single sample for the PCR, a set of chemical and molecular materials were utilized with a final volume of 25 µL (1.5 mM MgCl2, 1X PCR buffer, 0.2 mM dNTPs, 1U DNA polymerase enzyme, 200 ng bacterial DNA, and 25 pmol of each set of forward and reversed primers). In addition, PCR amplification was performed using two sets of specific primer pairs (Table 1). PCR results were observed under UV light after electrophoresis in agarose gel pre-stained with ethidium bromide.
Table 1.
Polymerase Chain Reaction Primers
|
|
Sequence 5`-3`
|
Gene
|
Size (bp)
|
Annealing (˚C)
|
|
Streptococcus sp. |
GTACAGTTGCTTCAGGACGTATC |
tuf
|
761 |
55 |
| ACGTTCGATTTCATCACGTTG |
|
Streptococcus pneumoniae
|
ATTTCTGTAACAGCTACCAACGA |
ply
|
348 |
52 |
| GAATTCCCTGTCTTTTCAAAGTC |
| TGGCAAGGTAAACTTCTAAAGCA |
| GCCCCTTCACAGTTGGTTAG |
Plant Sample Collection
The samples used in this study were bought from Damascus markets or collected during the flowering season from different Syrian regions (Table 2). They included T. syriacusBoiss., O. syriacumL., L. stoechas L., R. officinalis L. (Lamiaceae), M. communis L., S. aromaticumL. (Myrtaceae), C. zeylanicum L. (Lauraceae), J. foetidissimaWilld (Cupressaceae), P. roseumL. (Geraniaceae), and D. maritima L(Liliaceae). Leaves harvested during the pre-flowering stage were properly cleaned from all contaminants before the extraction procedure.
Table 2.
Sources and Characterizations of Studied Plants
|
Original Name
|
Family
|
Site
|
Hight (m)
|
Time of Collection
|
Used Part
|
|
T. syriacusBoiss. |
Lamiaceae
|
Damascus mountain (Alsoja) |
800 |
July |
Aerial parts |
|
O. syriacum L. |
Lamiaceae
|
Kafr Nobol-Idlib |
450 |
June |
Aerial parts |
|
L. stoechas L. |
Lamiaceae |
Countryside of Tartous |
250 |
May |
Aerial parts |
|
M. communis L. |
Myrtaceae
|
Latakia |
350 |
June |
Leaves |
|
S. aromaticum L. |
Myrtaceae |
Market |
|
|
Flowers |
|
C. zeylanicum L. |
Lauraceae
|
Market |
|
|
Barks |
|
R. officinalis L. |
Lamiaceae
|
Jableh |
350 |
May |
Aerial parts |
|
J. foetidissima Wild |
Cupressaceae
|
Damascus Alsabourah |
780 |
July |
Leaves |
|
P. roseumL. |
Geraniaceae
|
Damascus Doummar |
900 |
April |
Aerial parts |
|
D. maritima L. |
Liliaceae
|
Countryside of Tartous |
320 |
April |
Bulbs |
Isolation of Essential Oils
Before steam distillation, parts of the studied plants were finely chopped after drying them well using appropriate grinders. A water steam distillation device (Clevenger-type apparatus) was applied to isolate EOs. The European Pharmacopoeia method was followed (12), and the process of obtaining EOs was explained in detail in a previous article by the same researchers of this study (13). In brief, starting from 100 g of plant material, EOs are obtained using the distillation apparatus. The resulting oils are diluted in dimethyl sulfoxide and stored properly.
Essential Oil Susceptibility Assay
The susceptibility determination of EOs on sterile filter paper discs (6 mm in diameter) was explained in detail in a previous study conducted by the same researchers as this study (13). For this purpose, 50 μL of 5% concentration of each EO was added to each sterile paper, and 0.1 mL of the bacterial suspension was added with a final inoculum bacterial number of 106 CFU/mL.
Essential Oil Minimum Inhibitory Concentrations Determination
A broth susceptibility testing method was followed using three replicates for each EO (14). Ninety-six-well microtiter plates and Brucella® broth medium were used in this study. Concentration ranges of EOs were previously explained (13), and 1 x 106 CFU/mL of S. pneumoniae were added to each well. An average concentration of each EO has been adopted, ranging from 0.75 to 100 μL/mL. Positive (bacteria without EOs) and negative (without bacteria) controls were also performed with the same conditions. The plates were incubated for 24 hours at 37 °C. Minimum inhibitory concentration50 (MIC) and MIC90 were determined as previously explained (13).
Minimum Inhibitory Concentrations Determination for Antibiotics
Recommendations of the Clinical Laboratory Standards Institute (15) were followed to determine the MICS of each antibiotic. The method of microdilution broth using 96 microwell plates was applied in antibiotic susceptibility estimation. The process of antibiotic susceptibility, MIC, and MIC90 estimation was explained in detail in a previous article by the same researchers of this study (16), using 106 CFU/mL of bacteria in each micro-well plate. The average of three tests for each antibiotic was taken into account. Investigated antibiotics were penicillin G, amoxicillin, cefixime, cefpodoxime, cefotaxime, ceftriaxone, cefazolin, cefuroxime, ofloxacin, cotrimoxazole, erythromycin, spiramycin, azithromycin, chloramphenicol, vancomycin, levofloxacin, tetracycline, rifampicin, trimethoprim-sulfamethoxazole, and sparfloxacin (Oxoid, UK).
Results
Bacteria Identification
Overall, 27 positive isolates (27/87, 31%) were obtained when culturing was performed on S. pneumoniae selective media. They were alpha-hemolytic, without motion, catalase-negative, oxidase-negative, bile soluble, and Optochin-sensitive. In addition, they were all capable of fermenting galactose, raffinose, sucrose, inulin, and glucose. Finally, the arginine dihydrolase test was positive.
Results of Polymerase Chain Reaction
Electrophoresis of PCR products on the agarose gel confirmed that 25 out of the 27 (92.5%) isolates confirmed by biochemical tests belonged to S. pneumoniae (Figure 1).
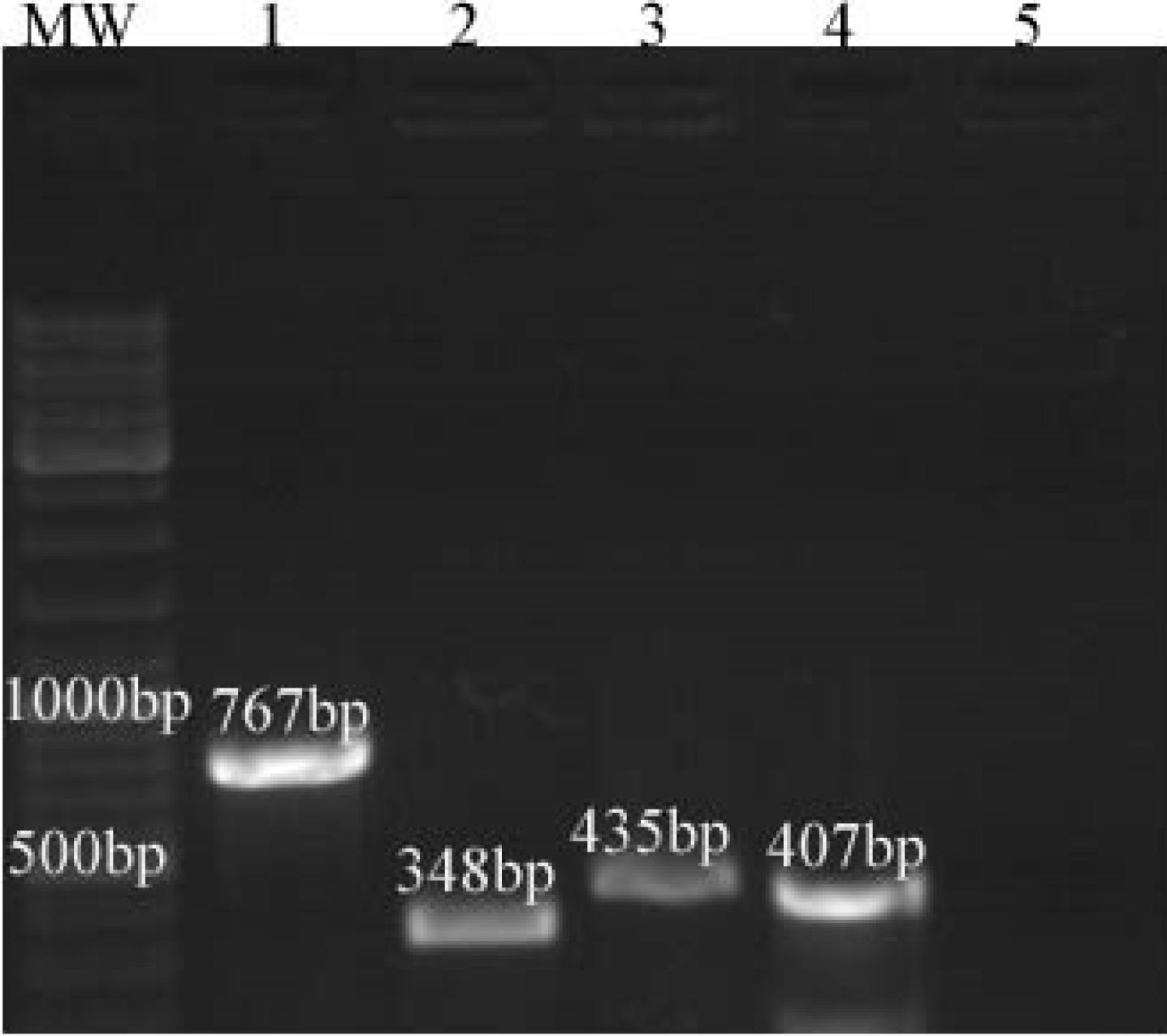
Figure 1.
PCR on a 1% Agarose Gel Where: (1) Streptococcus spp. (Sample From Damascus Hospital), (2) Streptococcus pneumoniae Type (Bronchial Secretions), (3) Streptococcus mutans Type (Oral Swab, Positive Control), (4) Streptococcus pyogenes Type (Pharyngeal Swab, Positive Control), and (5) Negative Control Without DNA; MW: DNA Molecular Weight Marker
.
PCR on a 1% Agarose Gel Where: (1) Streptococcus spp. (Sample From Damascus Hospital), (2) Streptococcus pneumoniae Type (Bronchial Secretions), (3) Streptococcus mutans Type (Oral Swab, Positive Control), (4) Streptococcus pyogenes Type (Pharyngeal Swab, Positive Control), and (5) Negative Control Without DNA; MW: DNA Molecular Weight Marker
Sensitivity of Streptococcus pneumoniae Isolates to Essential Oils
Based on the primary screening results, the most effective EOs were T. syriacusBoiss. (19-23 mm), O. syriacumL. (18-24 mm), and R. officinalis L. (17-223 mm), whereas P. roseumL.and D. maritima L. EOs were ineffective against S. pneumoniae isolates used in this study.
Essential Oil Minimum Inhibitory Concentrations
The results of Table 3 demonstrate the MICs of several EOs against 25 isolates of S. pneumoniae. Based on the data, the most effective EOs against these isolates, depending on the inhibition of 90% of bacteria, were T. syriacus Boiss., O. syriacum L., R. officinalis L., C. zeylanicum L., and J. foetidissima Willd. Conversely, O. syriacum L. EO presented the most MIC50 potent inhibition against S. pneumoniae (MIC50: 0.0625–0.125 µL/mL). However, P. roseumL. and D. maritima L. EOs produced the highest MIC50 and MIC90 values in the range of 1–2 µL/mL and 4–6 µL/mL, respectively.
Table 3.
MIC50 and MIC90 (µL/mL) Ranges of Some Essential Oils on 25 Isolates of Streptococcus pneumoniae
|
Essential Oils
|
MIC50
|
MIC90
|
|
T. syriacusBoiss. |
0.125-0.25 |
0.25-0.5 |
|
O. syriacum L. |
0.0625-0.125 |
0.125-0.25 |
|
L. stoechas L. |
1-2 |
4-8 |
|
M. communis L. |
0.5-2 |
4-6 |
|
S. aromaticum L. |
0.5-1 |
2-4 |
|
C. zeylanicum L. |
0.125-0.25 |
0.125- |
|
R. officinalis L. |
0.25-0.125 |
0.5-1 |
|
J. foetidissima Wild |
0.25-0.5 |
0.5-2 |
|
P. roseumL. |
1-2 |
4-6 |
|
D. maritima L. |
1-2 |
4-6 |
Note. MIC:Minimum inhibitory concentration.
Antibiotic Minimum Inhibitory Concentration Test
Table 4 provides MIC50 and MIC90 of several antibiotics on 25 isolates of S. pneumoniae. The results revealed that the most effective antibiotics against these isolates, depending on the inhibition of 90% of bacteria, were cefixime, cefpodoxime, cefuroxime, ceftriaxone, ofloxacin, vancomycin, levofloxacin, rifampicin, and sparfloxacin. Contrarily, spiramycin, penicillin G, amoxicillin, cefazolin, and erythromycin were the least effective. Vancomycin and Sparfloxacin showed the highest effect (MIC90 = 0.125–0.25 µg/mL).
Table 4.
MIC50 and MIC90 (µg/mL) of Some Antibiotics on 25 Isolates of Streptococcus pneumoniae
|
Antibiotic
|
MIC50
|
MIC90
|
| Penicillin G |
0.125-0.25 |
2-4 |
| Amoxicillin |
0.25-1 |
2-4 |
| Cefixime |
0.125-1 |
0.5-1 |
| Cefpodoxime |
0.062-0.25 |
0.25-0.5 |
| Cefotaxime |
0.125-0.25 |
0.5-1 |
| Ceftriaxone |
0.062-0.125 |
1-2 |
| Cefazolin |
0.25-1 |
2-4 |
| Cefuroxime |
0.125-0.25 |
0.25-1 |
| Ofloxacin |
0.25-0.5 |
0.5-1 |
| Cotrimoxazole |
0.5-1 |
1-2 |
| Erythromycin |
1-2 |
2-4 |
| Spiramycin |
1-2 |
4-6 |
| Azithromycin |
0.5-1 |
1-2 |
| Chloramphenicol |
0.5-1 |
1-2 |
| Vancomycin |
0.062-0.125 |
0.125-0.25 |
| Levofloxacin |
0.125-0.25 |
0.5-1 |
| Tetracycline |
0.25-0.5 |
1-2 |
| Rifampicin |
0.062-0.125 |
0.125-0.25 |
| Trimethoprim-Sulfamethoxazole |
0.25-0.5 |
1-2 |
| Sparfloxacin |
0.062-0.125 |
0.125-0.25 |
Note. MIC:Minimum inhibitory concentration.
Discussion
Global interest is increasing regarding finding alternatives to traditional treatments for S. pneumoniae due to the increasing resistance of these bacteria to almost all these therapies. Zafar et al indicated that almost 29.7% of S. pneumoniae isolates represented significantly low sensitivity to erythromycin (6), with a resistance of 28% of the isolates. In another study of the same group, the resistance rate of S. pneumoniae to erythromycin increased to 54.8% in 2016 (17).
Therefore, when selecting the appropriate antibiotic protocol used to treat diseases caused by S. pneumoniae (e.g., pneumonia and meningitis), the researchers should keep in mind the high number of strains resistant to penicillin and macrolides (penicillin-intermediate S. pneumoniae). Several clinical studies have shown the necessity of accurately calculating the doses of penicillin and erythromycin if reduced sensitivity of isolates to these antibiotics is suspected (18). Clinical guidelines in many countries suggest elevated doses of some beta-lactam groups because penicillin-intermediate S. pneumoniae strains might not be susceptible to doses that have been used since more than thirty years ago. However, some studies investigating antibiotic resistance in Pakistan confirmed the full efficacy of the amoxicillin/clavulanic acid and cefuroxime combination, increasing the confidence in their continued use as a good regimen therapy against S. pneumoniae infections (17).
Some microbiological assay results revealed the potent efficacy of some EOs (including peppermint, cinnamon bark, thyme, and the like). Accordingly, further research should be conducted to estimate their suggested role as alternatives or supported therapies to traditional agents. Its multi-ingredient formula expresses its antibacterial benefit.
In our study, the most effective EOs on S. pneumoniae were T. syriacusBoiss., O. syriacumL., R. officinalis L., C. zeylanicum L.,and J. foetidissimaWilld. Our results are consistent with those of De Aguiar et al, indicating that MICs for several EOs, including Origanum, Cinnamomum, Thymus, and Rosmarinus, were low and ranged from 0.312 µg/mL to 0.625 µg/mL (19). Inouye et al also found that thyme and cinnamon bark were highly effective against these types of bacteria (20). In addition, a previous study showed similar results for MIC and the potent antibacterial activity of cinnamon bark against these bacteria (21).
EOs commonly consist of a combination of substances (22). The major constituents of cinnamon bark and thyme are cinnamaldehyde and carvacrol, respectively, and those of rosemary oils include α-pinene, 1,8-cineole, and camphor, indicating that the activity of these oils was due to their major constituents.
Conclusion
The current study evaluated the antibacterial effect of 20 widely used antibiotics against 25 isolates of S. pneumoniae. Penicillin appeared to be relatively effective, and amoxicillin and cephalosporins were highly effective. Azithromycin and erythromycin were also highly influential and could be used as a first-line alternative treatment. Interestingly, vancomycin and levofloxacin were also highly effective and could be potential alternative treatment options for streptococcal infections. In this study, it was found that most of the EOs had a moderate effect on these bacteria, and only the EOs of T. syriacusBoiss., O. syriacumL., R. officinalis L., C. zeylanicum L.,and J. foetidissimaWilld. had a good effect on the isolates tested in this study.
Authors’ Contribution
Conceptualization: Ayman Al-Mariri, Mazen Safi.
Data curation: Mazen Safi, Ayman Al-Mariri.
Formal analysis: Mazen Safi.
Investigation: Mazen Safi, Marwa Khawajkiah.
Methodology: Mazen Safi, Ayman Al-Mariri.
Project administration: Mazen Safi, Ayman Al-Mariri.
Resources: Mazen Safi and Ayman Al-Mariri.
Supervision: Mazen Safi and Ayman Al-Mariri.
Validation: Mazen Safi and Ayman Al-Mariri.
Visualization: Mazen Safi, Marwa Khawajkiah, Nour Al-Sahar.
Writing-original draft: Mazen Safi.
Writing-review & editing: Mazen Safi.
Competing Interests
There are no competing interests to be declared.
Ethical Approval
This study was conducted in vitro. There is no experiment conducted on humans or live animals.
Funding
None.
References
- Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA. Prognosis and outcomes of patients with community-acquired pneumonia A meta-analysis. JAMA 1996; 275(2):134-41. [ Google Scholar]
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec 2007; 82(12):93-104. [ Google Scholar]
- File TM. Community-acquired pneumonia. Lancet 2003; 362(9400):1991-2001. doi: 10.1016/s0140-6736(03)15021-0 [Crossref] [ Google Scholar]
- Nicolau D. Clinical and economic implications of antimicrobial resistance for the management of community-acquired respiratory tract infections. J Antimicrob Chemother 2002; 50 Suppl S1:61-70. doi: 10.1093/jac/dkf809 [Crossref] [ Google Scholar]
- Guthrie R. Community-acquired lower respiratory tract infections: etiology and treatment. Chest 2001; 120(6):2021-34. doi: 10.1378/chest.120.6.2021 [Crossref] [ Google Scholar]
- Zafar A, Hussain Z, Lomama E, Sibille S, Irfan S, Khan E. Antibiotic susceptibility of pathogens isolated from patients with community-acquired respiratory tract infections in Pakistan--the active study. J Ayub Med Coll Abbottabad 2008; 20(1):7-9. [ Google Scholar]
- Beekmann SE, Heilmann KP, Richter SS, García-de-Lomas J, Doern GV. Antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and group A beta-haemolytic streptococci in 2002-2003 Results of the multinational GRASP Surveillance Program. Int J Antimicrob Agents 2005; 25(2):148-56. doi: 10.1016/j.ijantimicag.2004.09.016 [Crossref] [ Google Scholar]
- Jacobs MR, Felmingham D, Appelbaum PC, Grüneberg RN. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003; 52(2):229-46. doi: 10.1093/jac/dkg321 [Crossref] [ Google Scholar]
- Pallares R, Fenoll A, Liñares J. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides and quinolones. Int J Antimicrob Agents 2003; 22 Suppl 1:S15-24. doi: 10.1016/j.ijantimicag.2003.08.004 [Crossref] [ Google Scholar]
- Felmingham D, Feldman C, Hryniewicz W, Klugman K, Kohno S, Low DE. Surveillance of resistance in bacteria causing community-acquired respiratory tract infections. Clin Microbiol Infect 2002; 8 Suppl 2:12-42. doi: 10.1046/j.1469-0691.8.s.2.5.x [Crossref] [ Google Scholar]
- Ali R, Al-Achkar K, Al-Mariri A, Safi M. Role of polymerase chain reaction (PCR) in the detection of antibiotic-resistant Staphylococcus aureus. Egypt J Med Hum Genet 2014; 15(3):293-8. doi: 10.1016/j.ejmhg.2014.05.003 [Crossref] [ Google Scholar]
- Council of Europe. European Pharmacopoeia. 7th ed. Strasbourg, France: Council of Europe; 2011. p. 99.
- Safi M, Al-Mariri A. In vitro antibacterial activity of several plant extracts and essential oils against Brucella melitensis. Herba Pol 2014; 60(1):29-38. doi: 10.2478/hepo-2014-0003 [Crossref] [ Google Scholar]
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC. Antimicrobial susceptibility testing. In: Color Atlas and Textbook of Diagnostic Microbiology. 5th ed. Philadelphia: Lippincott-Raven; 1997. p. 785-856.
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard M7-A6. 6th ed. Wayne: National Committee for Clinical Laboratory Standards; 2003.
- Al-Mariri A, Safi M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran J Med Sci 2014; 39(1):36-43. [ Google Scholar]
- Zafar A, Hasan R, Nizamuddin S, Mahmood N, Mukhtar S, Ali F. Antibiotic susceptibility in Streptococcus pneumoniae, Haemophilus influenzae and Streptococcus pyogenes in Pakistan: a review of results from the Survey of Antibiotic Resistance (SOAR) 2002-15. J Antimicrob Chemother 2016; 71(Suppl 1):i103-9. doi: 10.1093/jac/dkw076 [Crossref] [ Google Scholar]
- Heffelfinger JD, Dowell SF, Jorgensen JH, Klugman KP, Mabry LR, Musher DM. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med 2000; 160(10):1399-408. doi: 10.1001/archinte.160.10.1399 [Crossref] [ Google Scholar]
- de Aguiar FC, Solarte AL, Tarradas C, Luque I, Maldonado A, Galán-Relaño Á. Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiologyopen 2018; 7(6):e00613. doi: 10.1002/mbo3.613 [Crossref] [ Google Scholar]
- Inouye S, Yamaguchi H, Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother 2001; 7(4):251-4. doi: 10.1007/s101560170022 [Crossref] [ Google Scholar]
- Choi O, Cho SK, Kim J, Park CG, Kim J. In vitro antibacterial activity and major bioactive components of Cinnamomum verum essential oils against cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. Asian Pac J Trop Biomed 2016; 6(4):308-14. doi: 10.1016/j.apjtb.2016.01.007 [Crossref] [ Google Scholar]
- Laird K, Phillips C. Vapour phase: a potential future use for essential oils as antimicrobials?. Lett Appl Microbiol 2012; 54(3):169-74. doi: 10.1111/j.1472-765X.2011.03190.x [Crossref] [ Google Scholar]