Avicenna Journal of Clinical Microbiology and Infection. 10(3):106-111.
doi: 10.34172/ajcmi.3452
Original Article
Antimicrobial and Anti-pathogenic Activity of New Naphtho [1,2,4] Triazol-Thiadiazin Derivatives
Maryam Kouhkan 1, *  , Miri Mahmoody 2, Jabbar Khalafy 3, Ali Souldozi 2, Mahsa Mohammadlou 3, Nazila Khorram Maslak 3
, Miri Mahmoody 2, Jabbar Khalafy 3, Ali Souldozi 2, Mahsa Mohammadlou 3, Nazila Khorram Maslak 3
Author information:
1Young Researchers and Elite Club, Urmia Branch, Islamic Azad university, Urmia, Iran
2Faculty of Science, Urmia Branch, Islamic Azad University, Urmia, Iran
3Department of Chemistry, Faculty of Science, Urmia University, Urmia 57154, Iran
Abstract
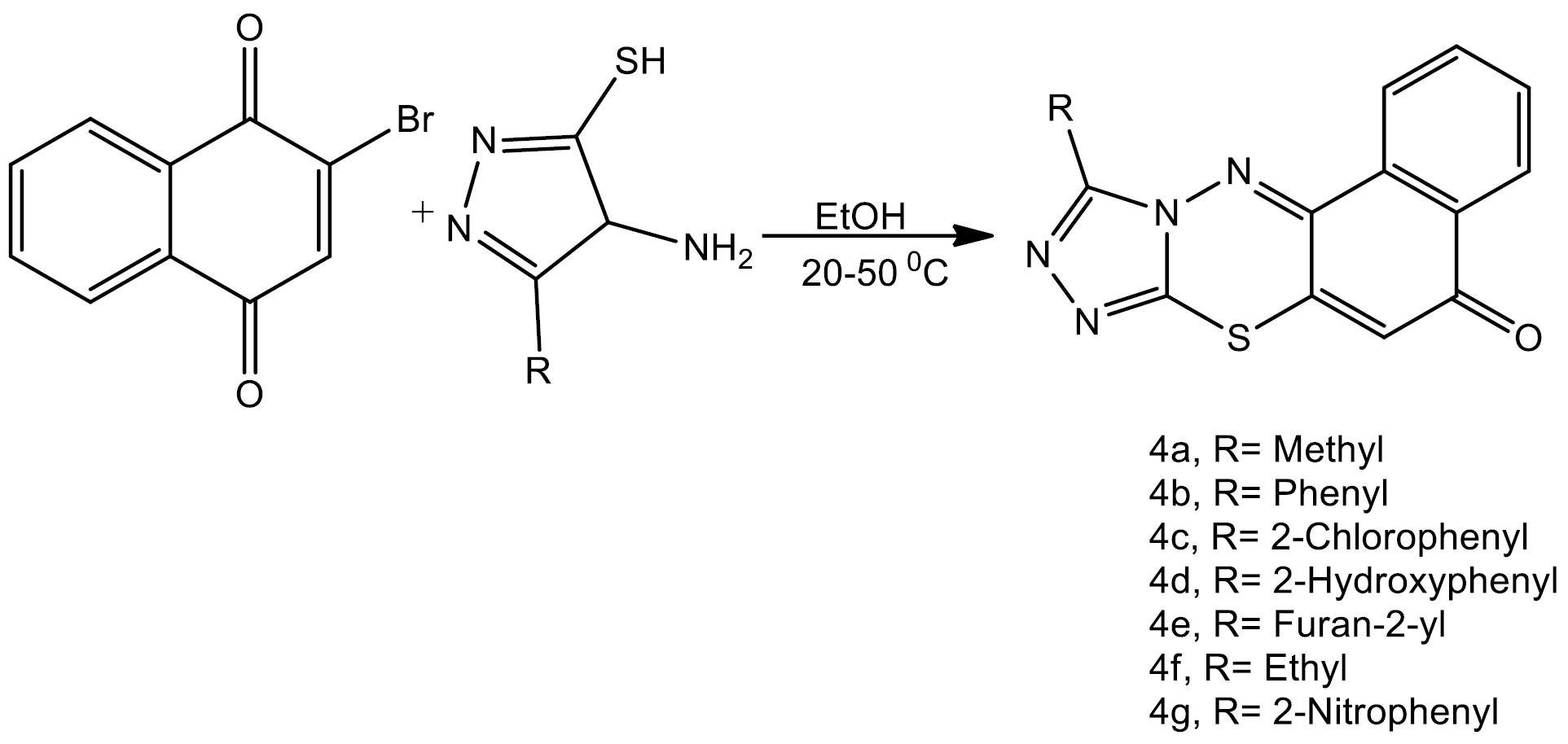
Background: Antimicrobials are one of the extremely important categories of drugs for the treatment, control, and prevention of microbial diseases, but the development of drug resistance against clinically used antibacterial agents has increased the demand for the design and synthesis of new drugs. We have previously synthesized new series of 10-substituted-5H-naphtho[1,2-e][1,2,4]triazolo[3,4-b] [1,3,4]thiadiazin derivatives (4a-4f). In this study, we evaluated the antimicrobial activity of these derivatives against some pathogenic microorganisms.
Methods: The reaction of 2-bromo-1,4-naphthoquinone with 4-amino-5-aryl-4H-1,2,4-triazole-3- thiols in ethanol at 50 °C gave the corresponding 2-[(4-amino-5-aryl-4H-1,2,4-triazol-3 yl)thio] naphthalene-1,4- diones. Moreover, their treatment with EtOH/HCl under reflux conditions produced 10-substituted-5H-naphtho[1,2-e][1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-5- ones through intramolecular cyclization. The well agar diffusion and agar dilution methods were used during the preliminary evaluation of antimicrobial activity for the determination of inhibition zone (IZ) and minimum inhibitory concentration (MIC).
Results: Seven tetracyclic heterocyclic ring systems were produced under reflux conditions. The structures of all the products were identified by their FT-IR, 1H, and 13C NMR spectral data and by elemental analysis. The results revealed that the antibacterial activity of compounds 4a, 4b, 4c, and 4d are higher than that of the others, and compounds 4d, 4a, 4e, and 4f exerted the greatest effect on fungal samples.
Conclusion: All synthesized compounds exhibited promising antibacterial and antifungal activity. In this study, compounds 4a-4g exhibited highly potent antimicrobial activity and acceptable selectivity index against Staphylococcal and Candida infections.
Keywords: Antibacterial activity, Antifungal activity, Antifungal, Triazol, Thiadiazine
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Kouhkan M, Mahmoody M, Khalafy J, Souldozi A, Mohammadlou M, Khorram Maslak N. Antimicrobial and anti-pathogenic activity of new naphtho [1,2,4] triazol-thiadiazin derivatives. Avicenna J Clin Microbiol Infect. 2023; 10(3):106-111. doi:10.34172/ajcmi.3452
Introduction
Resistance is the ability of a bacteria against the inhibitory effect of antibacterial agents on the proliferation and growth of bacteria. Through the indiscriminate use of antibiotics, resistant microorganisms have emerged during recent decades and caused numerous problems in the treatment of infections induced by these microorganisms. Therefore, an attempt at producing drugs to deal with these outstanding threats to global health is an essential need. To this end, one strategy is employing a combination of different active parts in one molecule. In this strategy, each drug moiety is designed to bind independently to different biological targets and accumulate at both target sites synchronously (1-3). It was found that 1, 2, 4-triazole and naphthalene derivatives exhibit a wide variety of pharmacological and biological activities such as antibacterial (4,5), antimycobacterial (6,7), anti-inflammatory (8), antifungal (9,10), antineoplastics (10), anticancer (11), and antiviral (12) activities. Thiadiazines are also efficient antibacterial and antifungal compounds (12), and their use in the treatment of the bacteria Helicobacter pylori and their role as reverse transcriptase inhibitors of the human immunodeficiency virus have been reported (13). On the other hand, the studies conducted on a large number of p-naphthol derivatives for antibacterial and antifungal activities indicated that these compounds exhibit preferential activity against gram-positive bacteria and human pathogenic fungi. In fact, in the present study, the synthesis of three-ring heterocycles has been designed in such a way that all three heterocycles have antimicrobial power and can exert their synergistic effect by being present in one molecule at the same time. As mentioned in the previous study, 10-substituted-5H-naphtho[1,2-e][1,2,4] triazolo[3,4-b][1,3,4] thiadiazin-5-ones derivatives were synthesized in the presence of different substitutions at the 5-triazole position as follows:
4a: 10-methyl -5H-naphtho[1,2-e] [1,2,4] triazolo [3,4-b][1,3,4] thiadiazin-5-ones 4b: 10-phenyl -5H-naphtho [1,2-e] [1,2,4] triazolo[3,4-b] [1,3,4] thiadiazin-5-ones 4c: 10-chlorophenyl -5H-naphtho[1,2-e] [1,2,4] triazolo [3,4-b][1,3,4] thiadiazin-5-ones 4d: 10-hydroxyphenyl -5H- naphtho[1,2-e] [1,2,4] triazolo[3,4-b] [1,3,4] thiadiazin-5-ones 4e: 10-furan-2-yl -5H-naphtho [1,2-e] [1,2,4] triazolo [3,4-b][1,3,4] thiadiazin-5-ones 4f: 10-ethyl -5H- naphtho[1,2-e] [1,2,4] triazolo[3,4-b] [1,3,4] thiadiazin-5-ones 4g:10-nitrophenyl -5H-naphtho [1,2-e][1,2,4] triazolo[3,4-b] [1,3,4] thiadiazin-5-ones.
Since the synthesis and evaluation of antimicrobial activity is an important part of our research program (14-16), in this study, we aimed to evaluate the antibacterial and antifungal activity of these derivatives against some microorganisms that cause the largest number of infections and, in some cases, have exhibited resistance to currently available antibiotics such as Methicillin-resistant Staphylococcus, Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli, Candida albicans, Candida tropicalis, Candida krusei, and Candida glabrata.
Materials and Methods
Preparation of Naphtho [1,2,4] triazol-Thiadiazin
General procedure for the synthesis of 10-substituted-5H-naphtho[1,2-e][1,2,4] triazolo[3,4-b][1,3,4] thiadiazin-5-ones thiadiazin-5-ones (4a-4g)) was as follows: Concentrated HCl (4-5 drops) was added to the solution of 2-[(4-amino-5-aryl (alkyl)-4H-1,2,4-triazol-3yl)thio]naphthalene-1,4-dione(1 mmol) in EtOH (20 mL). Then, the mixture was refluxed for 4 hours and left to cool at room temperature, and the resulting precipitate was filtered and washed with cold EtOH and dried to give products 4a-4g as orange needles in 56-82% yields, as depicted in Scheme 1 (14).
Scheme 1.
Synthesis of 10-Substituted-5H-Naphtho[1,2e][1,2,4]Triazolo [3,4-b][1,3,4]Thiadiazin-5-Ones (4a-4g)
.
Synthesis of 10-Substituted-5H-Naphtho[1,2e][1,2,4]Triazolo [3,4-b][1,3,4]Thiadiazin-5-Ones (4a-4g)
Antibacterial Screening
The antibacterial activity of synthesized compounds was tested against gram-positive and gram-negative bacteria, including E. coli ATCC 25922, P. aeruginosa ATCC 27853, S. aureus ATCC 25923, and Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 700698.These chosen strains were proceeded to screen the antibacterial activity because they are commonly isolated pathogens from hospitalized patients with intestinal ailments and blood and skin infections (17). All microorganisms were obtained from the Urmia University of Medical Sciences. The standardization of each bacterial inoculum was done following the Clinical and Laboratory Standards Institute (CLSI). Briefly, Mueller-Hinton agar (MHA) medium was used to prepare MHA agar plates which were inoculated with the bacterial suspension (106 CFU/mL) uniformly onto the surface of agar plates, and wells of uniform diameter were punched on the solidifying agar media by the sterile borer. Test compounds were then dissolved in 99.5% dimethyl sulfoxide (DMSO) to obtain 1 mg/mL stock solutions. Afterward, 100 μL of each of these solutions were dispensed into the labeled wells (18-20). Ceftizoxime and ciprofloxacin (Sigma, Aldrich, US) were used with the same method as standard drugs, and DMSO was used as solvent control. Next, Petri plates were transferred to the incubator for 18 hours at 35°C, and the final results were evaluated by measuring the average diameter of the inhibition zone around each well. Experiments were performed in triplicate.
Agar Dilution Method
The agar dilution method was performed according to the procedures described by the CLSI (21). Stock solutions of antimicrobial compounds were prepared in DMSO, added to molten MHA (Becton Dickinson, Franklin Lakes, MD, USA) at 50 °C, and then poured into Petri plates to provide two-fold dilutions ranging from 7.8 to 1000 µg ⁄mL. Bacterial inoculums were prepared from a 24-hour culture on MHA. Bacterial suspensions were applied to agar plates using a multipoint inoculator to give a final concentration of 1.5 × 105 CFU per spot (22). The agar plates were then incubated at 35 °C for 18 hours and were evaluated visually. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of antimicrobial agent observed in complete inhibition of visible growth or the growth of less than five colonies per spot (23).
Antifungal Activity
Agar Dilution Assay
In vitro antifungal activity of 10-substituted-5H-naphtho[1,2-e][1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-5-ones was evaluated against standardized clinically important fungi, including C. tropicalis (PTCC 5028), C. albicans (ATCC 10239), C. glabrata (IFRC 1627), and C. krusei(ATCC 6258). These yeasts were chosen because they are major human fungal pathogens that cause mucosal deep tissue infection, they may cause systemic infection in immune-compromised situations due to their great adaptability to different host niches (24). All fungi were obtained from the Shiraz University of Medical Sciences. The antifungal activity of triazole derivatives was determined using the agar-well diffusion method (25). Then, 100 µL of each fungal suspension with a cell density of 1.5 × 108 CFU/mL was applied on the surface of the pre-sterilized and autoclaved Sabouraud Dextrose Agar (SDA) Petri dishes and spread using a shaped glass spreader. Wells with 6 mm of diameter were made at the center of each of the SDA Petri plates with the help of a sterilized cork borer. Next, the wells were filled with triazole derivatives as prepared above with three replications for each treatment, and the zone of inhibition was calculated based on the mean values. Fluconazole (1 mg/mL) and DMSO were used as positive and negative controls, respectively. All Petri dishes, including triazoles and controls, were allowed to be diffused and incubated at 35 °C for 48 hours. The antifungal activity of derivatives was determined after incubation.
In the next step, the MICs were determined by the Agar dilution method according to the procedures described by the CLSI (26). Two-fold serial dilutions of the compounds and reference drug (fluconazole) were prepared in SDA to obtain the required concentrations of 25-1000 µg/mL. The fungal inoculates were adjusted to 0.5 McFarland standard (1.5 × 108 CFU/mL) (27), and the suspensions were then diluted to give 106 CFU/mL per spot. Petri dishes were spot inoculated with 10 µL of each prepared fungal suspension (106 CFU/spot) and incubated at 25 °C for 48 hours. At the end of the incubation period, MIC was determined, which is the lowest concentration of the test compound that resulted in no visible growth on the plate. To get the minimum fungicidal concentration (MFC), a loopful was taken from the MIC tubes and streaked on SDA plates. The growth was observed after incubation at 37 °C for 48 hours, and the lowest concentration was recorded as MFC which exhibited no growth (28).
Statistical Analysis
Statistical analysis was run using Microsoft Excel 2010 based on three independent experiments, and the results were expressed as mean.
Results
Antibacterial Screening
All newly synthesized compounds (4a-4g) were evaluated for their in vitro antibacterial activity against E. coli, P. aeruginosa, S. aureus, S, pyogenes, and MRSA using the agar well diffusion and dilution methods. Ceftizoxime and ciprofloxacin were used as reference standards. The antibacterial results of novel series of 10-substituted-5H-naphtho[1,2e][1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-5-ones are summarized in Table 1 and Figure 1.
Table 1.
Minimum Inhibitory Concentrations (µg/ mL) and Inhibition Zones (1 mg/mL) of Synthesized Compounds against Tested Bacteria
|
Compounds
|
MIC(IZ)
|
|
Staphylococcus aureus
|
Methicillin-resistant Staphylococcus aureus
|
Escherichia coli
|
Pseudomonas aeruginosa
|
|
4a
|
7.8 (25) |
15.6 (17) |
250 (10) |
500 (NA) |
|
4b
|
7.8 (25) |
6.6 (17) |
250 (10) |
500 (NA) |
|
4c
|
7.8 (25) |
15.6 (17) |
250 (10) |
500 (NA) |
|
4d
|
7.8 (25) |
15.6 (17) |
250 (10) |
500 (NA) |
|
4e
|
250 (15) |
250 (15) |
250 (10) |
500 (NA) |
|
4f
|
250 (25) |
250 (10) |
250 (10) |
500 (NA) |
|
4g
|
125 (26) |
500 (11) |
250 (10) |
500 (NA) |
| Ceftizoxime |
125 (25) |
125 (11) |
100 (16) |
(14) 500 |
| Ciprofloxacin |
0.07 (40 ) |
0.07 (40) |
(45) 15 |
1.5 (42) |
Note. MIC: Minimum inhibitory concentration; IZ: Inhibition zone; NA: No activity.

Figure 1.
Inhibition Zone of Compounds against Escherichia coli and Methicillin-resistant Staphylococcus aureus in 1mg/mL Concentration
.
Inhibition Zone of Compounds against Escherichia coli and Methicillin-resistant Staphylococcus aureus in 1mg/mL Concentration
All tested compounds (4a-4g) inhibited the growth of S. aureus and MRSA, but compounds 4a, 4b, 4c, and 4d were more active than the others. The growth of E. coli and P. aeruginosa was also inhibited by the compounds at the range of 250 µg/mL concentration. Furthermore, some of the compounds showed the same or higher antibacterial activity with ceftizoxime; however, they exhibited poor activity compared to ciprofloxacin. Additionally, the MBC of compounds was two or three-fold higher than that of the corresponding MIC results.
Antifungal Screening
The antifungal potential of tested compounds was evaluated according to their zone of inhibition and minimum inhibition concentration against tested fungal pathogens. The results were compared with the activity of fluconazole and are summarized in Table 2 and Figure 2.
Table 2.
Minimum Inhibitory Concentrations (µg/ mL) and Inhibition Zones (1 mg/mL) of Synthesized Compounds against Tested Fungi
|
Compounds
|
MIC(IZ)
|
|
Candida tropicalis
|
Candida
albicans
|
Candida
krusei
|
Candida glabrata
|
|
4a
|
400 (13) |
200 (16) |
200 (16) |
200 (12) |
|
4b
|
400 (13) |
800 (16) |
400 (13) |
400 (12) |
|
4c
|
800 (17) |
400 (25) |
400 (13) |
400 (15) |
|
4d
|
100 (25) |
100 (25) |
50 (25) |
100 (17) |
|
4e
|
400 (15) |
200 (25) |
200 (16) |
200 (16) |
|
4f
|
400 (25) |
400 (18) |
200 (16) |
200 (18) |
|
4g
|
400 (15) |
800 (25) |
200 (16) |
800 (12) |
| Fluconazole |
200 (15) |
100 (17) |
200 (16) |
200 (14) |
Note. MIC: Minimum inhibitory concentration; IZ: Inhibition zone.
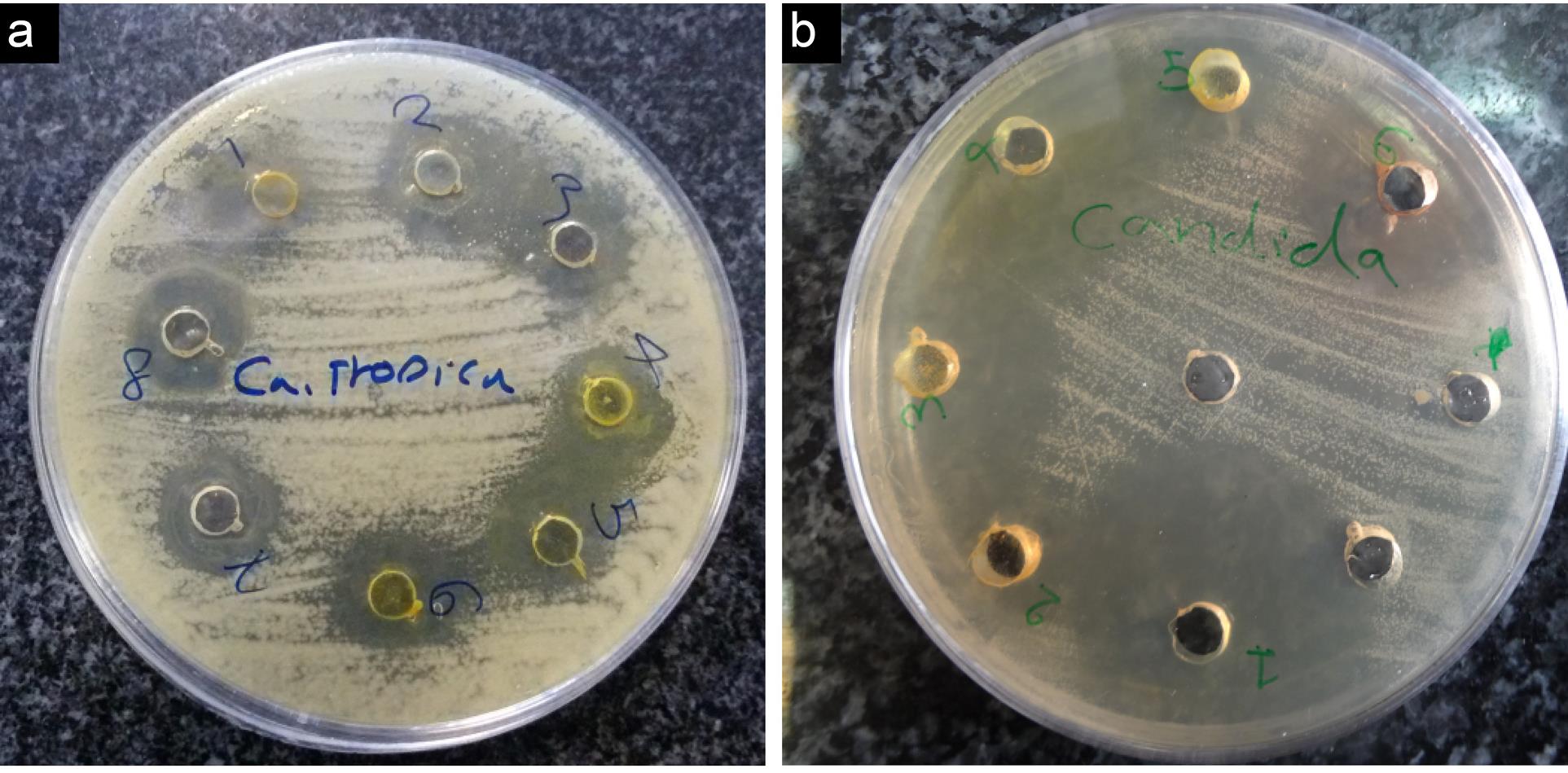
Figure 2.
Inhibition Zone of Compounds against Candida tropicalis (a) and Candida albicans (b) in 1 mg/mL Concentration
.
Inhibition Zone of Compounds against Candida tropicalis (a) and Candida albicans (b) in 1 mg/mL Concentration
The results revealed that all the synthesized compounds have potent antifungal activity against tested pathogenic fungi. Compound 4d showed maximum inhibition zone in Candida albicans, Candida tropicalis, Candida krusei, and Candida glabrata with a diameter of 25 ± 0.64 mm. Moreover, MIC was recorded as the lowest concentration of a compound that inhibits the growth of the tested microorganisms. Compared with the MIC values with the standard fluconazole (MIC = 1000 µg/mL), some compounds exhibited the highest potential in vitro antifungal activity against all evaluated fungi, especially compounds 4d (MIC = 50 to 100 µg/mL) and 4a, 4e,4f (MIC = 200 to 400 µg/mL). Additionally, compounds 4b and 4c (MIC = 400 to 800 µg/mL) demonstrated acceptable antifungal activity, and the MFC of compounds was the same or two-fold higher than that of the corresponding MIC results.
Discussion
All synthesized polycyclic heterocycles were tested for their antimicrobial activities, and MICs were recorded as a minimum concentration of compounds that inhibit the growth of tested microorganisms. The obtained results revealed that our new tetracyclic heterocyclic ring system (4a-4g) has good antimicrobial activity, but the highest inhibitory activity was observed against Gram-positive bacteria which can be explained by the presence of lipophilic moieties in triazolo scaffold. The widest spectrum of antibacterial activity was exerted by 4a, 4b, 4c, and 4d, suggesting that the phenyl ring, which contains an electron-withdrawing or electron-releasing substituent at the C-2 position of the [1,2,4] triazole ring exerts a good antibacterial effect against S. aureus and MRSA. These observations are similar to the results reported by Kumar et al (29). Interestingly, the most potent antibacterial compound in the present study belongs to the C-2 Hydroxyphenyl compound. As evidenced by inhibition zone sizes, the antibacterial activity of compounds against Gram-positive was somewhat stronger than that of ceftizoxime, while they displayed moderate antimicrobial activity against the Gram-negative bacterial strain. In addition, the antifungal screening results revealed that compounds are active against the fungal strains used in the study due to the presence of triazole thiadiazin moiety in 10 naphtha, which increased the antimicrobial activity. Moreover, compounds 4a, 4d, 4e, and 4f had better antifungal activity against tested fungal strains which is comparable to fluconazole, but significant activity was observed for compound 4d that was more potent than the control drug (fluconazole) against standard strains such as C. albicans, C. tropicalis, C. krusei, and C. glabrata. Additionally, the structural and antimicrobial similarity between our synthesized compounds and Fluconazole suggested that the mechanism of the action of the new compounds may be similar to that of fluconazole. Furthermore, this compound may be a good alternative for treating and controlling infections caused by MRSA, which is an extremely significant public health concern. In case further studies establish the safety and efficiency of these compounds, they may be represented as an excellent prototype with anti-MRSA activity in the development of new drugs for medical use.
In sum, some novel 10-substituted-5Hnaphtho[1,2-e][1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-5-ones (4a-4g) were synthesized, and their antimicrobial activities were investigated. The majority of the compounds were found to possess interesting antibacterial and antifungal activities against the tested microbial strains when compared to the standard drugs (i.e., ceftizoxime and fluconazole). The antimicrobial activities of the compounds were improved by structural modifications in the naphtho thiadiazin group attached to the triazole ring. Thus, these active compounds could be extremely good candidates for further antimicrobial research. Our previous study synthesized 3-substituted 5H [1,2,4]triazolo[3’,4’:2,3][1,3,4] thiadiazino[5,6-b]quinoxalines and investigated their antimicrobial activities (30). This molecule bears the greatest structural resemblance to the molecule studied in the present work, and the basic difference between these two structures is a substitution. In the previous study, the [1,3,4] thiadiazin ring was connected to the two rings of quinoxaline and [1,2,4] triazole, while in this study, [1,3,4] thiadiazin ring is located between the two rings of naphthol and [1,2,4] triazole. Comparing the antimicrobial power of the previous study and the current study yields very interesting results. By comparing these two studies, it can be found that the presence of naphthol substitution near the thiadiazole ring significantly increases the antimicrobial power of this molecule. More interestingly, by attaching different substitutions to the 2-triazole position, we can still notice high antimicrobial power, especially against gram-positive bacteria and fungi. Therefore, it can be concluded that in this molecule and generally similar molecules with this structure, the presence of the naphthol moiety adjacent to the thiadiazole ring significantly increases the antimicrobial power and exerts a synergistic effect. The result obtained in this research can play a key role in the synthesis of tricyclic, [1,2,4] triazole-[1,3,4] thiadiazin derivatives with high antimicrobial activity and double synergistic effect.
Acknowledgements
The authors would like to thank Professor Mohammadmahdi Baradarani for his help with synthesizing the compounds.
Authors’ Contribution
Conceptualization: Maryam Kouhkan.
Data curation: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy.
Formal analysis: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy.
Funding acquisition: Maryam Kouhkan, Ali Souldozi.
Investigation: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy.
Methodology: Maryam Kouhkan.
Project administration: Maryam Kouhkan.
Validation: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy, Ali Souldozi, Mahsa Mohammadlou, Nazila Khorram Maslak.
Visualization: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy.
Resources: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy.
Supervision: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy.
Writing–original draft: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy, Ali Souldozi.
Writing–review & editing: Maryam Kouhkan, Miri Mahmoody, Jabbar Khalafy, Ali Souldozi Babatunde Akinde.
Competing Interests
There is no conflict of interests.
Ethical Approval
This work did not require ethical approval under the research governance guidelines operating at the time of the research.
References
- Prakash CR, Raja S. Synthesis, characterization and in vitro antimicrobial activity of some novel 5-substituted Schiff and Mannich base of isatin derivatives. J Saudi Chem Soc 2013; 17(3):337-44. doi: 10.1016/j.jscs.2011.10.022 [Crossref] [ Google Scholar]
- Salih N, Salimon J, Yousif E. Synthesis and antimicrobial activities of 9H-carbazole derivatives. Arab J Chem 2016; 9(Suppl 1):S781-S6. doi: 10.1016/j.arabjc.2011.08.013 [Crossref] [ Google Scholar]
- Rosemeyer H. The chemodiversity of purine as a constituent of natural products. Chem Biodivers 2004; 1(3):361-401. doi: 10.1002/cbdv.200490033 [Crossref] [ Google Scholar]
- Palekar VS, Damle AJ, Shukla SR. Synthesis and antibacterial activity of some novel bis-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and bis-4-thiazolidinone derivatives from terephthalic dihydrazide. Eur J Med Chem 2009; 44(12):5112-6. doi: 10.1016/j.ejmech.2009.07.023 [Crossref] [ Google Scholar]
- Asif M. Pharmacological activities of triazole analogues as antibacterial, antifungal, antiviral agents. Pharm Sci Asia 2017; 44(2):59-74. [ Google Scholar]
- Gonec T, Kos J, Nevin E, Govender R, Pesko M, Tengler J. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014; 19(7):10386-409. doi: 10.3390/molecules190710386 [Crossref] [ Google Scholar]
- Klimesová V, Zahajská L, Waisser K, Kaustová J, Möllmann U. Synthesis and antimycobacterial activity of 1,2,4-triazole 3-benzylsulfanyl derivatives. Farmaco 2004; 59(4):279-88. doi: 10.1016/j.farmac.2004.01.006 [Crossref] [ Google Scholar]
- Paprocka R, Wiese M, Eljaszewicz A, Helmin-Basa A, Gzella A, Modzelewska-Banachiewicz B. Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivatives. Bioorg Med Chem Lett 2015; 25(13):2664-7. doi: 10.1016/j.bmcl.2015.04.079 [Crossref] [ Google Scholar]
- Radwan AA, Alanazi FK, Al-Agamy MH. 1,3,4-Thiadiazole and 1,2,4-triazole-3(4H)-thione bearing salicylate moiety: synthesis and evaluation as anti-Candida albicans. Braz J Pharm Sci 2017; 53(1):e15239. doi: 10.1590/s2175-97902017000115239 [Crossref] [ Google Scholar]
- Srinivas A. Synthesis and antimicrobial activity of bis-[4-methoxy-3-(6-aryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]-thiadiazin-3-yl)phenyl]methanes and bis-[(triazolo[3,4-b]thiadiazipin-3-yl)phenyl]methanes. Acta Chim Slov 2016; 63(1):173-9. doi: 10.17344/acsi.2015.2124 [Crossref] [ Google Scholar]
- Kulikov AS, Epishina MA, Batog LV, Rozhkov VY, Makhova NN, Konyushkin LD. Synthesis and antineoplastic properties of (1H-1,2,3-triazol-1-yl)furazans. Russ Chem Bull 2013; 62(3):836-43. doi: 10.1007/s11172-013-0113-2 [Crossref] [ Google Scholar]
- Beretta GL, Ribaudo G, Menegazzo I, Supino R, Capranico G, Zunino F. Synthesis and evaluation of new naphthalene and naphthoquinone derivatives as anticancer agents. Arch Pharm (Weinheim) 2017; 350(1):e1600286. doi: 10.1002/ardp.201600286 [Crossref] [ Google Scholar]
- Penta S, Gadidasu KK, Basavoju S, Rajeswar Rao V. An efficient one-pot synthesis of pyrazolyl-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazin-6-yl)-2H-pyran-2-one derivatives via multicomponent approach and their potential antimicrobial and nematicidal activities. Tetrahedron Lett 2013; 54(42):5663-6. doi: 10.1016/j.tetlet.2013.07.148 [Crossref] [ Google Scholar]
- Khalafy J, Mohammadlou M, Mahmoody M, Salami F, Marjani AP. Facile synthesis of new 10-substituted-5H-naphtho [1, 2-e][1, 2, 4] triazolo [3, 4-b][1, 3, 4] thiadiazin-5-ones. Tetrahedron Letters 2015; 56(12):1528-30. [ Google Scholar]
- Souldozi A. Efficient one-pot three-component reaction for the synthesis of (5-aryl-1,3,4-oxadiazol-2-yl)(pyridin-2-yl)methanol derivatives. J Chem Res 2015; 39(3):177-9. doi: 10.3184/174751915x14249664363057 [Crossref] [ Google Scholar]
- Kouhkan M, Souldozi A, Talebi R. In vitro antimicrobial activity of new substituted phenylthiazole derivatives. Iran J Toxicol 2018; 12(1):33-7. doi: 10.29252/arakmu.12.1.33 [Crossref] [ Google Scholar]
- Karlowsky JA, Jones ME, Draghi DC, Thornsberry C, Sahm DF, Volturo GA. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann Clin Microbiol Antimicrob 2004; 3:7. doi: 10.1186/1476-0711-3-7 [Crossref] [ Google Scholar]
- Jacob JH, Irshaid FI, Al-Soud YA. Antibacterial activity of some selected 1,2,4-triazole derivatives against standard, environmental, and medical bacterial strains. Adv Stud Biol 2013; 5(6):291-301. [ Google Scholar]
- Basoglu S, Yolal M, Demirci S, Demirbas N, Bektas H, Karaoglu SA. Design, synthesis and antimicrobial activities of some azole derivatives. Acta Pol Pharm 2013; 70(2):229-36. [ Google Scholar]
- Chakraborty S, Chakraborty B, Saha A, Saha C, Ghosh TK, Bhattacharyya I. Evaluation of antimicrobial activity of synthesized fluorocarbazole derivatives based on SAR. Indian J Chem 2017; 56B:701-8. [ Google Scholar]
- Syal K, Mo M, Yu H, Iriya R, Jing W, Guodong S. Current and emerging techniques for antibiotic susceptibility tests. Theranostics 2017; 7(7):1795-805. doi: 10.7150/thno.19217 [Crossref] [ Google Scholar]
- Schumacher A, Vranken T, Malhotra A, Arts JJC, Habibovic P. In vitro antimicrobial susceptibility testing methods: agar dilution to 3D tissue-engineered models. Eur J Clin Microbiol Infect Dis 2018; 37(2):187-208. doi: 10.1007/s10096-017-3089-2 [Crossref] [ Google Scholar]
- Gajdács M, Spengler G, Urbán E. Identification and antimicrobial susceptibility testing of anaerobic bacteria: Rubik’s cube of clinical microbiology?. Antibiotics (Basel) 2017; 6(4):25. doi: 10.3390/antibiotics6040025 [Crossref] [ Google Scholar]
- Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 2013; 62(Pt 1):10-24. doi: 10.1099/jmm.0.045054-0 [Crossref] [ Google Scholar]
- Sahoo J, Paidesetty SK. Antimicrobial activity of novel synthesized coumarin based transitional metal complexes. J Taibah Univ Med Sci 2017; 12(2):115-24. doi: 10.1016/j.jtumed.2016.10.004 [Crossref] [ Google Scholar]
- Bouryabaf LS, Moradi M, Tajik H, Badali A. Biofilm removal and antimicrobial activities of agar hydrogel containing colloid nano-silver against Staphylococcus aureus and Salmonella typhimurium. J Med Bacteriol 2017; 6(3-4):51-58. [ Google Scholar]
- Kadaikunnan S, Rejiniemon T, Khaled JM, Alharbi NS, Mothana R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob 2015; 14:9. doi: 10.1186/s12941-015-0069-1 [Crossref] [ Google Scholar]
- Ikegbunam M, Ukamaka M, Emmanuel O. Evaluation of the antifungal activity of aqueous and alcoholic extracts of six spices. Am J Plant Sci 2016; 7(1):118-25. doi: 10.4236/ajps.2016.71013 [Crossref] [ Google Scholar]
- Kumar P, Narasimhan B, Sharma D. Substituted benzoic acid benzylidene/furan-2-yl-methylene hydrazides: synthesis, antimicrobial evaluation and QSAR analysis. ARKIVOC. 2008(13):159-78.
- Mahmoody M, Kouhkan M, Khalafy J, Pourali S, Samadi N. In vitro antimicrobial activity of new 3-substituted 5H-(1,2,4)triazolo(3’,4’:2,3) (1,3,4)thiadiazino(5,6-b)quinoxaline derivatives. Med Lab J 2020; 14(2):20-5. doi: 10.29252/mlj.14.2.20 [Crossref] [ Google Scholar]