Avicenna Journal of Clinical Microbiology and Infection. 10(2):43-48.
doi: 10.34172/ajcmi.3447
Original Article
Prevalence and Antibiotic Resistance Pattern of Diarrheagenic Escherichia coli Pathotypes Among Children under 5 Years in Khuzestan, Iran
Susan Tatar 1, *  , Seyedeh Elham Rezatofighi 1
, Seyedeh Elham Rezatofighi 1  , Mohammad Reza Akhoond 2
, Mohammad Reza Akhoond 2
Author information:
1Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Iran
2Mathematical Sciences and Computer Faculty, Shahid Chamran University of Ahvaz, Ahvaz, Iran
Abstract
Background: Diarrhea is a life-threatening cause of high mortality, especially among children living in areas with poor sanitation. Enterobacteriaceae is one of the serious causes of bacterial diarrhea in children and adults. In this family, infection with diarrheagenic Escherichia coli (DEC) pathotypes in children is associated with extensive health risks and is of particular importance. In this study, we compared the distribution of pathotypes, epidemiological patterns, and antibiotic resistance of DEC in two diarrheal and non-diarrheal groups among children less than 5 years.
Methods: In this study, 303 stool samples were collected from patients admitted to Golestan hospitals in Ahvaz and Dr. Ganjavian in Dezful, Khuzestan. To this end, 201 samples from children with diarrhea (case group) and 102 samples from healthy children (control group) were examined. DEC was characterized by polymerase chain reaction (PCR) for each stool sample, and DEC isolates were tested with antibiotic resistance tests against different antibiotic agents to identify the prevalence of multidrug-resistant (MDR) strains in both groups.
Results: DEC was found in 24% (48 out of 200) of the children with diarrhea and 3.8% (4 out of 103) of the healthy children. Enteroaggregative E. coli (EAEC) was the DEC most frequently associated with diarrhea (32 out of 48, 66.6%), which was followed by enteropathogenic E. coli (EPEC) 22.9% (11 out of 48, 22.9%), and enterotoxigenic E. coli (ETEC) (5 out of 48, 10.4%) from children with diarrhea. Four DEC isolates were identified in healthy children: EAEC (2 out of 4, 50%) and EPEC (2 out of 4, 50%) in the healthy group, but no enteroinvasive E. coli (EIEC) or enterohemorrhagic E. coli (EHEC) strains were found in both groups in this study group. In general, DEC isolates exhibited high resistance to ceftriaxone and cefotaxime, and 33 (63.4%) isolates of DEC were MDR.
Conclusion: A high prevalence of DEC strains was observed in the group of children with diarrhea and healthy children. Accordingly, further attention should be paid to continuous monitoring of the prevalence and pattern of antibiotic resistance of diarrheal bacterial isolates among children and the whole community.
Keywords: Diarrhea, Under 5 years, E. coli, MDR, DEC
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Tatar S, Rezatofighi SE, Akhoond MR. Prevalence and antibiotic resistance pattern of diarrheagenic Escherichia coli pathotypes among children under 5 years in Khuzestan, Iran. Avicenna J Clin Microbiol Infect. 2023; 10(2):43-48. doi:10.34172/ajcmi.3447
Introduction
Diarrhea causes the death of 533 768 children up to 5 years old globally (1), causes approximately 9% of all deaths among children under 5 worldwide, and kills more than 1300 young children every day (2). Owing to unsanitary living conditions, including contaminated water sources, unsanitary environment, and insufficient education in economically poor countries in Africa, Asia, and South America, these regions are more susceptible to diarrheal diseases (3). Infectious diarrhea is caused by different groups of pathogens. The Enterobacteriaceae family causes numerous mild to fatal contagious diseases, especially a variety of gastrointestinal disorders worldwide. Due to the increase in antibiotic resistance and the easy transfer of antibiotic-resistant genes among members of the Enterobacteriaceae family, it is difficult to treat patients with these infectious diseases with conventional antibiotics (4,5).
Diarrheagenic Escherichia coli (DEC) is one of the most prevalent causes of bacterial diarrhea in children living in less developed countries (6). Although various strains of E. coli exist as normal flora in the digestive system of many animals as well as humans in a harmless form, some strains of this bacteria cause serious gastrointestinal and extraintestinal disorders (7).
DEC strains are defined in 5 main subgroups according to their pathogenic characteristics: (a) Enterotoxigenic E. coli (ETEC) is the main cause of traveler’s diarrhea and also causes diarrhea in malnourished infants. ETEC strains increase fluid and electrolyte excretion by producing heat-labile and heat-stable toxins (8,9). (b) Enteroinvasive E. coli (EIEC) are closely related to Shigella species in terms of biochemical, genetic, and pathogenicity. Similar to Shigella, EIEC also causes dysentery in humans (10). (c) Enteroaggregative E. coli (EAEC) form an aggregated adhesion (AA) pattern when grown on the 2-HEp cell line. EAEC is one of the main causes of acute and chronic diarrhea in children and adults, especially in developing countries (11,12). (d) Enteropathogenic E. coli (EPEC) strains attach to the enterocyte membrane through a process called localized adherence (LA). EPEC strains are divided into two groups based on the presence of bundle-forming pili (BFP). The first group is typical EPEC that has BFP, while the second group is atypical EPEC that does not have BFP (13,14) (e) Shiga toxin-producing E. coli (STEC) strains, the main reservoir of which is raw meat, adhere to the colon mucosa and cause diarrhea by producing verotoxin. Serotype H7:O157 is one of the prominent STEC strains causing endemic outbreaks and sporadic cases of acute diarrhea worldwide (15-17). Statistics on DEC strains causing diarrhea in children are scattered because accurate identification of DEC strains is not often tested in most countries (18).
To better deal with outbreaks of DEC in communities, it is required to conduct tests for molecular identification of pathological strains and more tests to investigate the pattern of antibiotic resistance and their transmission. The indiscriminate and increasing use of antibiotics and the horizontal transfer of resistance genes by mobile genes cause the emergence of bacteria that are resistant to almost all antibiotic families (19,20). The scattered studies conducted in Iran have indicated that due to the resistance of the strains to the first line of antibiotic agents, the treatment of DEC strains is increasingly accompanied by problems (20,21).
In this study, we have described the distribution of DEC strains and their epidemiological characteristics in children with diarrhea compared to children without diarrhea who have been referred to the Khuzestan medical centers, Southwest of Iran. Furthermore, this study has warned about the dire situation of multidrug resistance (MDR) among DEC strains by conducting antibiotic sensitivity tests.
Materials and Methods
Sampling
From September 2015 to October 2016, hospitalized children up to 60 months of age who visited Golestan Hospital in Ahvaz and Dr. Ganjavian hospital in Dezful, Khuzestan province, were selected for sample collection. Stool samples containing E. coli strains were analyzed in two groups: diarrheal (case group) and non-diarrheal (control group). Hence, 200 samples of children with diarrhea and 103 samples of children without diarrhea who were referred to hospitals for reasons other than digestive disorders were examined.
Only one stool sample was collected from each child and analyzed. Samples from children treated with antibiotics in the last 28 days or infected with Salmonella, Shigella, and parasites were excluded from the study.
Specimen Collection, Isolation, Culture, and Identification of Escherichia coli
This study included 303 stool samples containing E. coli from children up to 60 months. To perform the relevant tests, stool samples were transported in clean disposable boxes. Fecal samples were cultured directly on a MacConkey agar plate (Merck; Frankfurt, Germany). Then, the incubation of culture media was done at 37 °C for one night. After that, lactose fermenters were subcultured on eosin methylene blue medium (Merck; Frankfurt, Germany). The presence of E. coli strains in the collected stool samples was confirmed by performing standard biochemical tests, including oxidase negative, methyl red positive, indole positive, Voges-Proskauer negative, citrate negative, catalase positive, type of carbohydrate consumption in triple sugar iron agar, and urease negative (22).
Multiplex Polymerase Chain Reaction
The boiling method was used to extract the DNA of E. coli to prepare template DNA. Genes of marker factors were used to identify the DEC pathotypes: escV for EPEC detection, stx1 and stx2 for STEC detection, elt,estIa, andestIbfor ETEC detection,invEfor EIEC detection, and aggRandastAfor EAEC detection. Polymerase chain reaction (PCR) levels and quality for identification of DEC strains, including primer sequence, size, and annealing temperature were similar to the method implemented by Müller et al using a thermocycler (Bio-Rad) (23). After performing PCR, the size of each locus was determined by electrophoresis on 1.5% gel agarose with a molecular marker (100 bp Ladder RTU, Sinaclon, Iran). Then, amplified genes obtained from PCR were evaluated by ultraviolet irradiation in Doc gel (Uvitec, Cambridge, UK).
Antimicrobial Susceptibility Testing
Standard diffusion (Kirby-Bauer method) was performed on Muller-Hinton agar based on the method determined by the Clinical and Laboratory Standards Institute to evaluate the antibiotic resistance/susceptibility pattern of DEC strains. The following 8 antibiotic disks (Padtanteb, Iran) were used: ampicillin (10 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), meropenem (10 μg), and imipenem (10 μg) (24). For the analyses, E. coli ATCC25922 was used as the control.
Results
Prevalence of Diarrheagenic Escherichia coli Strains Among Diarrheal and Non-diarrheal Samples
Out of 303 stool samples containing E. coli strains, 200 isolates (66%) and 103 isolates (34%) were obtained from children with diarrhea (cases) and children without diarrhea (controls), respectively. Finally, 52 DEC isolates were detected from both groups: 48 (92%) children with symptomatic diarrhea and 4 (8%) asymptomatic children. However, neither EIEC nor STEC strains were detected in this study.
The detection of DEC strains in male samples (55.7%) was higher than in female samples (44.2%). Furthermore, children under two years had the highest prevalence of diarrheal diseases (50%) and the presence of DEC strains (52.1%). The frequency of age and gender of children in both diarrheal and non-diarrheal groups and each of the DEC strains are presented in Table 1.
Table 1.
Age and Gender Distribution of Children with DEC in Case and Control Groups
Pathotype
Variables
|
Case Group (n=48)
|
Control Group (n=4)
|
DEC
(n=52)
|
Total (N=303)
|
EAEC
(n=32)
|
EPEC
(n=11)
|
ETEC
(n=5)
|
Total
|
EAEC
(n=2)
|
EPEC
(n=2)
|
ETEC
(n=0)
|
Total
|
Total Case
(n=200)
|
Total Control
(n=103)
|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
| Female |
14 (29.1%) |
6 (12.5%) |
2 (4.2%) |
22 (45.8%) |
1 (25%) |
0 |
0 |
1 (25%) |
23 (44.2%) |
86 (43%) |
46 (44.6%) |
| Male |
18 (37.5%) |
5 (10.4%) |
3 (6.3%) |
26 (54.2%) |
1 (25%) |
2 (50%) |
0 |
3 (75%) |
29 (55.7%) |
114 (57%) |
57 (55.4%) |
| Age (month) |
|
|
|
|
|
|
|
|
|
|
|
| 0-11 |
5 (10.4%) |
3 (6.2%) |
2 (4.2%) |
10 (20.8%) |
0 |
0 |
0 |
0 |
10 (19.2%) |
47 (23.5%) |
12 (11.7%) |
| 12-23 |
12 (25%) |
2 (4.2%) |
1 (2.1%) |
15 (31.3%) |
1 (25%) |
0 |
0 |
1 (25%) |
16 (30.8%) |
59 (29.5%) |
33 (32%) |
| 24-35 |
8 (16.7%) |
3 (6.2%) |
1 (2.1%) |
12 (25%) |
0 |
0 |
0 |
0 |
12 (23.1%) |
45 (22.5%) |
29 (28.1%) |
| 36-47 |
4 (8.3%) |
2 (4.2%) |
1 (2.1%) |
7 (14.6%) |
0 |
1 (25%) |
0 |
1 (25%) |
8 (15.4%) |
31 (15.5%) |
21 (20.4%) |
| 48-60 |
3 (6.2%) |
1 (2.1%) |
0 |
4 (8.3%) |
1 (25%) |
1 (25%) |
0 |
2 (50%) |
6 (11.5%) |
18 (9%) |
8 (7.8%) |
Note. EAEC: Enteroaggregative Escherichia coli; EPEC: Enteropathogenic Escherichia coli; ETEC: Enterotoxigenic Escherichia coli; DEC: Diarrheagenic Escherichia coli.
EAEC was the most common pathogenic strain found in both groups (34/52, 65.4%). In the case group, the highest prevalence of EAEC was among children aged 12-23 months (12/48, 25%), but the prevalence of ETEC was higher in children between 0-11 months (2/48, 4.2%). Furthermore, the lowest prevalence of all pathotypes was between 48-60 months. Figure 1 shows the frequency of each DEC strain in both groups.
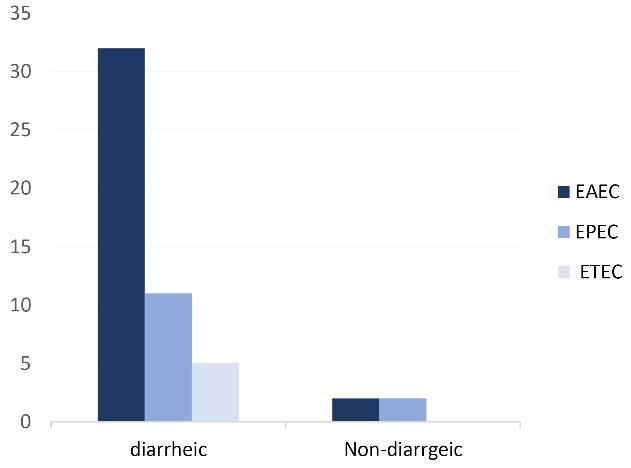
Figure 1.
Frequency of DEC Pathotypes. Note. DEC: Diarrheagenic Escherichia coli
.
Frequency of DEC Pathotypes. Note. DEC: Diarrheagenic Escherichia coli
Antibiotic Resistance of Diarrheagenic Escherichia coli Isolates
All isolates were sensitive to imipenem (n = 52, 100%). The highest rates of resistance were observed against cefotaxime (68.7%), ceftazidime (64.6%), and ceftriaxone (66.7%) in children with diarrhea. Furthermore, none of the isolates were sensitive to all antibiotic discs tested in this study, but all 4 DEC isolates strains were resistant to ceftriaxone in non-diarrheic children. Of the total 52 found DEC, 33 (63.4%) isolates were MDR (resistance to more than three antimicrobial drug families).
Discussion
Pathogenic strains of E. coli are transmitted to the host in different ways, cause serious digestive diseases in humans, especially children, easily penetrate the human food chain, exist in contaminated water, and can be transmitted through the fecal-oral route (9,11,15). DEC is one of the major enteric pathogens that cause diarrhea in children in developing countries. In this study, out of a total of 303 E. coli samples, 17.2% (n = 52) were positive for DEC infection. In a study conducted by Samal et al in Orissa, India, the prevalence of enteric bacterial pathogens in hospitalized patients with diarrhea was reported to be 75.5% E. coli strains and 13.3% DEC strains (25). In the present study, the frequency of DEC pathotypes was higher in diarrheal samples (48 out of 200, 24%) than in the non-diarrheal group (4 out of 103, 3.9%). In a study in Brazil, DEC pathotypes were identified in 18.0% of children with diarrhea and 19.0% of control subjects (26).
The highest prevalence of diarrheal diseases and DEC strains was observed among male children, with 57% and 55.7%, respectively. Moreover, the highest prevalence of diarrheal diseases and DEC strains was observed among children under 2 years old, with 53% and 50%, respectively. In the group of children with diarrhea, the frequency of DEC strains in children aged 48-60 months was lower than that in younger ages (4 out of 48, 8.3%), but in the control group at this age, the frequency of DEC isolates was the highest (2 out of 4, 50%).
E. coli strains can colonize and form biofilms on the mucosal surfaces of hosts such as animals and humans. EPEC strains cause diarrheal diseases by forming attaching and effacing legions and firmly adhering to the surface of intestinal cells (13). EAEC strains are defined by manifesting the aggregative adherence pattern on epithelial cells in cell culture. EACE strains cause diarrhea by the aggR gene, which regulates biofilm formation and aggregates adhesion factors that cause direct attachment to intestinal cells (11,27), while the pathogenicity of ETEC strains is due to the secretion of enterotoxins such as heat-labile and heat-stable and heat-stable (8). In the present study, PCR method was used to identify DEC strains (Figure 2) EAEC was the most common DEC pathotype diagnosed in both case and control groups, with 32 (16%) and 2 (1.9%) frequency, respectively. The present study showed the presence of EAEC and EPEC virulence genes even in children without symptoms of diarrhea.
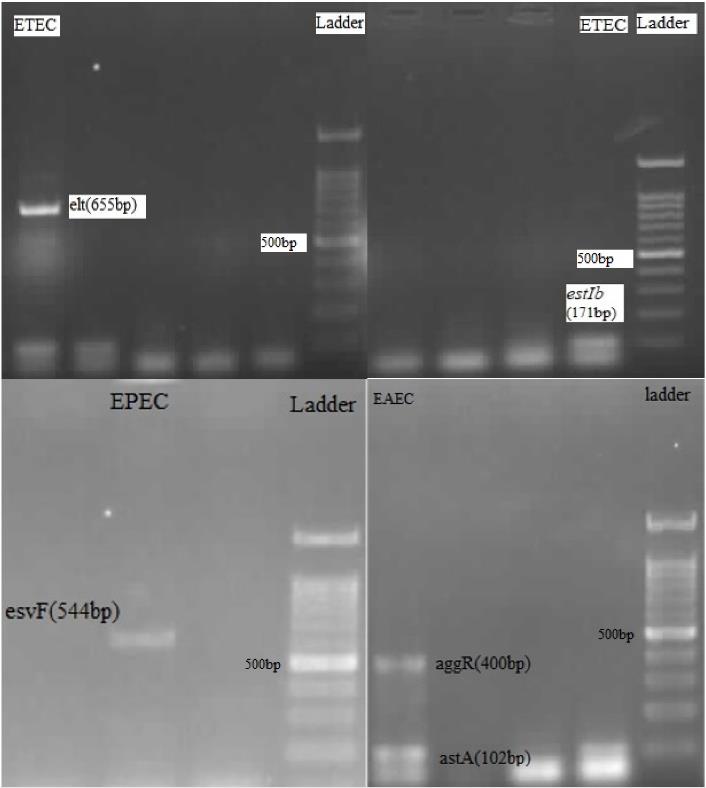
Figure 2.
PCR Results: Detection of ETEC by elt Gene(655bp) or estIb Gene (171bp), Detection of EPEC by esvF Gene (544 bp), and Detection of EAEC by astA Gene(102 bp) and aggR Gene (400 bp). Note. PCR:Polymerase chain reaction; ETEC: Enterotoxigenic Escherichia coli; EPEC: Enteropathogenic Escherichia coli; EAEC: Enteroaggregative Escherichia coli
.
PCR Results: Detection of ETEC by elt Gene(655bp) or estIb Gene (171bp), Detection of EPEC by esvF Gene (544 bp), and Detection of EAEC by astA Gene(102 bp) and aggR Gene (400 bp). Note. PCR:Polymerase chain reaction; ETEC: Enterotoxigenic Escherichia coli; EPEC: Enteropathogenic Escherichia coli; EAEC: Enteroaggregative Escherichia coli
The prevalence and distribution of infections caused by DEC strains are diverse worldwide (28). In the study by Khairy et al, EAEC (47%) was the predominant pathotype of DEC isolated from children with diarrhea in Egypt (29). The highest frequency of DEC observed both in the diarrhea group and all samples is related to EAEC, followed by EPEC and ETEC with the numbers presented in Table 1. The results of the present study are almost similar to the study from India in which EAEC was the predominant DEC strain found in the diarrhea group (69%), followed by ETEC and EPEC strains (30).
In this study, none of the EIEC and STEC strains were found among the samples. Moharana et al in India also reported no EIEC and STEC strains among the diarrheal samples, and ETEC was the most common DEC in the study (40 out of 77) (31). However, studies by Lima et al in Brazil and Eltai et al in Qatar on the epidemiology of DEC among children showed that EPEC is the most common DEC in children (32,33).
In the present study, antibiotic resistance of DEC isolates from children who were admitted to the hospital due to diarrhea was tested and compared to DEC isolates from healthy children as listed in Table 2. In our study, the highest values of antibiotic resistance were observed against cefotaxime, ceftriaxone, and ceftazidime. In addition, high resistance to third-generation cephalosporins in DEC strains has been reported in other studies. Different prevalence rates of MDR in DEC strains have been reported worldwide. In the present study, MDR strains were identified in 63.4% of DEC isolates. In the diarrheal group, the prevalence of MDR strains (31 out of 48, 64.6%) was higher than that in the control group (2 out of 4, 50%). Moreover, EPEC (8 out of 11, 72.7%) had the highest prevalence of MDR among DEC pathotypes compared to EAEC (20 out of 31, 64.5%) and ETEC (3 out of 5, 60%) strains in the case group. Furthermore, the different prevalence of MDR in DEC isolates in Qatar, Iran, and China studies has been reported to be 40%, 50%, and 66.7%, respectively (33-35).
Table 2.
Distribution of Antibiotic Resistance of 52 DEC Strains Found in Case and Control Children
|
Antibiotics
|
Case Children (n=48)
|
Control Children (n=4)
|
Total (N=52)
|
EAEC
(n=32)
|
EPEC
(n=11)
|
ETEC
(n=5)
|
EAEC
(n=2)
|
EPEC
(n=2)
|
ETEC
(n=0)
|
Total Case
(n=48)
|
Total Control
n=4
|
| Cefotaxime |
23 (48%) |
7 (14.6%) |
3 (6.2%) |
2 (50%) |
1 (25%) |
0 |
33 (68.7) |
3 (75%) |
| Ceftazidime |
23 (48%) |
6 (12.5%) |
2 (4.2%) |
1 (25%) |
1 (25%) |
0 |
31 (64.6%) |
2 (50%) |
| Ceftriaxone |
22 (46%) |
6 (12.5%) |
4 (8.3%) |
2 (50%) |
2 (50%) |
0 |
32 (66.7%) |
4 (100%) |
| Ciprofloxacin |
7 (14.6%) |
3 (6.2%) |
1 (2.1%) |
1 (25%) |
0 |
0 |
11 (23%) |
1 (25%) |
| Amikacin |
12 (25%) |
1 (2.1%) |
1 (2.1%) |
1 (25%) |
0 |
0 |
14 (29.2%) |
1 (25%) |
| Gentamicin |
14 (29.2%) |
2 (4.2%) |
2 (4.2%) |
1 (25%) |
1 (25%) |
0 |
18 (37.5) |
2 (50%) |
| Meropenem |
2 (4.2%) |
1 (2.1%) |
0 |
1 (25%) |
0 |
0 |
3 (6.2%) |
1 (25%) |
| Imipenem |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| MDR |
20 (41.7%) |
8 (16.7%) |
3 (6.2%) |
1 (25%) |
1 (25%) |
0 |
31 (64.6%) |
2 (50%) |
Note. DEC: Diarrheagenic Escherichia coli; EAEC: Enteroaggregative Escherichia coli; EPEC: Enteropathogenic Escherichia coli; ETEC: Enterotoxigenic Escherichia coli; MDR: Multidrug-resistant.
Conclusion
In the present study, a high prevalence of EAEC strains was observed in children under 5 years in Khuzestan province. Since the high prevalence of antibiotic resistance of DEC strains was detected in both groups with diarrhea and without diarrhea symptoms in this research, preventive measures and further studies are suggested to reduce the prevalence of DEC strains. Furthermore, the information obtained from this study can be used to identify emerging antimicrobial resistance and develop appropriate treatment guidelines and interventions.
Authors’ Contribution
Conceptualization: Seyedeh Elham Rezatofighi, Susan Tatar.
Data curation: Susan Tatar.
Formal analysis: Seyedeh Elham Rezatofighi, Susan Tatar, Mohammad reza Akhoond.
Funding acquisition: Seyedeh Elham Rezatofighi.
Investigation: Seyedeh Elham Rezatofighi, Mohammad Reza Akhoond, Susan Tatar.
Methodology: Seyedeh Elham Rezatofighi, Susan Tatar, Mohammad Reza Akhoond.
Project administration: Susan Tatar.
Resources: Susan Tatar.
Supervision: Seyedeh Elham rezatofighi.
Validation: Seyedeh Elham Rezatofighi.
Visualization: Susan Tatar.
Writing–original draft: Susan Tatar.
Writing–review & editing: Susan Tatar, Seyedeh Elham Rezatofighi.
Competing Interests
None.
Ethical Approval
The study protocol follows the ethical guidelines of the Declaration of Helsinki (No: EE/97.24.3.70382/scu.ac.ir).
References
- GBD 2017 Diarrhoeal Disease Collaborators. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the Global Burden of Disease Study 2017. Lancet Infect Dis 2020; 20(1):37-59. doi: 10.1016/s1473-3099(19)30401-3 [Crossref] [ Google Scholar]
- WHO, UNICEF One is Too Many: Ending Child Deaths from Pneumonia and Diarrhoea. New York: UNICEF; 2016.
- Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 2013; 26(4):822-80. doi: 10.1128/cmr.00022-13 [Crossref] [ Google Scholar]
- Bezabih YM, Sabiiti W, Alamneh E, Bezabih A, Peterson GM, Bezabhe WM. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J Antimicrob Chemother 2021; 76(1):22-9. doi: 10.1093/jac/dkaa399 [Crossref] [ Google Scholar]
- De Angelis G, Del Giacomo P, Posteraro B, Sanguinetti M, Tumbarello M. Molecular mechanisms, epidemiology, and clinical importance of β-lactam resistance in Enterobacteriaceae. Int J Mol Sci 2020; 21(14):5090. doi: 10.3390/ijms21145090 [Crossref] [ Google Scholar]
- Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 2009; 136(6):1874-86. doi: 10.1053/j.gastro.2009.02.072 [Crossref] [ Google Scholar]
- Jafari A, Aslani MM, Bouzari S. Escherichia coli: a brief review of diarrheagenic pathotypes and their role in diarrheal diseases in Iran. Iran J Microbiol 2012; 4(3):102-17. [ Google Scholar]
- Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. Lancet Infect Dis 2018; 18(11):1229-40. doi: 10.1016/s1473-3099(18)30475-4 [Crossref] [ Google Scholar]
- Alia RA, Sadia N, Shammy NP, Tithy FA, Shelim R, Parvin R. Diarrhoeal disease in relation to childhood malnutrition and its impact on socio-economic condition in emerging countries like Bangladesh. J Paediatr Perinatol Child Health 2022; 6(3):370-9. [ Google Scholar]
- Geurtsen J, de Been M, Weerdenburg E, Zomer A, McNally A, Poolman J. Genomics and pathotypes of the many faces of Escherichia coli. FEMS Microbiol Rev 2022; 46(6):fuac031. doi: 10.1093/femsre/fuac031 [Crossref] [ Google Scholar]
-
Moxley RA. Enterobacteriaceae: Escherichia. In: McVey DS, Kennedy M, Chengappa MM, Wilkes R, eds. Veterinary Microbiology. Wiley; 2022. p. 56-74. 10.1002/9781119650836.ch6.
- Joffré E, Iñiguez Rojas V. Molecular epidemiology of enteroaggregative Escherichia coli (EAEC) isolates of hospitalized children from Bolivia reveal high heterogeneity and multidrug-resistance. Int J Mol Sci 2020; 21(24):9543. doi: 10.3390/ijms21249543 [Crossref] [ Google Scholar]
- Mare AD, Ciurea CN, Man A, Tudor B, Moldovan V, Decean L, Toma F. Enteropathogenic Escherichia coli—a summary of the literature. Gastroenterol Insights 2021; 12(1):28-40. doi: 10.3390/gastroent12010004 [Crossref] [ Google Scholar]
- Silva SS, Monfardini MV, Scaletsky ICA. Large plasmids encoding antibiotic resistance and localized-like adherence in atypical enteropathogenic Escherichia coli strains. BMC Microbiol 2020; 20(1):138. doi: 10.1186/s12866-020-01809-4 [Crossref] [ Google Scholar]
- Kholdi S, Motamedifar M, Fani F, Mohebi S, Bazargani A. Virulence factors, serogroups, and antibiotic resistance of Shiga-toxin producing Escherichia coli from raw beef, chicken meat, and vegetables in Southwest Iran. Iran J Vet Res 2021; 22(3):180-7. doi: 10.22099/ijvr.2021.39266.5706 [Crossref] [ Google Scholar]
- Falup-Pecurariu O, Lixandru RI, Cojocaru E, Csutak K, Monescu V, Muhsen K. Shiga toxin producing Escherichia coli-associated diarrhea and hemolytic uremic syndrome in young children in Romania. Gut Pathog 2019; 11:46. doi: 10.1186/s13099-019-0327-4 [Crossref] [ Google Scholar]
- Castro VS, de Souza Figueiredo EE, Stanford K, McAllister T, Conte-Junior CA. Shiga-toxin producing Escherichia coli in Brazil: a systematic review. Microorganisms 2019; 7(5):137. doi: 10.3390/microorganisms7050137 [Crossref] [ Google Scholar]
- Yu J, Jing H, Lai S, Xu W, Li M, Wu J. Etiology of diarrhea among children under the age five in China: results from a five-year surveillance. J Infect 2015; 71(1):19-27. doi: 10.1016/j.jinf.2015.03.001 [Crossref] [ Google Scholar]
- Sawa T, Kooguchi K, Moriyama K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J Intensive Care 2020; 8:13. doi: 10.1186/s40560-020-0429-6 [Crossref] [ Google Scholar]
- Alikhani MY, Hashemi SH, Aslani MM, Farajnia S. Prevalence and antibiotic resistance patterns of diarrheagenic Escherichia coli isolated from adolescents and adults in Hamedan, Western Iran. Iran J Microbiol 2013; 5(1):42-7. [ Google Scholar]
- Memariani M, Najar Peerayeh S, Zahraei Salehi T, Shokouhi Mostafavi SK. Occurrence of SHV, TEM and CTX-M β-lactamase genes among enteropathogenic Escherichia coli strains isolated from children with diarrhea. Jundishapur J Microbiol 2015; 8(4):e15620. doi: 10.5812/jjm.8(4)2015.15620 [Crossref] [ Google Scholar]
- Mahon C, Manuselis G. Textbook of Diagnostic Microbiology. 5th ed. Pennsylvania: Elsevier Mosby; 2015. p. 484-5.
- Müller D, Greune L, Heusipp G, Karch H, Fruth A, Tschäpe H. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl Environ Microbiol 2007; 73(10):3380-90. doi: 10.1128/aem.02855-06 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M1008: Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Wayne, PA: CLSI; 2018.
- Samal SK, Khuntia HK, Nanda PK, Satapathy CS, Nayak SR, Sarangi AK. Incidence of bacterial enteropathogens among hospitalized diarrhea patients from Orissa, India. Jpn J Infect Dis 2008; 61(5):350-5. [ Google Scholar]
- Dias RC, dos Santos BC, dos Santos LF, Vieira MA, Yamatogi RS, Mondelli AL. Diarrheagenic Escherichia coli pathotypes investigation revealed atypical enteropathogenic E coli as putative emerging diarrheal agents in children living in Botucatu, São Paulo State, Brazil. APMIS 2016; 124(4):299-308. doi: 10.1111/apm.12501 [Crossref] [ Google Scholar]
- Ageorges V, Monteiro R, Leroy S, Burgess CM, Pizza M, Chaucheyras-Durand F. Molecular determinants of surface colonisation in diarrhoeagenic Escherichia coli (DEC): from bacterial adhesion to biofilm formation. FEMS Microbiol Rev 2020; 44(3):314-50. doi: 10.1093/femsre/fuaa008 [Crossref] [ Google Scholar]
- O’Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis 2005; 16(2):125-36. doi: 10.1053/j.spid.2005.12.008 [Crossref] [ Google Scholar]
- Khairy RMM, Fathy ZA, Mahrous DM, Mohamed ES, Abdelrahim SS. Prevalence, phylogeny, and antimicrobial resistance of Escherichia coli pathotypes isolated from children less than 5 years old with community acquired- diarrhea in Upper Egypt. BMC Infect Dis 2020; 20(1):908. doi: 10.1186/s12879-020-05664-6 [Crossref] [ Google Scholar]
- Mandal A, Sengupta A, Kumar A, Singh UK, Jaiswal AK, Das P. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli pathotypes in diarrheal children from low socioeconomic status communities in Bihar, India: emergence of the CTX-M type. Infect Dis (Auckl) 2017; 10:1178633617739018. doi: 10.1177/1178633617739018 [Crossref] [ Google Scholar]
- Moharana SS, Panda RK, Dash M, Chayani N, Bokade P, Pati S. Etiology of childhood diarrhoea among under five children and molecular analysis of antibiotic resistance in isolated enteric bacterial pathogens from a tertiary care hospital, Eastern Odisha, India. BMC Infect Dis 2019; 19(1):1018. doi: 10.1186/s12879-019-4501-6 [Crossref] [ Google Scholar]
- Lima FM, de Paulo Daurelio F, Mucci ER, Ahagon CM, Dos Santos Carmo AM, Eterovic A. Epidemiology and genetic screening of diarrheagenic Escherichia coli among symptomatic and asymptomatic children. J Med Microbiol 2019; 68(7):1033-41. doi: 10.1099/jmm.0.001020 [Crossref] [ Google Scholar]
- Eltai NO, Al Thani AA, Al Hadidi SH, Al Ansari K, Yassine HM. Antibiotic resistance and virulence patterns of pathogenic Escherichia coli strains associated with acute gastroenteritis among children in Qatar. BMC Microbiol 2020; 20(1):54. doi: 10.1186/s12866-020-01732-8 [Crossref] [ Google Scholar]
- Moini AS, Soltani B, Taghavi Ardakani A, Moravveji A, Erami M, Haji Rezaei M. Multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolated from patients in Kashan, Iran. Jundishapur J Microbiol 2015; 8(10):e27517. doi: 10.5812/jjm.27517 [Crossref] [ Google Scholar]
- Zhou Y, Zhu X, Hou H, Lu Y, Yu J, Mao L. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: a hospital based study. BMC Infect Dis 2018; 18(1):63. doi: 10.1186/s12879-017-2936-1 [Crossref] [ Google Scholar]