Avicenna Journal of Clinical Microbiology and Infection. 10(1):13-19.
doi: 10.34172/ajcmi.2023.3433
Original Article
Studying the Inhibitory Effects of Some Chalcone Derivatives on Streptococcus mutans Sortase A to Prevent Dental Caries: An In Silico Approach
Azizeh Asadzadeh 1, *  , Masoumeh Abbasi 2
, Masoumeh Abbasi 2  , Zahra Pournuroz Nodeh 3
, Zahra Pournuroz Nodeh 3  , Fatemeh Mahmoudi 4
, Fatemeh Mahmoudi 4 
Author information:
1Department of Biology, Faculty of Science, Nour Danesh Institute of Higher Education, Meymeh, Isfahan, Iran
2Department of Microbiology, Faculty of Basic Science, Malekan Branch, Islamic Azad University, Malekan, Iran
3Department of Chemistry, Lahijan Branch, Islamic Azad University, Lahijan, Iran
4Department of Biology, Faculty of Science, Zand Institute of Higher Education, Shiraz, Fars, Iran
Abstract
Background:
Streptococcus mutans is one of the most important microorganisms in tooth decay. Sortase A (SrtA) of S. mutans is responsible for the attachment of bacteria to the host cell and biofilm formation. Therefore, it seems necessary to investigate the inhibitors of this enzyme to prevent dental caries. Chalcones are always of interest in the medical community due to their wide range of biological activities. Many studies have reported that chalcone can help prevent caries. The present study was conducted to identify potential SrtA inhibitors with the chalcone skeleton.
Methods: The chalcone derivatives were obtained from the ZINC15, LEA3D, and PubChem databases, and then the selected compounds were optimized by HyperChem software. The affinity of these compounds to SrtA and total binding free energy (ΔGbind) were estimated by the AutoDock 4.0 program. Finally, drug-likeness screening and absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of the best ligands were obtained using online servers.
Results: Compared to chalcone, four of the studied ligands, including compounds 2, 7, 8, and 9 demonstrated high affinity for binding to S. mutans SrtA, with suitable drug-likeness and ADMET properties. Ligand 9 interacted with the key residues in the active site by the most negative ΔGbind (-4.64 kcal/mol). The best conformation of this ligand had the most overlap with the chalcone.
Conclusion: By complementary both in vitro and in vivo studies on the inhibitory effects of compounds 2, 7, 8, and 9, the present study can be useful in controlling tooth decay and dental diseases.
Keywords: Chalcone derivatives, In silico, Streptococcus mutans, Sortase A, Molecular docking, Dental caries
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Asadzadeh A, Abbasi M, Pournuroz Nodeh Z, Mahmoudi F. Studying the inhibitory effects of some chalcone derivatives on Streptococcus mutans sortase a to prevent dental caries: An in silico approach. Avicenna J Clin Microbiol Infect. 2023; 10(1):13-19. doi:10.34172/ajcmi.2023.3433
Introduction
Biofilm, the multidimensional complex structure, plays a highly important role in causing dental caries (1). The main component of the biofilm forming on the tooth surface is the Gram-positive bacterium called Streptococcus mutans. The sortase A (SrtA) of S. mutans is responsible for the attachment of microorganisms to the host cell wall and plaque formation. At the site of biofilm formation, S. mutansuses sugar compounds and during metabolism, converts them into acidic compounds, causing tooth decay (2,3).
Biofilm formation occurs in two ways, the first way requires the presence of sucrose. In this way, glycosyltransferases convert sucrose into polysaccharides and provide the conditions for plaque formation. In the second way, which is completely independent of this disaccharide, an interaction between surface protein Pac and agglutinins is created as a result of the catalytic activity of SrtA (4-6).
SrtA is an important group of transpeptidases located in the membrane of S. mutans (7). Microorganism surface proteins with conserved motif LPXTG are targeted as SrtA substrates. SrtA cleaves threonine and glycine peptide bond in surface proteins, and then in the catalytic site of SrtA, cysteine forms a peptide bond with a carbonyl end of threonine. Finally, this intermediate is transferred to the lipid II-surface protein to promote biofilm formation (7,8).
Considering the importance of SrtA in the pathogenesis of S. mutans and dental plaque formation, the investigation of its inhibitors has frequently been of interest to researchers (9-11). Although various antimicrobial compounds are commercially available to prevent and treat tooth decay, dental caries has always been one of the problems of the medical community. However, due to drug resistance and occasionally the side effects of inhibitory compounds, it seems necessary to design new ligands with higher efficiency.
Chalcone, which is also called benzyl acetophenone, is a natural product belonging to flavonoid compounds (12-14). Chalcones can be isolated from plants belonging to Leguminosae, Asteraceae, and Moraceaespecies. Since ancient times, chalcones have been used in medical sciences due to their antibacterial, antifungal, and antiparasitic properties. Many studies have confirmed that chalcone can help prevent caries (14-17).
Molecular docking allows us to investigate the interactions between the ligand-protein complex and ΔGbind (18). Previous studies represented that molecular docking suggests suitable compounds for enzyme inhibition, drug, and vaccine design (19-23).
In this study, one of the most important enzymes involved in the biofilm formation, S. mutans SrtA, was chosen as a receptor to identify inhibitors with the chalcone skeleton by molecular docking, and then drug-likeness screening and absorption, distribution, metabolism, excretion, and toxicity (ADMET) analyses were performed for selected ligands by online servers.
Materials and Methods
Selection of Compounds and Energy Optimization
The compounds that were selected for screening and analysis were based on the chalcone scaffold. Chalcone is an organic compound with an unsaturated ketone and two aromatic rings (14). Compounds in the simulation description format were downloaded from ZINC15, LEA3D, and PubChem databases, and then a suitable format was created using GaussView software, version 5.0. Finally, select compounds were optimized by HyperChem Professional software. For this purpose, the Polak-Ribière algorithm was used with a value of 0.1 for RMS.
Receptor Selection and Preparation
There are many structures of the crystallized S. mutans SrtA protein in the Protein Data Bank (PDB) site, but based on the study performed on the inhibition of SrtA by chalcone (17), PDB ID 4TQX was chosen for molecular docking. The three-dimensional crystal structures of the selected protein were downloaded from the Protein Data Bank in pdb format. Tetra ethylene glycol (PG4301), acetic acid (ACY302), sulfate ion (SO4303), and all water molecules were removed from the receptor structure using the Discovery Studio Visualizer software, and then it was employed as an input file in AutoDock tools, version 4.2.
Grid Box and Grid Center Determination
Based on the analysis of the crystal structure of 4TQX, there was no co-crystallized inhibitor in this structure. Discovery Studio Visualizer software was utilized to determine the position of the grid center. First, important amino acids of the active site were obtained using previous research (7,24), and then the center of those amino acids was used as the center of the grid. For calculating an optimal box size, the length of the ligands was calculated four times and employed as grid box size.
Molecular Docking Studies
Molecular docking studies can be applied to model the interaction between a ligand and a receptor, which allows us to determine the binding energy level and how the ligand is placed in the macromolecule (18). Docking was performed with AutoDock tools (ADT), version 4.2. After preparing protein and ligand files in pdb format, to create charge parameters for each atom in protein and ligand, pdbq files were created by adding Gasteiger and Kollman charges. Finally, auto-grid and auto-dock programs were performed after creating the grid and docking parameter files, respectively. Next, the types of clusters, ΔGbind, and the best conformation were studied through the dlg output file.
Drug-Likeness Screening and Absorption, Distribution, Metabolism, Excretion, and Toxicity Properties
Drug-likeness screening and ADMET properties were performed for compounds that demonstrated good docking results. In addition to drug effectiveness, suitable physicochemical properties are extremely important in drug design. In the present study, Lipinski’s rule of five (RO5) was used to study drug-likeness screening. According to the RO5, low absorption occurs in molecules with a molecular weight of more than 500 g/mol, the number of hydrogen bond acceptors of more than 10, the number of hydrogen bond donors of more than 5, and a lipophilicity factor (logP) more than 5 (25).
ADMET, which includes the main parameters of drug design (i.e., absorption, distribution, metabolism, excretion, and toxicity), was investigated using SwissADME and ProTox-II Server.
Results
Selection and Energy Optimization of Compounds
In this study, ZINC15, LEA3D, and PubChem databases were employed to select compounds with the chalcone skeleton, and then the selected compounds were subjected to HyperChem to obtain the compound with the lowest energy. The ligand’s name, molecular formula, database, ligand ID, and structural details of the selected compounds are presented in Table 1.
Table 1.
List of Studied Compounds
|
Ligand Number
|
Ligand Name
|
Molecular Formula
|
Database
|
Ligand ID
|
Structure
|
| 1 |
(E)-3-(4-methoxyphenyl)-1-(4-methylsulfanylphenyl)prop-2-en-1-one |
C17H16O2S |
Zinc15 |
ZINC4252637 |
|
| 2 |
1,2-diphenylethane-1,2-dione |
C14H10O2 |
PubChem |
8651 |
|
| 3 |
2-(2-Phenylethyl)-3-phenylaziridine |
C16H17N |
Zinc15 |
ZINC205482393 |
|
| 4 |
bis(4-fluorophenyl)methanone |
C13H8F2O |
PubChem |
9582 |
|
| 5 |
(E)-3-phenyl-1-(3,4,5-trifluorophenyl)prop-2-en-1-one |
C15H9F3O |
PubChem |
101571323 |
|
| 6 |
6-quinoxalinyl benzoate |
C15H10N2O2 |
LEA3D |
g9_mol30 |
|
| 7 |
(E)-1-[2-hydroxy-5-(methoxymethoxy)phenyl]-3-phenylprop-2-en-1-one |
C17H16O4 |
PubChem |
11335085 |
|
| 8 |
1-[4-[3-(4-propanoylphenyl)propyl]phenyl]propan-1-one |
C21H24O2 |
PubChem |
134887749 |
|
| 9 |
1-[4-[2-(4-acetylphenyl)ethyl]phenyl]ethanone |
C18H18O2 |
PubChem |
13100 |
|
| 10 |
Chalcone |
C15H12O |
PubChem |
637760 |
|
Receptor Selection and Preparation
The crystal structure of S. mutansSrtA with access code 4TQX as a receptor was downloaded from https://www.rcsb.org. The crystal structure of 4TQX with resolution 1.37 Å was reported by Wallock-Richards et al. This enzyme consists of a catalytic part with 8 beta sheets and a tail at the N-terminal end with a helix structure (17). The final structure of the protein after preparation with Discovery software is shown in Figure 1.
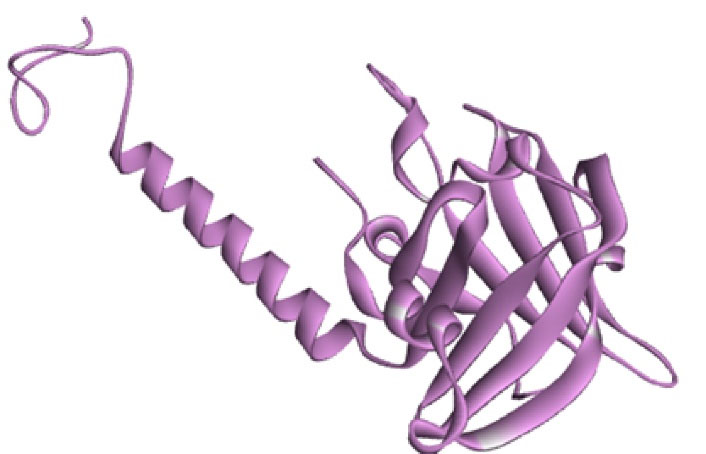
Figure 1.
The Final Structure of the Receptor After Preparation.
.
The Final Structure of the Receptor After Preparation.
Grid Box and Grid Center Determination
Amino acids such as cysteine 205 (Cα: X = 5.81, Y = 29.70, and Z = -13.33) and arginine 213 (Cα: X = 4.36, Y = 31.66, and Z = -17.16) are important residues in the catalytic site of the enzyme. SrtA recognizes and cleaves the amide bond of the substrate by cysteine 205 and stabilizes the transition state by arginine 213 (7,24). Therefore, the center of these two residues (X = 5.48, Y = 29.14, and Z = -16.244( was used as the grid center. Using Discovery Studio Visualizer software, the size of the grid box was determined to be 40 × 40 × 40.
Molecular Docking Studies
A molecular docking study was performed to analyze the affinity and orientation of chalcone derivatives in the active site of SrtA (18). The results related to the binding energy, intermol energy, electrostatic energy, and torsional energy of all compounds are provided in Table 2. A more negative binding energy indicates a stronger interaction between the ligand and receptor. Compared to chalcone, compounds 2, 7, 8, and 9 showed a high affinity for binding to S. mutans SrtA. The results of the clustering histogram of compounds represented that ligand 9 has the most negative binding energy, and 88/100 conformations are placed in cluster 1. The best conformation of ligand 9 has the most overlap with the chalcone (Figure 2A). Discovery Studio Visualizer and LigPlus software were employed to study the hydrogen bonds and hydrophobic interactions between ligands and amino acids in the active site of the enzyme, respectively. These interactions are summarized in Table 3, and the binding mode of ligand 9 is illustrated in Figure 2.
Table 2.
Docking Results Based on Energy Values in kcal/mol
|
Ligand Number
|
Binding Energy
|
Intermol Energy
|
Electrostatic Energy
|
Torsional Energy
|
| 1 |
-2.73 |
-4.22 |
0.38 |
-0.24 |
| 2 |
-4.09 |
-4.99 |
-0.06 |
0.89 |
| 3 |
-3.65 |
-5.43 |
0.33 |
1.79 |
| 4 |
-3.49 |
-4.09 |
-0.07 |
0.6 |
| 5 |
-3.45 |
-4.34 |
0.0 |
0.89 |
| 6 |
-3.82 |
-4.72 |
-0.15 |
0.89 |
| 7 |
-4.32 |
-6.41 |
-0.19 |
2.09 |
| 8 |
-4.59 |
-6.97 |
-0.07 |
2.39 |
| 9 |
-4.64 |
-6.13 |
0.01 |
1.49 |
| Chalcone |
-3.88 |
-4.77 |
0.06 |
0.89 |
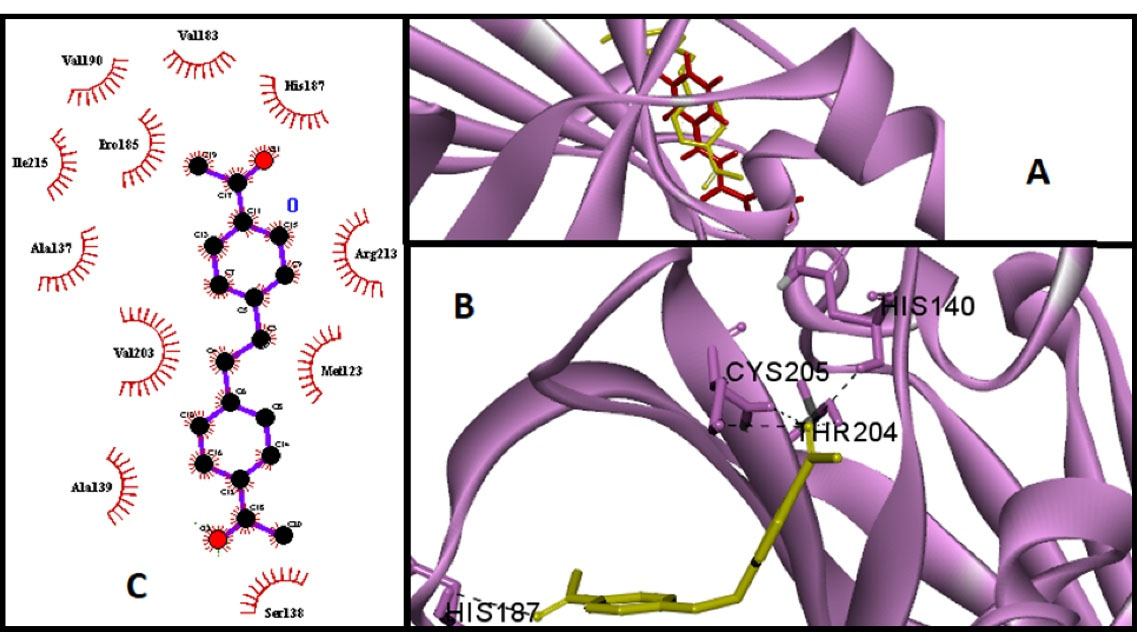
Figure 2.
(A) Docked Conformations of Chalcone (Red) and Compound 9 (Yellow) in the Active Site of SrtA (violet), (B) Hydrogen Bonding, and (C) Hydrophobic Interactions of Compound 9 Analyzed by Discovery Studio and LigPlus, respectively.
.
(A) Docked Conformations of Chalcone (Red) and Compound 9 (Yellow) in the Active Site of SrtA (violet), (B) Hydrogen Bonding, and (C) Hydrophobic Interactions of Compound 9 Analyzed by Discovery Studio and LigPlus, respectively.
Table 3.
Docking Results Based on Interaction Analysis
|
Ligand Number
|
Amino Acids Involved in Hydrogen Bonds
|
Amino Acids Involved in Hydrophobic Interaction
|
| 1 |
- |
Ala139, His140, Thr204, Ser138, Cys205, Leu116, Ala137, Pro185, Thr184, Val183, His187, Arg213, Val203, Leu111, and Met123 |
| 2 |
His140, Thr204, and Cys205 |
Ser138, Val203, Arg213, Ala137, Ala139, Ala208, and Asp207 |
| 3 |
- |
Val190, Ile215, Thr184, Cys205, Met123, Ala137, Leu116, Val203, Ser138, Thr204, Arg213, His187, Pro135, and Val183 |
| 4 |
Thr204 and Cys205 |
Ala139, Leu116, Ser138, Arg213, Ala137, and Val203 |
| 5 |
Arg213 |
Ile191, Val190, Met123, Val203, Ala137, Ser138, and Leu116 |
| 6 |
Arg213 |
Ile191, Val190, Pro185, His187, Val203, Cys205, Thr204, Ser138, Leu116, Ala137, and Met123 |
| 7 |
His140, Thr204, and Cys205 |
Leu116, Ala139, Ser138, Thr117, Arg213, Val203, Ala137, Met123, Asp112, and Asn113 |
| 8 |
His140, Thr204, and Cys205 |
Ser138, Ala139, Leu116, Ala137, Leu111, Arg213, Met123, Val188, Val190, Ile191, His187, Thr184, Val203, Pro185, and Val183 |
| 9 |
His140, His187, Thr204, and Cys205 |
Ser138, Ala139, Val203, Ala137, Ile215, Pro185, Val190, Val183, His187, Arg213, and Met123 |
| Chalcone |
His140, Thr204, and Cys205 |
Met123, Ala137, Leu116, Arg213, Ser138, ALa139, and Val203 |
Drug-Likeness Screening and Absorption, Distribution, Metabolism, Excretion, and Toxicity Prediction
Based on the results (Table 4), all the selected compounds had acceptable drug-likeness properties and follow Lipinski’s RO5.
Table 4.
Drug-Likeness Screening
|
Ligand Number
|
Molecular Weight (g/mol)
|
H-bond Acceptors
|
H-bond Donors
|
Lipophilicity
|
TPSA (Å2)
|
Water Solubility
|
| 2 |
210.23 |
2 |
0 |
3.38 |
34.14 |
High |
| 7 |
284.31 |
4 |
1 |
3.81 |
55.76 |
Moderately soluble |
| 8 |
308.41 |
2 |
0 |
4.99 |
34.14 |
Moderately soluble |
| 9 |
266.33 |
2 |
0 |
3.51 |
34.14 |
High |
| Chalcone |
208.26 |
1 |
0 |
3.08 |
17.07 |
High |
Note. TPSA: Topological polar surface area; LogP: Partition coefficient in octanol/water.
The values of lipophilicity, molecular weight, and the topological polar surface area (TPSA) indicated good membrane permeability with high to moderate water solubility. The molecular weight of all compounds is less than 500 g/mol.
Membrane permeability was limited when the TPSA was greater than 140 Å2. The results (Table 4) also revealed that the TPSA value of all selected compounds is less than 140 Å2. According to the RO5, an oral drug should have a LogP value < 5. The lipophilicity value of all ligands was less than 5.
The ADMET properties of the selected compound are presented in Table 5. In this study, all selected compounds exhibited high gastrointestinal absorbance and blood-brain barrier penetration, and no compounds were the substrates of permeability glycoprotein. The results of the Cytochrome P450 inhibitor demonstrated that all the compounds are inhibitors for CYP2C19. Compounds 2, 7, and chalcone did not interact with CYP2D6 and CYP3A4, while compounds 8 and 9 were potential inhibitors. Among all compounds, only compound 7 could inhibit CYP2C9. Compounds 7, 8, and 9 were the inhibitors of CYP1A2.
Table 5.
ADMET Properties
|
Ligand Number
|
ADME Properties
|
Toxicity
|
|
GI Absorption
|
BBB Permeant
|
P-gp Substrate
|
CYP Inhibitor
|
Hepatotoxicity
|
Carcinogenicity
|
Immunotoxicity
|
Mutagenicity
|
Cytotoxicity
|
| 2 |
High |
Yes |
No |
CYP2C19 inhibitor |
Inactive |
Inactive |
Inactive |
Inactive |
Inactive |
| 7 |
High |
Yes |
No |
CYP1A2 inhibitor
CYP2C19 inhibitor
CYP2C9 inhibitor |
Inactive |
Inactive |
Inactive |
Inactive |
Inactive |
| 8 |
High |
Yes |
No |
CYP3A4 inhibitor
CYP1A2 inhibitor
CYP2C19 inhibitor
CYP2D6 inhibitor |
Inactive |
Inactive |
Inactive |
Inactive |
Inactive |
| 9 |
High |
Yes |
No |
CYP3A4 inhibitor
CYP1A2 inhibitor
CYP2C19 inhibitor
CYP2D6 inhibitor |
Inactive |
Inactive |
Inactive |
Inactive |
Inactive |
| Chalcone |
High |
Yes |
No |
CYP2C19 inhibitor |
Inactive |
Inactive |
Inactive |
Inactive |
Inactive |
Note. ADMET: Absorption, distribution, metabolism, excretion, and toxicity; BBB: Blood-brain barrier; CYP: Cytochrome P450; GI: Gastrointestinal; P-gp: Permeability glycoprotein.
Based on ProTox-II server results, all compounds were non-active to mutagenicity, immunotoxicity, cytotoxicity, carcinogenicity, and hepatotoxicity.
Discussion
Tooth decay is one of the most common and important global oral health problems (26); it is directly related to the formation of a sticky layer on the teeth, which is called plaque. Considering the importance of S. mutans SrtA in the formation of dental plaques (1-3), in this study, new compounds with the chalcone skeleton were studied to obtain potential inhibitors of this enzyme.
Several studies reported that chalcone can inhibit SrtA activity. Using in vitro methods, Li et al investigated the inhibitory activity of chalcone against SrtA. Their results represented that chalcone with a half maximal inhibitory concentration of 28.41 ± 5.34 µM occupies the enzyme’s active site (27). The findings of Wallock-Richards et al and Zhang et al. revealed that the plant’s natural product chalcone is an inhibitor of SrtA and can effectively inhibit S. mutans biofilm formation (17,28).
Due to the important role of the chalcone ketone group in enzyme inhibition, derivatives with O, N, F, and S functional groups were selected from the ZINC15, LEA3D, and PubChem databases. GaussView software was used to convert the 3D structure of the selected compounds into the appropriate input file for HyperChem software.
After energy optimization, the molecular docking study was performed for the screening of compounds based on the mode of interactions and the binding energy.
In the active site of S. mutans SrtA, peptide bond cleavage and transition state stabilization require the presence of cysteine 205 and arginine 213, respectively (7,24). Considering the main role of these residues in the enzyme’s catalytic activity, the biological activity of the enzyme will be inhibited if the compound interacts with them.
Wang et al elucidated that astilbin forms a hydrogen bond with arginine 213 and interferes with the catalytic activity of SrtA. In this research, similar to our study, 4TQX was chosen as the receptor (29). According to Luo et al, cysteine 205 and arginine 213 participated in the interaction between all studied inhibitors and S. mutans SrtA (7).
Based on our docking results, all compounds interact with these two key amino acids. H-bond interaction with cysteine 205 and hydrophobic interaction with arginine 213 were observed in the docking result of ligands 2, 4, 7, 8, 9, and chalcone. Arginine 213 interacts with ligands 5 and 6 by hydrogen bonding. Ligands 1 and 3 are also connected to these amino acids through a hydrophobic bond.
Among the studied compounds, some of the derivatives (2, 7, 8, & 9) have more negative binding energy and higher affinity than chalcone (∆Gbinding < -3.88 kcal/mol). The structure analysis of these compounds indicates that the presence of an additional carbonyl (C = O) group in the ligand can be effective in receptor affinity. Compound 9 (with ΔGbind = -4.64 kcal/mol) was predicted as the most potent inhibitor. The Ser138, Ala139, Val203, Ala137, Ile215, Pro185, Val190, Val183, His187, Arg213, Met123 His140, His187, Thr204, and Cys205 in the active site of S. mutans SrtA were the sites for hydrogen bonding and hydrophobic interactions with compound 9.
Santana de Oliveira et al studied the antibacterial properties of Siparuna guianensis by the molecular docking approach and reported that the main compound of the essential oil (Atractylone) binds to SrtA via Ile215, Val190, Ile191, Val188, Arg213, and Val203 (30). It was found that compound 9 and Atractylone shared common interactions with Ile215, Val190, Arg213, and Val203 residues.
Finally, 1,2-diphenylethane-1,2-dione (compound 2), (E)-1-[2-hydroxy-5-(methoxymethoxy)phenyl]-3-phenylprop-2-en-1-one (compound 7), 1-[4-[3-(4-propanoylphenyl)propyl]phenyl]propan-1-one (compound 8), and 1-[4-[2-(4-acetylphenyl)ethyl]phenyl]ethanone (compound 9) were selected for further analyses.
A computer-based drug-likeness screening and the ADME properties of the selected ligands revealed that all compounds have high absorption capability and acceptable drug-likeness profiles based on Lipinski’s RO5. Additionally, a toxicity study showed that none of the selected ligands were toxic.
Conclusion
In the current study, the structural binding features of chalcone derivatives with S. mutans SrtA were analyzed, and then several parameters such as absorption, distribution, metabolism, excretion, toxicity, and drug-likeness were studied for the selected compounds.
Based on the results, compounds 2, 7, 8, and 9 (with the appropriate results of docking, drug-likeness, and ADMET properties) can act as SrtA inhibitors and prevent biofilm formation and tooth decay. However, future validation by both in vitro and in vivo studies is inevitable in this regard.
Acknowledgments
The authors would like to thank Dr. Afshin Fassihi for his helpful advice, and special thanks also go to all members of the Isfahan Pharmaceutical Sciences Research Center for their support and encouragement.
Authors’ Contribution
Conceptualization: Azizeh Asadzadeh, Masoumeh Abbasi, Zahra Pournuroz Nodeh and Fatemeh Mahmoudi.
Data curation: Azizeh Asadzadeh.
Formal analysis: Azizeh Asadzadeh.
Funding acquisition: Azizeh Asadzadeh, Masoumeh Abbasi, Zahra Pournuroz Nodeh and Fatemeh Mahmoudi.
Investigation: Azizeh Asadzadeh, Masoumeh Abbasi, Zahra Pournuroz Nodeh and Fatemeh Mahmoudi.
Methodology: Azizeh Asadzadeh.
Project administration: Azizeh Asadzadeh.
Supervision: Azizeh Asadzadeh.
Validation: Azizeh Asadzadeh, Masoumeh Abbasi, Zahra Pournuroz Nodeh and Fatemeh Mahmoudi.
Visualization: Azizeh Asadzadeh, Masoumeh Abbasi, Zahra Pournuroz Nodeh and Fatemeh Mahmoudi.
Writing–original draft: Azizeh Asadzadeh, Masoumeh Abbasi, Zahra Pournuroz Nodeh and Fatemeh Mahmoudi.
Writing–review & editing: Azizeh Asadzadeh and Masoumeh Abbasi.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The authors declare that they have no conflict of interest.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Colombo APV, Tanner ACR. The role of bacterial biofilms in dental caries and periodontal and peri-implant diseases: a historical perspective. J Dent Res 2019; 98(4):373-85. doi: 10.1177/0022034519830686 [Crossref] [ Google Scholar]
- Lin Y, Zhou X, Li Y. Strategies for Streptococcus mutans biofilm dispersal through extracellular polymeric substances disruption. Mol Oral Microbiol 2022; 37(1):1-8. doi: 10.1111/omi.12355 [Crossref] [ Google Scholar]
- Zhang Z, Yang Y, Sun Q, Zeng W, Li Y. Inhibition of biofilm formation and virulence factors of cariogenic oral pathogen Streptococcus mutans by shikimic acid. Microbiol Spectr 2022; 10(4):e0119922. doi: 10.1128/spectrum.01199-22 [Crossref] [ Google Scholar]
- Li B, Li X, Lin H, Zhou Y. Curcumin as a promising antibacterial agent: effects on metabolism and biofilm formation in S mutans. Biomed Res Int 2018; 2018:4508709. doi: 10.1155/2018/4508709 [Crossref] [ Google Scholar]
- Senadheera D, Cvitkovitch DG. Quorum sensing and biofilm formation by Streptococcus mutans. In: Utsumi R, ed. Bacterial Signal Transduction: Networks and Drug Targets. New York, NY: Springer; 2008. p. 178-88. 10.1007/978-0-387-78885-2_12.
- Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev 2018; 54(1):22-9. doi: 10.1016/j.jdsr.2017.08.002 [Crossref] [ Google Scholar]
- Luo H, Liang DF, Bao MY, Sun R, Li YY, Li JZ. In silico identification of potential inhibitors targeting Streptococcus mutans sortase A. Int J Oral Sci 2017; 9(1):53-62. doi: 10.1038/ijos.2016.58 [Crossref] [ Google Scholar]
- Novick RP. Sortase: the surface protein anchoring transpeptidase and the LPXTG motif. Trends Microbiol 2000; 8(4):148-51. doi: 10.1016/s0966-842x(00)01741-8 [Crossref] [ Google Scholar]
- Cho E, Hwang JY, Park JS, Oh D, Oh DC, Park HG. Inhibition of Streptococcus mutans adhesion and biofilm formation with small-molecule inhibitors of sortase A from Juniperus chinensis. J Oral Microbiol 2022; 14(1):2088937. doi: 10.1080/20002297.2022.2088937 [Crossref] [ Google Scholar]
- Maghsoodlou M, Fozouni L, Salehnia Sammak A. Screening of Streptococcus mutans sortase A via myricetin-like inhibitors: in vitro evaluation and molecular docking-based virtual. Avicenna J Med Biochem 2022; 10(1):52-7. doi: 10.34172/ajmb.2022.07 [Crossref] [ Google Scholar]
- Salmanli M, Tatar Yilmaz G, Tuzuner T. Investigation of the antimicrobial activities of various antimicrobial agents on Streptococcus mutans sortase A through computer-aided drug design (CADD) approaches. Comput Methods Programs Biomed 2021; 212:106454. doi: 10.1016/j.cmpb.2021.106454 [Crossref] [ Google Scholar]
- Yerragunta V, Kumara Swamy T, Suman D, Anusha V, Patil P, Samhitha T. A review on chalcones and its importance. PharmaTutor 2013; 1(2):54-9. [ Google Scholar]
- Gaonkar SL, Vignesh UN. Synthesis and pharmacological properties of chalcones: a review. Res Chem Intermed 2017; 43(11):6043-77. doi: 10.1007/s11164-017-2977-5 [Crossref] [ Google Scholar]
- Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem 2005; 12(4):481-99. doi: 10.2174/0929867053363153 [Crossref] [ Google Scholar]
- Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L. Potential pharmacological uses of chalcones: a patent review (from June 2011-2014). Expert Opin Ther Pat 2015; 25(3):351-66. doi: 10.1517/13543776.2014.995627 [Crossref] [ Google Scholar]
- Satokata AAC, Souza JH, Silva LLO, Santiago MB, Ramos SB, de Assis LR. Chalcones with potential antibacterial and antibiofilm activities against periodontopathogenic bacteria. Anaerobe 2022; 76:102588. doi: 10.1016/j.anaerobe.2022.102588 [Crossref] [ Google Scholar]
- Wallock-Richards DJ, Marles-Wright J, Clarke DJ, Maitra A, Dodds M, Hanley B. Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. Chem Commun (Camb) 2015; 51(52):10483-5. doi: 10.1039/c5cc01816a [Crossref] [ Google Scholar]
- Pagadala NS, Syed K, Tuszynski J. Software for molecular docking: a review. Biophys Rev 2017; 9(2):91-102. doi: 10.1007/s12551-016-0247-1 [Crossref] [ Google Scholar]
- Asadzadeh A, Sirous H, Pourfarzam M, Yaghmaei P, Afshin F. In vitro and in silico studies of the inhibitory effects of some novel kojic acid derivatives on tyrosinase enzyme. Iran J Basic Med Sci 2016; 19(2):132-44. [ Google Scholar]
- Shojaei Barjouei M, Norouzi S, Bernoos P, Mokhtari K, Asadzadeh A. Comparison of the inhibitory activity of bioactive compounds of Salvia officinalis with antidiabetic drugs, voglibose and miglitol, in suppression of alpha-glucosidase enzyme by in silico method. Iran J Diabetes Metab 2022;22(3):145-54. [Persian].
- Ghaffari S, Asadzadeh A, Seyedhosseini Ghaheh H, Sholehvar F. Docking study on salicylaldehyde derivatives as anti-melanogenesis agents. J Fasa Univ Med Sci 2018;8(1):618-27. [Persian].
- Naderi Kotaki M, Asadzadeh A, Heidaryan F. Study the effect of thymus vulgaris in inhibiting acetylcholinesterase enzyme in order to treat Alzheimer’s disease. J Sabzevar Univ Med Sci 2020;27(5):594-602. [Persian].
- Shams Moattar F, Asadzadeh A, Heydari M, Zamani M, Esnaashari F, Jeldani F. Designing multi‐epitope subunit vaccine candidate for Zika virus utilizing in silico tools. Res Mol Med 2022; 10(1):9-18. doi: 10.32598/rmm.10.1.1249.1 [Crossref] [ Google Scholar]
- Guimaraes CP, Witte MD, Theile CS, Bozkurt G, Kundrat L, Blom AE. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat Protoc 2013; 8(9):1787-99. doi: 10.1038/nprot.2013.101 [Crossref] [ Google Scholar]
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 2004; 1(4):337-41. doi: 10.1016/j.ddtec.2004.11.007 [Crossref] [ Google Scholar]
- Heng C. Tooth decay is the most prevalent disease. Fed Pract 2016; 33(10):31-3. [ Google Scholar]
- Li H, Chen Y, Zhang B, Niu X, Song M, Luo Z. Inhibition of sortase A by chalcone prevents Listeria monocytogenes infection. Biochem Pharmacol 2016; 106:19-29. doi: 10.1016/j.bcp.2016.01.018 [Crossref] [ Google Scholar]
- Zhang B, Teng Z, Li X, Lu G, Deng X, Niu X. Chalcone attenuates Staphylococcus aureus virulence by targeting sortase A and alpha-hemolysin. Front Microbiol 2017; 8:1715. doi: 10.3389/fmicb.2017.01715 [Crossref] [ Google Scholar]
- Wang J, Shi Y, Jing S, Dong H, Wang D, Wang T. Astilbin inhibits the activity of sortase A from Streptococcus mutans. Molecules 2019; 24(3):465. doi: 10.3390/molecules24030465 [Crossref] [ Google Scholar]
- Santana de Oliveira M, da Cruz JN, Almeida da Costa W, Silva SG, da Paz Brito M, de Menezes SAF. Chemical composition, antimicrobial properties of Siparunaguianensis essential oil and a molecular docking and dynamics molecular study of its major chemical constituent. Molecules 2020; 25(17):3582. doi: 10.3390/molecules25173852 [Crossref] [ Google Scholar]