Avicenna Journal of Clinical Microbiology and Infection. 10(1):38-42.
doi: 10.34172/ajcmi.2023.3418
Original Article
Comparison of the Scolicidal Activity of Pulicaria gnaphalodes and Alhagi maurorum Extracts against Protoscoleces of Echinococcus granulosus Sensu Lato In Vitro
Seyed Jafar Adnani Sadati 1, 2  , Babake Aghili 1
, Babake Aghili 1  , Abolghasem Siyadatpanah 3
, Abolghasem Siyadatpanah 3  , Roghayeh Norouzi 4, *
, Roghayeh Norouzi 4, * 
Author information:
1Department of Microbiology Immunology and Parasitology, School of Medicine, Qom University of Medical Sciences, Qom, Iran
2Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran
3Paramedical school, Infectious Diseases Research Center, Gonabad University of Medical Sciences, Gonabad, Iran
4Department of Pathobiology, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran
Abstract
Background: Cystic echinococcosis (CE), caused by the larval stage of the Echinococcus granulosus, is a zoonotic disease and has a global distribution. Today, herbal compounds are highly regarded in order to inactivate hydatid cyst protoscoleces. This study aimed to compare the scolicidal activity of hydroalcoholic extract of Pulicaria gnaphalodes and Alhagi maurorum against hydatid cyst protoscoleces in vitro.
Methods: The scolicidal activity of P. gnaphalodes and A. maurorum extracts were evaluated at 50, 100, 150, and 200 mg/mL concentrations following 15, 30, and 60 minutes of exposure. Then, they were compared with Albendazole (5 g/100 mL) as positive control and distilled water as negative one in similar doses. The viability of protoscoleces was confirmed with a 0.1% eosin stain test under a light microscope. The experiments were performed twice, and data were analyzed by GraphPad software version 5.0.
Results: The results of this study indicated that P. gnaphalodes extract killed 100% of the protoscoleces at a concentration of 200 mg/mL after 30 minutes of exposure, but the hydroalcoholic extract of A. maurorum at the same concentration and time could kill 90% of protoscoleces.
Conclusion: The findings of the present study confirmed that P. gnaphalodes had a strong scolicidal effect; however; in vivo studies are needed to evaluate the effectiveness of P. gnaphalodes plant.
Keywords: Hydatid cyst, Scolicidal, Pulicaria gnaphalodes, Alhagi maurorum, In vitro
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Adnani Sadat SJ, Aghili B, Siyadatpanah A, Norouzi R. Comparison of the scolicidal activity of Pulicaria gnaphalodes and Alhagi maurorum extracts against protoscoleces of Echinococcus granulosus sensu lato In Vitro. Avicenna J Clin Microbiol Infect. 2023; 10(1):38-42. doi:10.34172/ajcmi.2023.3418
Introduction
Cystic echinococcosis (CE) is a zoonotic parasitic disease caused by the larval stages of cestodes (tapeworms) of the Echinococcus granulosus (1). CE usually involves various host organs such as the liver (50–70%), lungs (less frequently), heart, brain, bones, spleen, and kidneys which may even lead to death (2,3). At present, hydatid cyst surgery and chemotherapy with benzimidazole derivatives (albendazole and mebendazole) are used for the treatment of cysts. However, they have their own disadvantages. Surgery is not highly recommended since it is not completely safe and has various adverse side effects; likewise, the use of chemical drugs is not strongly suggested due to increased resistance of protoscoleces, unfavorable side effects, and teratogenic effects (4-7). The main concern in surgery is cyst rupture or leakage of cyst contents, leading to secondary infection or the involvement of adjacent organs, so different scolicidal agents are used to lower this risk (8,9). These agents must have specific characteristics in order to be used in surgery such as being able to destroy protoscoleces in a low concentration and during a short period of time, having no reduction in efficacy after being diluted with cyst fluid, and being non-toxic, safe, and cost-effective. Among the scolicidal agents, herbal compounds are of great interest to researchers due to their easy availability, few side effects, low toxicity, and reasonable cost (10).
Pulicaria gnaphalodes (kak kosh -e byabani) is usually consumed as a flavoring agent, herbal tea, and medicinal plant. It is an obstinate plant that grows mostly in sandy, rocky, and abandoned areas in Saudi Arabia, Afghanistan, Pakistan, Iran, India, Iraq, and Turkey. This plant has antibacterial, anti-diarrheal, anti-inflammatory, antioxidant, antihypercholesterolemic, and leishmanicidal properties (11). Alhagi maurorum ( khar shotor -e Irani) is traditionally used in folk medicine as a remedy for rheumatic pains, liver disorders, cholagogue, gastrointestinal disorders, urinary tracts diseases, antiasthmatic, anti-bronchitis, and anti-diarrheal activities (12). The current study was conducted due to the antimicrobial properties of these plants on parasite survival. Accordingly, this study aimed to compare the scolicidal effect of hydroalcoholic extract of P. gnaphalodes and A. maurorum on hydatid cyst protoscoleces in vitro.
Materials and Methods
Preparation of Protoscoleces
In this experimental study, which was conducted in 2021, 30 sheep livers and lungs were collected from the industrial slaughterhouse of Qom province and transferred to the Parasitology Laboratory of the Faculty of Medicine. The cyst fluid was aspirated with a sterile syringe, transferred to a beaker, and placed at room temperature for 10 mines to settle protoscoleces. After 10 minutes, the supernatant was discarded, and the protoscoleces were washed three times with phosphate buffered saline and stained with 0.1% eosin to assess the protoscoleces viability (13,14).
Plant Collection
Pulicaria gnaphalodes was collected from the deserts of South of Khorasan province (east of Iran) and was identified and approved in the botany section of Qom Agricultural Research Center. A. maurorum was purchased from the medicinal plant shop of Qom province, then both plants were milled using an electric grinder.
Preparation of Plant Extracts
The maceration method was used to prepare the hydroethanolic extract. Two hundred grams of plant material powder was mixed with 800 mL of 70% ethanol and placed in a shaker at room temperature for 3 days. The materials were passed through three layers of gauze and incubated at 37°C until water and alcohol were completely evaporated. After the complete evaporation of water and alcohol, the dry material was scraped from the bottom of the container and stored at 4°C for later use (15).
Evaluation of the Scolicidal Activity of the Plant Extracts
To evaluate the scolicidal activity of the hydroethanolic extracts of the P. gnaphalodes and A. maurorum, plant concentrations of 50, 100, 150, and 200 mg/mL in distilled water were prepared separately. Half a milliliter of the extracts was added to the microtubes, and a drop of protoscoleces solution, containing 2 × 103 protoscoleces was added. The tubes were quickly spun and incubated for 15, 30, and 60 minutes at 37°C followed by removing the supernatant. Then, a drop of 0.1% eosin was added to the remaining precipitate and spun gently. Furthermore, a drop of protoscoleces was placed on a slide, and the dead and live protoscoleces were counted using light microscopy. The scolicidalactivity of P. gnaphalodes and A. maurorum extracts were evaluated, along with Albendazole (5g/100 mL) as the positive control and distilled water as the negative control. Albendazole was purchased from Tolide Darouhai Dami Company (Iran). To conduct the test correctly and obtain more accurate results, the experiments were performed twice (14).
Statistical Analysis
Data were analyzed by a two-way ANOVA using GraphPad Prism software program version 5.0 and expressed as mean ± standard deviation.
Results
Table 1 and Figure 1 present the inactivity of protoscoleces of the two extracts. The scolicidal effect after 15, 30, and 60 minutes of exposure to 50 mg/mL of P. gnaphalodes and A. maurorumwere 33.33%, 37%, and 40.67% as well as 47.33%, 50%, and 80.67%, respectively.
Table 1.
The Scolicidal Activity of Pulicaria gnaphalodes and Alhagi maurorumExtracts on Cystic Echinococcosis
|
Concentration
|
Time
|
P. gnaphalodes
|
A. maurorum
|
Positive Control
|
Negative Control
|
| 50 mg/mL |
15 min |
33.33 ±1.15 |
47.33 ±1.15 |
100 ±0.00 |
4.66 ±0.57 |
| 30 min |
37 ±1.00 |
50 ±1.00 |
100 ±0.00 |
4.66 ±1.00 |
| 60 min |
40.67 ±2.08 |
80.67 ±2.08 |
100 ±0.00 |
4.33 ±0.57 |
| 100 mg/mL |
15 min |
46.2 ±2.00 |
60 ±2.00 |
100 ±0.00 |
4.66 ±0.57 |
| 30 min |
45± 1.52 |
75 ±2.00 |
100 ±0.00 |
4 ±1.00 |
| 60 min |
53± 2.08 |
82 ±2.51 |
100 ±0.00 |
4.33 ±0.57 |
| 150 mg/mL |
15 min |
57± 2.08 |
80 ±1.08 |
100 ±0.00 |
4.66 ±0.57 |
| 30 min |
61± 1.52 |
85.67 ±3.05 |
100 ±0.00 |
4 ±1.00 |
| 60 min |
80± 1.5 |
90.67 ±0.57 |
100 ±0.00 |
4.33 ±0.57 |
| 200 mg/mL |
15 min |
1.0087± |
86.33 ±1.15 |
100 ±0.00 |
4.66 ±0.57 |
| 30 min |
100± 1.50 |
87.67 ±1.50 |
100 ±0.00 |
4 ±1.00 |
| 60 min |
100± 0.75 |
90 ±0.57 |
100 ±0.00 |
4.33 ±0.57 |
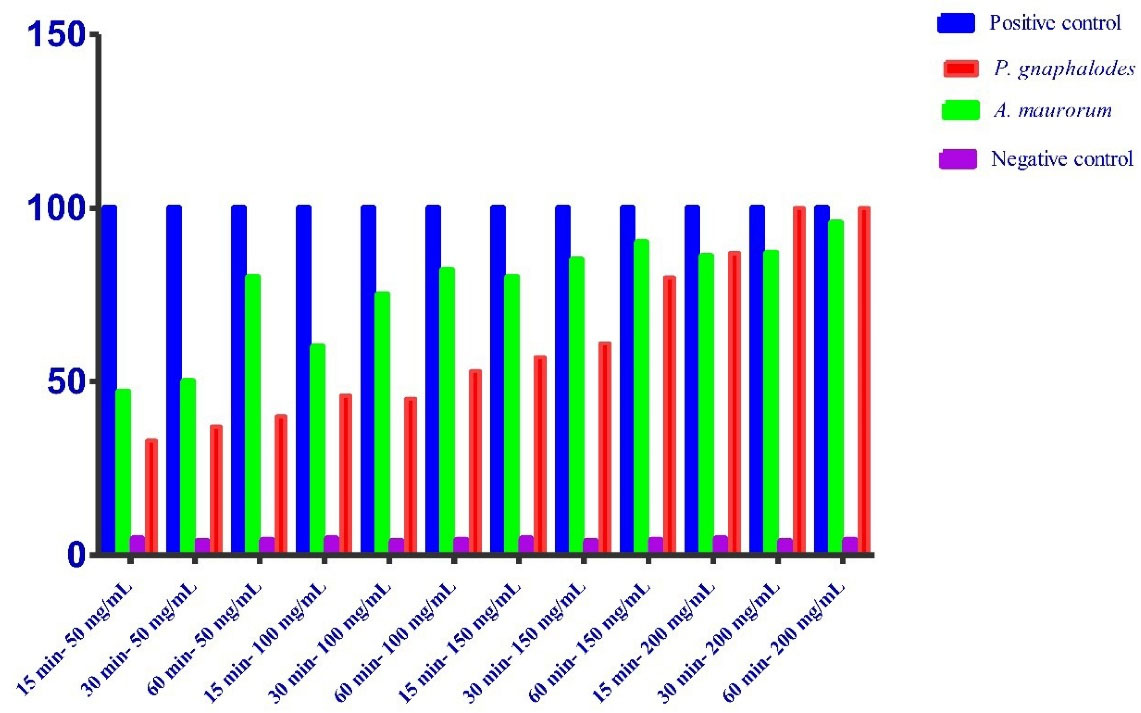
Figure 1.
The Scolicidal Activity of Pulicaria gnaphalodes and Alhagi maurorumon Cystic Echinococcosis
.
The Scolicidal Activity of Pulicaria gnaphalodes and Alhagi maurorumon Cystic Echinococcosis
The scolicidal effect after 15, 30, and 60 minutes of exposure to 100 mg/mL of P. gnaphalodes and A. maurorumwere46.2%, 45%, and53% as well as60%, 75%, and 82%,respectively. Moreover, the scolicidal effect after 15, 30, and 60 minutes of exposure to 150 mg/mL of P. gnaphalodes and A. maurorumwere 57%, 61%, and80% as well as80%, 85%, and 90.67%,respectively. In the same way the scolicidal effect after 15, 30, and 60 minutes of exposure to 200 mg/mL of P. gnaphalodes and A. maurorumwere87%, 100%, and100% as well as86.33%, 87.67%, and 90%,respectively. Furthermore, the inactivity of protoscoleces in the negative and positive control groups at the indicated time were 4.66% and 100%, respectively.
Discussion
Nowadays, many scolicidal agents such as hypertonic saline, mannitol, chlorhexidine gluconate, plant extracts, nanoparticles, and the like have been used to inactivate hydatid cyst protoscoleces, but many of these agents have adverse effects that limit their use (5). Among the scolicidal agents, herbal compounds are of great interest to researchers due to their easy availability, fewer side effects, low toxicity, and reasonable cost. This study strived to compare the scolicidal effect of hydroalcoholic extract of P. gnaphalodes and A. maurorum on hydatid cyst protoscoleces in vitro.
The results of this study indicated that the hydroethanolic extract of P. gnaphalodes and A. maurorum had scolicidalactivity in all concentrations, but P. gnaphalodes had a higher scolicidaleffect compared to A. maurorum in all concentrations and times. On the other hand, the results revealed that P. gnaphalodes extract kills 100% of the protoscoleces at a concentration of 200 mg/mL after 30 and 60 minutes of exposure, but A. maurorum extract could kill 90% of the protoscoleces at the same concentration and time. In both extracts, scolicidalactivity increased by increasing the concentration and time of exposure. The lowest scolicidaleffect of each extract at a concentration of 50 mg/mL after 15 minutes of exposure to protoscoleces was 33.33% and 47.33%, respectively.
The scolicidaleffects of A. maurorum extract in all receiving groups at all concentrations except 50 mg/mL for 15 minutes were significant compared to the negative control (P < 0.05). In both extracts, the increase in lethal effects was dose-dependent and significant (P < 0.05), while a significant difference (P < 0.05) was observed in the groups receiving A. maurorum extract compared to the positive control group (receiving albendazole).
The effect of the aqueous extract of Punica granatum (sour pomegranate) against protoscoleces was studied, and it was concluded that the concentration of 80 mg has the greatest effect after 15 minutes and causes the elimination of 100% of protoscoleces (16). The scolicidal activity of aqueous and alcoholic extracts of Peganum harmala was studied, and the results confirmed that the aqueous extract of P. harmala has a weaker and insignificant effect on protoscoleces compared to its alcoholic extract, while alcoholic extract caused 100% mortality of protoscoleces at the same concentration and time (17). Another study investigated the activity of aqueous and hydroalcoholic extract of Berberis vulgaris (barberry fruit) on hydatid cyst protoscoleces. The results displayed that aqueous extract in 5 minutes and hydroalcoholic extract in just 2 minutes kill all protoscoleces (18). The lethal effect of Lepidium sativum on protoscoleces exhibited that the concentration of 15 mg has the greatest effect after 60 minutes (10).In another research, the lethal effect of methanol extract from pomegranate root was investigated, and the concentration of 0.1% in 6 hours had the strongest scolicidal effects (19).
In a study, Ceratonia silique extract at a concentration of 50 mg/mL caused the destruction of all protoscoleces after 30 minutes (20). Furthermore, a concentration of 100 mg/mL of Zingiber officinale killed all protoscoleces in 40 minutes, while Artemisia aucheri extract had little effect at all concentrations (21).
The result of the chloroformic extract of Allium sativum on protoscoleces confirmed its highest scolicidal activity at a concentration of 200 mg/mL (22). Another study found that the methanolic extract of Zataria multiflora destroys 100% of the protoscoleces at concentrations of 10 mg/mL and 25 mg/mL during 3 minutes and 1 minute, respectively (23).
In another study, the concentration of 10 mg/mL of Nigella sativa essential oil removed all protoscoleces (24). The scolicidal effect of the hydroalcoholic extract of Taxus baccata at a concentration of 150 mg/mL was 66.6% (25). The comparison of scolicidal effects of hydroalcoholic extracts of Calendula officinalis, Artemisia dracunculus, Artemisia absinthium,andFerula asafoetidarevealed that Artemisia absinthium andFerula asafetida remove 100% of the protoscoleces at a concentration of 250 mg/mL after 60 minutes, while the concentration of 250 mg/mL of hydroalcoholic extract of Calendula officinalis and Artemisia dracunculus removes 42.33% and 65.67%, respectively (26).
It appears that the difference in the results of this study with identical studies is due to the differences in the type of plant, extract, concentration, and time of exposure, as well as the difference in the measurement units. Moreover, the results of this study indicated that the scolicidal activity of the hydroalcoholic extract of P. gnaphalodesremoved 100% of the protoscoleces at a concentration of 200 mg/mL after 30 minutes of exposure. Therefore, it was concluded that this plant has the potential to be used in hydatid cyst surgery as an effective scolicidal agent. This investigation was done in vitro, so it is necessary to do it in vivo to check its possible side effects on internal organs. This study would have been more accurate if the strain of the parasite had been determined.
Acknowledgments
The authors would like to thank the Research Deputy at the Qom University of Medical Sciences who provided the required data. This investigation was supported by a grant from the Qom University of Medical Sciences (No: IR.MUQ. REC. 1400.163).
Authors’ Contribution
Conceptualization: Seyed Jafar Adnani Sadati.
Data curation: Roghayeh Norouzi.
Formal analysis: Abolghasem Siyadatpanah.
Funding acquisition: Seyed Jafar Adnani Sadati.
Investigation: Roghayeh Norouzi.
Methodology: Babake Aghili.
Project administration: Abolghasem Siyadatpanah.
Resources: Roghayeh Norouzi.
Supervision: Abolghasem Siyadatpanah.
Validation: Babake Aghili.
Visualization: Roghayeh Norouzi.
Writing–original draft: Roghayeh Norouzi.
Writing–review & editing: Roghayeh Norouzi.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
This study was approved by the Research Committee of Qom University of Medical Sciences (No: IR.MUQ.REC.1400.163).
References
- Norouzi R, Ataei A, Hejazy M, Noreddin A, El Zowalaty ME. Scolicidal effects of nanoparticles against hydatid cyst protoscolices in vitro. Int J Nanomedicine 2020; 15:1095-100. doi: 10.2147/ijn.s228538 [Crossref] [ Google Scholar]
- Ahmadpour E, Godrati-Azar Z, Spotin A, Norouzi R, Hamishehkar H, Nami S. Nanostructured lipid carriers of ivermectin as a novel drug delivery system in hydatidosis. Parasit Vectors 2019; 12(1):469. doi: 10.1186/s13071-019-3719-x [Crossref] [ Google Scholar]
- Eshraghi M, Norouzi R, Aghili B, Hendijani Fard M, Adnani Sadati SJ. Epidemiological characteristics of patients with hydatid cysts in Qom province hospitals from 2001 to 2019. Avicenna J Clin Microbiol Infect 2022; 9(1):26-30. doi: 10.34172/ajcmi.2022.05 [Crossref] [ Google Scholar]
- Walker M, Rossignol JF, Torgerson P, Hemphill A. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J Antimicrob Chemother 2004; 54(3):609-16. doi: 10.1093/jac/dkh386 [Crossref] [ Google Scholar]
- Kohansal MH, Nourian A, Rahimi MT, Daryani A, Spotin A, Ahmadpour E. Natural products applied against hydatid cyst protoscolices: a review of past to present. Acta Trop 2017; 176:385-94. doi: 10.1016/j.actatropica.2017.09.013 [Crossref] [ Google Scholar]
- Naseri M, Akbarzadeh A, Spotin A, Asl Rahnemaii Akbari N, Mahami-Oskouei M, Ahmadpour E. Scolicidal and apoptotic activities of albendazole sulfoxide and albendazole sulfoxide-loaded PLGA-PEG as a novel nanopolymeric particle against Echinococcus granulosus protoscoleces. Parasitol Res 2016; 115(12):4595-603. doi: 10.1007/s00436-016-5250-8 [Crossref] [ Google Scholar]
- Maggiore MA, Albanese AA, Gende LB, Eguaras MJ, Denegri GM, Elissondo MC. Anthelmintic effect of Mentha spp essential oils on Echinococcus granulosus protoscoleces and metacestodes. Parasitol Res 2012; 110(3):1103-12. doi: 10.1007/s00436-011-2595-x [Crossref] [ Google Scholar]
- Alyousif MS, Al-Abodi HR, Almohammed H, Alanazi AD, Mahmoudvand H, Hakami Shalamzari M. Chemical composition, apoptotic activity, and antiparasitic effects of Ferulamacrecolea essential oil against Echinococcus granulosus protoscoleces. Molecules 2021; 26(4):888. doi: 10.3390/molecules26040888 [Crossref] [ Google Scholar]
- Eshraghi M, Shahmoradi L, Ghoddoosi M, Adnani Sadati SJ. Diagnosis of primary hydatid cyst of thyroid gland: a case report. Biomol Concepts 2019; 10(1):106-10. doi: 10.1515/bmc-2019-0013 [Crossref] [ Google Scholar]
- Bahrami S, Razi Jalali MH, Ramezani Z, Pourmehdi Boroujeni M, Toeimepour F. In vitro scolicidal effect of Lepidium sativum essential oil. J Ardabil Univ Med Sci 2015;15(4):395-403. [Persian].
- Anthony JP, Fyfe L, Smith H. Protective and anti-inflammatory effects of plant oils. Trends Parasitol 2005; 10(21):462-8. doi: 10.1016/j.pt.2005.08.004 [Crossref] [ Google Scholar]
- Naqvi SAR, Shah SMA, Kanwal L, Saeed M, Atta-ul-Haq Atta-ul-Haq, Nisar J. Antimicrobial and antihypercholesterolemic activities of Pulicariagnaphalodes. Dose Response 2020; 18(1):1559325820904858. doi: 10.1177/1559325820904858 [Crossref] [ Google Scholar]
- Adnani Sadati SJ, Farahnak A, Molaei Rad MB, Golestani A, Eshraghiyan MR. A comparison between the effects of albendazole and mebendazole on the enzymatic activity of excretory/secretory products of Echinococcus granulosus protoscoleces in vitro. Iran J Public Health 2016; 45(2):223-9. [ Google Scholar]
- Fateh R, Norouzi R, Mirzaei E, Nissapatron V, Nawaz M, Khalifeh-Gholi M. In vitro evaluation of albendazole nanocrystals against Echinococcus granulosus protoscolices. Ann Parasitol 2021; 67(2):203-12. doi: 10.17420/ap6702.330 [Crossref] [ Google Scholar]
- Meshkibaf MH, Abdollahi A, Fasihi Ramandi M, Sadati SA, Moravvej A, Hatami S. Antibacterial effects of hydro-alcoholic extracts of Ziziphoratenuior, Teucrium polium, Barberiscorcorde and Stachys inflate. Koomesh 2010;11(4):240-5. [Persian].
- Jafari Z, Rouhani S, Niyyati M, Kamalinejad M, Zayeri F. Invitro effectiveness of Punica granatum aqueous extract on viability of Echinococcus granulosus protoscolex. J North Khorasan Univ Med Sci 2017; 9(1):65-74. doi: 10.18869/acadpub.jnkums.9.1.65.[Persian] [Crossref] [ Google Scholar]
- Mahdavi M, Masood J. Scolicidal effect of alcoholic, aqueous and total alkaloids of Peganum harmala L. (Syrian rue) against hydatid cysts protoscolices. Tehran Univ Med J 2002;60(3):215-26. [Persian].
- Salehi N, Rouhani S, Kamalinejad M, Zayeri F, Motaghifar A. Scolicidal effects of Berberis vulgaris fruit extract on hydatid cyst protoscolices. Tehran Univ Med J 2014;72(2):121-8. [Persian].
- Zibaei M, Sajedi B, Jafari Z. Scolicidal effects of different concentrations hydroalcoholic extract of Punica granatum root on hydatid cyst protoscolices. Alborz Univ Med J 2014; 3(4):205-10. doi: 10.18869/acadpub.aums.3.4.205.[Persian] [Crossref] [ Google Scholar]
- Malekifard F, Keramati F. Investigation of the effects of Ceratonia silique extract on protoscolexes of hydratid cyst in vitro. Armaghane Danesh 2018;23(1):69-79. [Persian].
- Saki J, Biranvand E, Arjmand R. The in vitro anti-Leishmania effect of Zingiber officinale extract on promastigotes and amastigotes of Leishmania major and Leishmania tropica. Turkiye Parazitol Derg 2022; 46(2):91-6. doi: 10.4274/tpd.galenos.2021.53825 [Crossref] [ Google Scholar]
- Sadjjadi SM, Zoharizadeh MR, Panjeshahin MR. In vitro screening of different Allium sativum extracts on hydatid cysts protoscoleces. J Invest Surg 2008; 21(6):318-22. doi: 10.1080/08941930802348261 [Crossref] [ Google Scholar]
- Moazeni M, Larki S, Oryan A, Saharkhiz MJ. Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: an in vivo study. Vet Parasitol 2014; 205(1-2):107-12. doi: 10.1016/j.vetpar.2014.07.006 [Crossref] [ Google Scholar]
- Mahmoudvand H, Saedi Dezaki E, Kheirandish F, Ezatpour B, Jahanbakhsh S, Fasihi Harandi M. Scolicidal effects of black cumin seed (Nigella sativa) essential oil on hydatid cysts. Korean J Parasitol 2014; 52(6):653-9. doi: 10.3347/kjp.2014.52.6.653 [Crossref] [ Google Scholar]
- Norouzi R, Hejazy M, Azizi D, Ataei A. Effect of Taxus baccata L extract on hydatid cyst protoscolices in vitro. Arch Razi Inst 2021; 75(4):473-80. doi: 10.22092/ari.2019.125573.1310 [Crossref] [ Google Scholar]
- Norouzi R, Abedi Maleki R, Siyadatpanah A, Fiad A, El Zowalaty ME. In vitro scolicidal effect of Calendula officinalis, Artemisia dracunculus, Artemisia absinthium, and Ferulaassafoetida extracts against hydatid cyst protoscolices. Ann Parasitol 2022; 68(3):543-51. doi: 10.17420/ap6803.461 [Crossref] [ Google Scholar]