Avicenna Journal of Clinical Microbiology and Infection. 9(3):109-114.
doi: 10.34172/ajcmi.2022.3397
Original Article
Inhibitory Activity of Oliveria decumbens Essential Oil on Staphylococcus aureus Biofilm and Planktonic Cells
Mahshad Javid Moghadam 1  , Siavash Maktabi 2, *
, Siavash Maktabi 2, *  , Mehdi Zarei 2
, Mehdi Zarei 2  , Pezhman Mahmoodi Koohi 3
, Pezhman Mahmoodi Koohi 3
Author information:
1Ph.D Graduated from faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran
2Department of Food Hygiene, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran
3Department of Pathobiology, Faculty of Veterinary Science, Bu-Ali Sina University, Hamadan, Iran
Abstract
Background:
Staphylococcus aureus is a pathogenic bacterium that forms biofilms on biotic and abiotic surfaces. Biofilm formation is important for researchers because it increases the risk of food contamination in the food industry, increases the pathogenicity of bacteria, and damages the equipment. The main purpose of this study was to find out the antimicrobial and antibiofilm properties of Oliveria decumbens essential oil (Od-EO) against Staphylococcus aureus.
Methods: In this study, the antibacterial and anti-biofilm properties of Od-EO were tested against four strong biofilm producers. S. aureus isolates were obtained from food and humans. The antibacterial properties of Od-EO on planktonic S. aureus were investigated using the disk diffusion method; further, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined. The microtiter plate (MTP) method and slime production evaluation were used to assess the inhibitory effect of Od-EO on S. aureus biofilm formation.
Results: Od-EO indicated strong antimicrobial activity against planktonic S. aureus. After performing tests related to the anti-biofilm activity of Od-EO, it was found that Od-EO significantly reduced slime production and thus inhibited biofilm formation.
Conclusions: Od-EO and its components can be used as a new anti-biofilm agent in medical, dental, and food industry equipment.
Keywords: Biofilm, Essential oil, Oliveria decumbens, Staphylococcus aureus
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Javid Mmoghadam M, Maktabi S, Zarei M, Mahmoodi Koohi P. Inhibitory activity of oliveria decumbens essential oil on staphylococcus aureus biofilm and planktonic cells. Avicenna J Clin Microbiol Infect. 2022; 9(3):109-114. doi:10.34172/ ajcmi.2022.3397
Introduction
Staphylococcus aureus is a pathogenic and harmful bacterium that can cause various diseases from simple skin infections to fatal systemic diseases (1,2). In addition, S. aureus is a foodborne pathogen, inducing staphylococcal food poisoning (3). S. aureus can attach, grow, and form biofilms on different biotic and abiotic surfaces and can resist in clinical and food-processing environments (4). These surfaces include foods and food processing equipment or indwelling medical devices such as joint prosthetics, artificial heart valves, catheters, and dental implants (5). Biofilms increase bacterial resistance against the actions of typical antimicrobial agents, harsh environmental conditions, and host immunity compared to planktonic cells (3).
Biofilm formation is the result of the interaction of three main factors: the microbial cell, attachment surface, and surrounding medium (6). During biofilm formation, bacteria produce a self-produced extracellular matrix that consists of materials such as proteins, carbohydrates, and extracellular DNA, which encloses the cells in an adhesive matrix (7). Accumulated data indicate that biofilm formation is facilitated by the production of various microbial surface components that recognize adhesive matrix molecules and polysaccharide intercellular adhesion during the development of an actual biofilm (1,8). Since biofilms are of great concern, many studies have been conducted to gain more information about their development, spread, and control strategies (9).
In recent years, researchers have been interested in the investigation of natural antimicrobial compounds. Most of the natural compounds originated from plants and investigated in different studies have medicinal and antimicrobial properties, including antibiofilm activity. Antibiofilm activity of plant extracts and essential oils has been reported against Listeria monocytogenes (10), Escherichia coli (11), S. aureus (12,13), and other pathogens.
Oliveria decumbens belongs to Umbelliferae family and is endemic to Iran. Most plants belonging to this family produce volatile compounds such as terpenes. Several studies have been conducted on the antimicrobial and antioxidant properties of Umbelliferae family EOs (14), but no study has been conducted on the antibiofilm effects of Oliveria decumbensso far.
The purpose of this study was to determine the chemical composition of Oliveria decumbens and assess its antibacterial and antibiofilm activity against S. aureus isolates from foodstuff, humans, and standard strains that form strong biofilms.
Materials and Methods
Bacterial Strains
This study investigated four strong biofilm producers of S. aureus from 20 isolates of respiratory patients and food samples. All isolates were confirmed in a previous epidemiology study conducted by Sharifi et al (15). The Congo red method was used to investigate biofilm formation by the isolates. S. aureus ATCC 29213 was used as a standard strong biofilm constituent strain, and Staphylococcus epidermidis ATCC 12228 was used as a negative control in this study based on previous studies (16). Bacterial stocks were kept at −80°C in tryptic soy broth (TSB) containing 25% glycerol (v/v), and the frozen strains were subcultured in tryptic soy agar after thawing.
Preparation of Collected Plants
Oliveria decumbens plants were collected from Khuzestanin province during the full flowering stage of the plant. The collected plants were identified and confirmed as Oliveria decumbens using the Faculty of Agriculture at the Shahid Chamran University of Ahvaz. They were then washed (with distilled water) and dried in the dark at 25°C. Subsequently, the Od-EO was extracted using a Clevenger device for three hours.
GC-MS Analysis of the Od-EO
Od-EO main compounds were analyzed using gas chromatography/mass spectrometry (GC-MS, Agilent 5977B, USA) by an HP-5MS capillary column (5% phenyl methyl silicone and 95% dimethylpolysiloxane) with a column length of 30 meters, the internal diameter of 0.25 mm, and film thickness of 0.25 µm. Further, helium was used as carrier gas (99.99%) with 1.1 mL/min, and 0.2 μL of Od-EO was injected for GC-MS analysis which was repeated three times (17).
Anti-microbial Activity of Od-EO on S. aureus
Agar Disc Diffusion Assay
One of the tests performed to determine the antimicrobial properties of Od-EO was agar disk diffusion according to CLSI (18). S. aureus suspensions (~1.5 × 108 CFU/mL) were cultured on Mueller–Hinton agar plates using sterile swabs. Sterilized paper discs containing 20 μL of Od-EO were prepared in advance, and after drying, they were placed on Mueller Hinton agar. The diameter of the inhibition zone was measured at the end of the incubation period (24 hours at 37°C). Negative control (sterile discs with dimethyl sulfoxide [DMSO]) and positive controls were also used. Negative contained sterile discs with DMSO, and positive controls included ampicillin (10 μg/disk), vancomycin (30 μg/disk), amoxicillin (25 μg/disk), trimethoprim/sulfamethoxazole (23.75 μg/disk), and oxytetracycline (30 μg/disk) (Padtan Teb, Iran).
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Determination
minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using double broth dilution of Od-EO (0.015–4 µL/mL). At first, Od-EO was dissolved in a TSB medium supplemented with 0.1% DMSO. Then, 180 µL of the broth containing various concentrations of Od-EO was distributed into each well of a 96-well polystyrene microtiter plate (MTP), and 20 µL of the bacterial culture (~106 CFU/mL) was added to each well. After incubating the plates (37°C for 24 hours), the lowest concentration of Od-EO that inhibited the visible growth of S. aureus was determined as the MIC. The wells with the lowest concentration of Od-EO that showed no growth on tryptic soy agar were recognized as MBC. Sterile TSB without bacterial culture was used as the negative control (8).
Phenotypic Identification of Slime Production
Slime production by the S. aureus isolates was investigated using the Congo red method. All strains were separately cultured in Congo red agar (CRA) medium. ATCC29213 and ATCC22812 were used as positive and negative controls, respectively, and the color of the S. aureus isolate colonies was compared to that of the controls (15). Internal reference six-color scales were used to accurately assess all possible colors of cultured colonies. The color scale varied from strong black to red. Bacteria that produce strong black and black colonies were considered slime producers, whereas those that form dark (almost black) colonies were considered weak slime producers. In contrast, red-colored colonies were considered strains unable to produce slime (19).
Evaluation of Anti-biofilm Activity of Od-EO
The effect of Od-EO on the S. aureus biofilm formation was studied using an MTP test. An aliquot (180 µL) of TSB containing 1/2 × MIC (0.25 µL/mL), 1/4 × MIC (0.125 µL/mL), 1/8 × MIC (0.0625 µL/mL), and 1/16 × MIC (0.0312 µL/mL) concentrations of the Od-EO (5 wells for each concentration) was added to each well of a sterile 96-well polystyrene plate. Then, 20 µL of bacterial culture (106 CFU/mL) was added to each well (final volume of 200 µL in each well). A positive control contained TSB-DMSO with S. aureus, and negative controls included TSB-DMSO plus S. aureus and 0.1 mg/mLvancomycin. The plates were incubated at 37°C for 48 hours without shaking to allow the cells to attach to the surface and form biofilms. After incubation, the contents of the wells were drained and washed three times with sterile phosphate-buffered saline to remove unattached and poorly-attached cells. The plates were then air-dried. Afterward, the wells were stained with 200 µL of 1% crystal violet at room temperature for 15 minutes to validate the cell attachment to the surface. Then, plates were rinsed with phosphate-buffered saline to remove unabsorbed crystal violet, and the wells were distained with 100 µL of ethanol-acetic acid. Finally, optical density was measured using a microplate reader (BOX998, BioTek, Winooski, VT, USA) at 570 nm (20).
Effect of Od-EO on Slime Production
To investigate the effect of Od-EO on the rate of exopolysaccharide production, a culture medium containing TSB supplemented with Congo red, saccharose, and DMSO was used. When a bacterium was inoculated into the medium and incubated for 24 hours, the bacteria that were able to produce slime changed the color of the environment to black. The darker color of the environment indicates a higher production of slime, and the red color of the environment denotes a lack of slime production. To perform this test, a single colony of bacteria was inoculated into a microtube containing 1 mL of the above-mentioned TSB supplemented with 1/2 × MIC, 1/4 × MIC, and 1/8 × MIC concentrations of Od-EO. After incubation for 24 hours at 37°C, the color of each tube was compared to that of the controls without EO, and the production of slime was checked (21,22).
Statistical Analysis
IBM SPSS Statistics 23 software was used to perform the statistical analysis of data. One-way analysis of variance (ANOVA) was used to evaluate the differences and check the significance of the differences, the P value was considered to be less than 0.05. All assays were performed in triplicates.
Results
GC-MS Analysis
GC-MS analysis revealed 15 compounds in the Od-EO, representing 99.56% of the total oil. Table 1 presents the concentrations of the Od-EO compounds. Thymol has the highest concentration of the constituent compounds (53.4%), and then γ-terpinene (20.48%), p-cymene (18.02%), and myristicin (2.7%) were the dominant compounds of Od-EO with the highest concentration.
Table 1.
Chemical Compositions of the Oliveria decumbens Essential Oil
|
Compounds
|
%
|
RI
|
RT
|
| Thymol |
53.4 |
1289 |
18.988 |
| γ-Terpinene |
20.48 |
1054 |
9.604 |
| Cymene |
18.02 |
1020 |
8.368 |
| Myristicin |
2.7 |
1517 |
28.458 |
| Limonene |
1.5 |
1024 |
8.488 |
| β-Pinene |
1.16 |
974 |
6.823 |
| Carvacrol |
0.66 |
1298 |
19.337 |
| Terpinene-4-ol |
0.6 |
1174 |
14.228 |
| β-Myrcene |
0.4 |
988 |
7.23 |
| α-Thujene |
0.24 |
924 |
5.45 |
| α-Pinene |
0.15 |
932 |
5.633 |
| α-Terpinene |
0.12 |
1014 |
8.071 |
| Terpinolene |
0.05 |
1086 |
10.669 |
| 1,8-Cineole |
0.04 |
1026 |
8.569 |
| α-Terpineol |
0.04 |
1186 |
14.806 |
| Total |
99.56 |
|
|
Note. RI: Retention index; RT: Retention time.
Anti-microbial Activity of Od-EO on Staphylococcus aureus
Table 2 illustrates the results of the Od-EO antimicrobial activity tests using the agar disk diffusion method. The MIC and MBC of Od-EO against planktonic S. aureus were 0.5 µL/mL and 1 µL/mL, respectively.
Table 2.
Agar Disc Diffusion Assay
|
Microorganism
|
Diameter of the Inhibitory Zone
|
|
Od-EO
|
Vancomycin
|
SXT
|
Ampicillin
|
DMSO
|
| Standard strain (ATCC 29213) and4 biofilm-producer isolates of Staphylococcus aureus |
39 ± 2.18 |
22 ± 1.5** |
27 ± 1.26* |
24 ± 4.9* |
0.00 ± 0.00*** |
Note. Od-EO: Oliveria decumbens essential oil; SXT: Trimethoprim/sulfamethoxazole; DMSO:Dimethyl sulfoxide.
Mean values of three replicates ± the standard deviation of the mean (*P < 0.05, **P < 0.01, and ***P < 0.001).
Phenotypic Identification of Slime-Producing Strains
The CRA plate test is a classic method for the phenotypic detection of slime-producing bacteria of this species (21). By direct analysis of the colonies grown on CRA, slime-producing strains were identified. On CRA, the slime-producing strains formed strong black to almost black colonies, whereas the non-producing slime strains developed strong red colonies (Table 3). Therefore, CRA is not a quantitative test and depends entirely on the subjective color assessment. Furthermore, the color change of colonies from black to red is progressive and sometimes difficult for researchers to classify the results (19). The standard strain ATCC29213 produced slime and formed completely black colonies, while ATCC12228 was a non-slime producer as illustrated in Figure 1. As Table 3 depicts, four S. aureus isolates that formed strong black colonies on the CRA were selected for further investigation.
Table 3.
Phenotypic Identification of Biofilm Formation Production on CRA
|
Isolate Number
|
Source of
Staphylococcus aureus
|
Colony Color on CRA
|
| 1 |
ATCC29213 |
+ + |
| 2 |
ATCC12228 |
- |
| 3 |
Human isolate |
+ + |
| 4 |
Human isolate |
+ + |
| 5 |
Food isolate |
+ + |
| 6 |
Food isolate |
+ |
Note. CRA: Congo red agar;Strong Black + + , Almost black + , Red –.
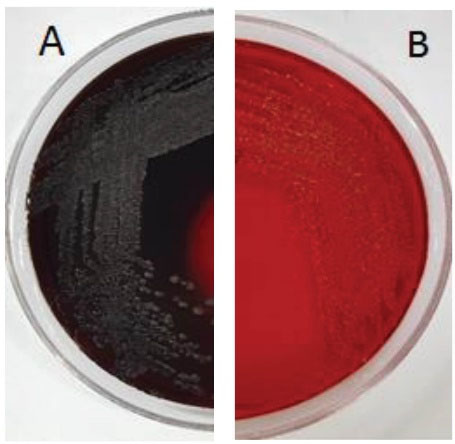
Figure 1.
(A) Staphylococcus aureus ATCC29213 Cultured on CRA (positive control); (B) Staphylococcus aureus ATCC12228 cultured on Congo Red Agar Medium (negative control)
.
(A) Staphylococcus aureus ATCC29213 Cultured on CRA (positive control); (B) Staphylococcus aureus ATCC12228 cultured on Congo Red Agar Medium (negative control)
Effect of Od-EO on Staphylococcus aureus Biofilm Inhibition
Staphylococcus aureus biofilm formation in the presence of 1/2 × MIC, 1/4 × MIC, 1/8 × MIC, and 1/16 × MIC concentrations of Od-EO was measured using a crystal violet assay. As shown in Figure 2, the inhibitory effect of Od-EO on S. aureus biofilm formation reached a significant level at 0.0312 µL/mL (MIC/16) compared to that of the control (P < 0.001). The data demonstrated that biofilm formation decreased with increasing Od-EO concentration.
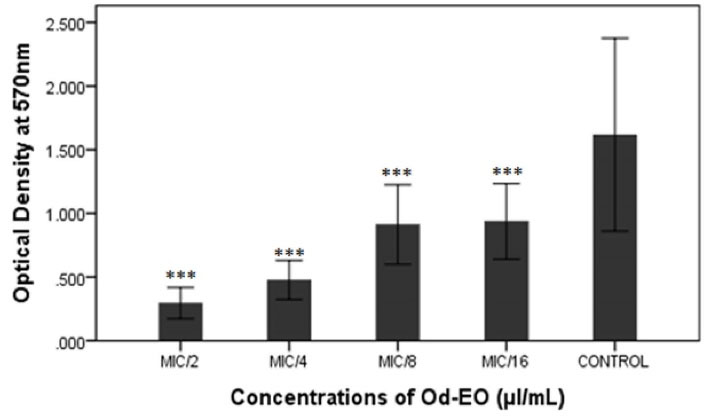
Figure 2.
Anti-biofilm Effect of Different Concentrations of Od-EO on the Staphylococcus aureus Biofilm. Note. Od-EO: Oliveria decumbens essential oil; The error bars represent the standard deviation of three replicates (***P < 0.001)
.
Anti-biofilm Effect of Different Concentrations of Od-EO on the Staphylococcus aureus Biofilm. Note. Od-EO: Oliveria decumbens essential oil; The error bars represent the standard deviation of three replicates (***P < 0.001)
Effect of Od-EO on Slime Production
As illustrated in Table 4 and Figure 3, by comparing the treatments with positive and negative controls, Od-EO at concentrations of 1/2 × MIC (0.25 µL/mL), 1/4 × MIC (0.125 µL/mL), and 1/8 × MIC (0.0625 µL/mL) significantly inhibited slime production (P < 0.05).
Table 4.
Effect of Od-EO on the Amount of Slime Production in Staphylococcus aureus
|
Isolates Number
|
Source of
Staphylococcus aureus
|
Concentrations of Od-EO (µL/ML)
|
|
MIC/2
|
MIC/4
|
MIC/8
|
Without Od-EO
|
| 1 |
ATCC29213 |
- |
+ |
+ |
+ + |
| 2 |
ATCC12228 |
- |
- |
- |
- |
| 3 |
Human isolate |
- |
- |
+ |
+ + |
| 4 |
Human isolate |
- |
- |
+ |
+ + |
| 5 |
Food isolate |
- |
- |
- |
+ |
| 6 |
Food isolate |
- |
- |
+ |
+ + |
Note. Od-EO: Oliveria decumbens essential oil; MIC: Minimum inhibitory concentration. Strong Black + + , Almost Black + , Red –.
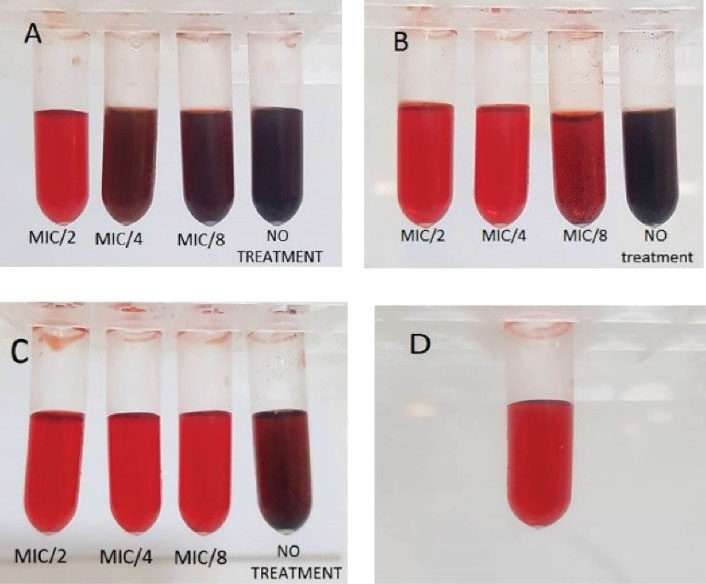
Figure 3.
Inhibitory Effect of Od-EO on the Presence of Exopolysaccharide (Slime Production)
Note. Od-EO: Oliveria decumbens essential oil; A: ATCC29213 (Positive control); B: Human isolate; C: Food isolate, and D: Negative control without bacteria.
.
Inhibitory Effect of Od-EO on the Presence of Exopolysaccharide (Slime Production)
Note. Od-EO: Oliveria decumbens essential oil; A: ATCC29213 (Positive control); B: Human isolate; C: Food isolate, and D: Negative control without bacteria.
Discussion
The formation of bacterial biofilms on biotic and abiotic surfaces has become important in the food industry, public health, and medicine. Biofilms are more resistant to chemical agents and antibiotics compared to planktonic cells (3). Biofilm resistance is related to various factors, including physical barriers for penetrating the chemical agents into the biofilm such as extracellular polysaccharides and changes in gene expression in cells embedded in the biofilm (23). Attachment and stabilization of bacteria into surfaces are the first and most important steps in biofilm formation and development. The production of exopolysaccharides termed slime plays an important role in the mechanisms involved in microbial attachment to surfaces (19).
In recent years, EOs and their constituent compounds have been used for their antimicrobial properties, but there is not enough data regarding the effect of EOs on biofilm formation. The present study explored the preliminary biofilm-forming abilities of the four S. aureus isolates. In addition, this study investigated the antibacterial and anti-biofilm effects of Od-EO against standard strains and four S. aureus isolates obtained from food and humans.
The antimicrobial properties of Od-EO have been previously studied (14,24,25), but their anti-biofilm properties have not been explored. The antimicrobial properties of Od-EO appear to be related to oxygenated monoterpenes such as thymol and carvacrol (24). According to the results obtained from GC-MS analysis, thymol (53.4%) was the major compound.
Amin et al (24) reported thymol (47.06%) as the major compound in Od-EO. In another study, 10 components in the Od-EO were reported, among which γ-terpinene, myristicin, thymol, ρ-cymene, and carvacrol were predominant (24). To some extent, the results of the current study are analogous to the results of the above-mentioned studies. The chemical composition of a plant EO depends on various factors such as season, climate, and growth stage.
In parallel with similar studies, the current data indicated that Od-EO had potent antimicrobial activity against S. aureus. An average diameter of 39 mm inhibition zone was observed in the disc diffusion test, which was significantly (P < 0.05) larger than that in the positive controls. The MIC and MBC of Od-EO for four isolates and standard strains were determined to be 0.5 and 1 µL/mL, respectively. These results are consistent with previous studies (24,25)
The MTP order was used to determine the anti-biofilm properties of Od-EO. This assay indicated that the effect of Od-EO on initial bacterial cell attachment and prevention of biofilm formation was dosage-dependent (P < 0.001). The effect of Od-EO on slime production also confirmed the potent anti-biofilm properties of this EO against S. aureus (Figure 3). The use of Congo red in this experiment is due to the fact that this color indicates the presence of exopolysaccharide (22). In all tested isolates, Od-EO at MIC/2 and MIC/4 prevented slime production. Many studies have investigated the anti-biofilm effects of different EOs on bacteria such as yarrow EO against Listeria monocytogenes (23), cardamom EO against methicillin-resistant S. aureus biofilm (26), and peppermint EO against S. aureus (5). In all these studies, researchers concluded that plant EOs are effective in preventing biofilm formation. Because the use of natural compounds prevents microorganism attachment or biofilm formation, it seems to be a useful method for the sanitization of biotic and abiotic surfaces (10).
Conclusion
Od-EOat low concentrations has significant anti-bacterial and anti-biofilm effects on S. aureus (standards, humans, and foodstuff isolates). Od-EO or its compounds can be used as anti-biofilm and anti-bacterial sanitizers in various fields, including medicine, dentistry, and the food industry. In addition, the synergistic effects of the combination of Od-EO with other chemical and natural compounds to produce more powerful sanitizers need to be investigated. These sanitizers may apply on different surfaces in medical and food surfaces to remove or prevent bacterial populations.
Acknowledgments
We would like to express our appreciation of the Research Council of Shahid Chamran University of Ahvaz, Iran, for their financial support (Grant No. SCU.VF 99.534).
Conflict of Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The experiments were conducted according to the protocol approved by the Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz.
References
- Atshan SS, Shamsudin MN, Karunanidhi A, van Belkum A, Lung LT, Sekawi Z. Quantitative PCR analysis of genes expressed during biofilm development of methicillin resistant Staphylococcus aureus (MRSA). Infect Genet Evol 2013; 18:106-12. doi: 10.1016/j.meegid.2013.05.002 [Crossref] [ Google Scholar]
- Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 2004; 186(6):1838-50. doi: 10.1128/jb.186.6.1838-1850.2004 [Crossref] [ Google Scholar]
- Avila-Novoa MG, Iñíguez-Moreno M, Solís-Velázquez OA, González-Gómez JP, Guerrero-Medina PJ, Gutiérrez-Lomelí M. Biofilm formation by Staphylococcus aureus isolated from food contact surfaces in the dairy industry of Jalisco, Mexico. J Food Qual 2018; 2018:1746139. doi: 10.1155/2018/1746139 [Crossref] [ Google Scholar]
- Cha Y, Son B, Ryu S. Effective removal of staphylococcal biofilms on various food contact surfaces by Staphylococcus aureus phage endolysin LysCSA13. Food Microbiol 2019; 84:103245. doi: 10.1016/j.fm.2019.103245 [Crossref] [ Google Scholar]
- Kang J, Jin W, Wang J, Sun Y, Wu X, Liu L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019; 101:639-45. doi: 10.1016/j.lwt.2018.11.093 [Crossref] [ Google Scholar]
- Van Houdt R, Michiels CW. Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol 2010; 109(4):1117-31. doi: 10.1111/j.1365-2672.2010.04756.x [Crossref] [ Google Scholar]
- Moormeier DE, Bayles KW. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol 2017; 104(3):365-76. doi: 10.1111/mmi.13634 [Crossref] [ Google Scholar]
- Liu M, Wu X, Li J, Liu L, Zhang R, Shao D. The specific anti-biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control 2017; 73:613-8. doi: 10.1016/j.foodcont.2016.09.015 [Crossref] [ Google Scholar]
- Srey S, Jahid IK, Ha SD. Biofilm formation in food industries: a food safety concern. Food Control 2013; 31(2):572-85. doi: 10.1016/j.foodcont.2012.12.001 [Crossref] [ Google Scholar]
- Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol 2010; 50(1):30-5. doi: 10.1111/j.1472-765X.2009.02747.x [Crossref] [ Google Scholar]
- Agrawal I. Susceptibility of bacterial biofilms against some leaf extracts. Plant Sciences Feed 2011; 1(5):69-73. [ Google Scholar]
- Quave CL, Plano LR, Pantuso T, Bennett BC. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 2008; 118(3):418-28. doi: 10.1016/j.jep.2008.05.005 [Crossref] [ Google Scholar]
- Mohammadi Bazargani M, Rohloff J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016; 61:156-64. doi: 10.1016/j.foodcont.2015.09.036 [Crossref] [ Google Scholar]
- Alizadeh Behbahani B, Tabatabaei Yazdi F, Vasiee A, Mortazavi SA. Oliveria decumbens essential oil: chemical compositions and antimicrobial activity against the growth of some clinical and standard strains causing infection. Microb Pathog 2018; 114:449-52. doi: 10.1016/j.micpath.2017.12.033 [Crossref] [ Google Scholar]
- Sharifi A, Mohammadzadeh A, Zahraei Salehi T, Mahmoodi P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J Appl Microbiol 2018; 124(2):379-88. doi: 10.1111/jam.13639 [Crossref] [ Google Scholar]
- Chen Q, Xie S, Lou X, Cheng S, Liu X, Zheng W. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources. Microbiologyopen 2020; 9(1):e00946. doi: 10.1002/mbo3.946 [Crossref] [ Google Scholar]
- Nikravan L, Maktabi S, Ghaderi Ghahfarrokhi M, Mahmoodi Sourestani M. The comparison of antimicrobial and antioxidant activity of essential oil of Oliveria decumbens and its nanoemulsion preparation to apply in food industry. Iran Vet J 2020; 17(3):78-87. doi: 10.22055/ivj.2021.297017.2384 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. Wayne, PA: CLSI; 2018.
- Arciola CR, Campoccia D, Gamberini S, Cervellati M, Donati E, Montanaro L. Detection of slime production by means of an optimised Congo red agar plate test based on a colourimetric scale in Staphylococcus epidermidis clinical isolates genotyped for ica locus. Biomaterials 2002; 23(21):4233-9. doi: 10.1016/s0142-9612(02)00171-0 [Crossref] [ Google Scholar]
- Onsare JG, Arora DS. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol 2015; 118(2):313-25. doi: 10.1111/jam.12701 [Crossref] [ Google Scholar]
- Mariana NS, Salman SA, Neela V, Zamberi S. Evaluation of modified Congo red agar for detection of biofilm produced by clinical isolates of methicillin resistance Staphylococcus aureus. Afr J Microbiol Res 2009; 3(6):330-8. [ Google Scholar]
- Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol 1989; 42(8):872-4. doi: 10.1136/jcp.42.8.872 [Crossref] [ Google Scholar]
- Jadhav S, Shah R, Bhave M, Palombo EA. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013; 29(1):125-30. doi: 10.1016/j.foodcont.2012.05.071 [Crossref] [ Google Scholar]
- Amin G, Sourmaghi MH, Zahedi M, Khanavi M, Samadi N. Essential oil composition and antimicrobial activity of Oliveria decumbens. Fitoterapia 2005; 76(7-8):704-7. doi: 10.1016/j.fitote.2005.06.009 [Crossref] [ Google Scholar]
- Hajimehdipoor H, Samadi N, Mozaffarian V, Rahimifard N, Shoeibi S, Pirali Hamedani M. Chemical composition and antimicrobial activity of Oliveria decumbens volatile oil from West of Iran. J Med Plants 2010; 9(Suppl 6):39-44. [ Google Scholar]
- Cui H, Zhang C, Li C, Lin L. Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. LWT 2020; 122:109057. doi: 10.1016/j.lwt.2020.109057 [Crossref] [ Google Scholar]