Avicenna Journal of Clinical Microbiology and Infection. 10(1):1-8.
doi: 10.34172/ajcmi.2023.3394
Original Article
ESβL and MβL Production in Gram-Negative Bacteria Isolated From HIV Seropositive Individuals
Folasade M. Adeyemi 1, *  , Omotayo O. Oyedara 1, 2
, Omotayo O. Oyedara 1, 2  , Abideen A. Wahab 1, Sunday B. Akinde 1
, Abideen A. Wahab 1, Sunday B. Akinde 1
Author information:
1Department of Microbiology, Faculty of Basic and Applied Sciences, Osun State University, Osogbo, Nigeria
2Departamento de Microbiología e Inmunología, Facultad de Ciencias Biologicas, Universidad Autonoma de Nuevo Leon, San Nicolas de los Garza, Nuevo Leon, 66455, Mexico
Abstract
Background: Extended-spectrum β-lactamase (ESβL) or metallo-β-lactamase (MβL) production by gram-negative bacteria in immunocompromised patients poses a serious therapeutic challenge for infection control and is associated with infections with a higher morbidity/mortality, especially in developing countries. This study aimed to phenotypically evaluate the production of ESβL as well as MβL in 75 gram-negative bacterial isolates from clinical samples of the human immunodeficiency virus (HIV) positive individuals.
Methods: Bacterial identification was by chromogenic media, analytical profile index 20 E, and 20 NE kits, and ESβL production was tested by double-disc synergy test (DDST) and combination disc method, while MβL production was screened with imipenem ethylene diamine tetra-acetic acid (EDTA) combined disc and EDTA-disc potentiation with ceftazidime.
Results: Altogether, 57 isolates (76.0%) produced ESβL either with DDST (6), combination disc method (49), or both (2). DDST detected the ESβL enzyme in 10.7% of the tested isolates which were all Pseudomonas aeruginosa. None of the bacterial isolates revealed MβL production with the imipenem/imipenem-EDTA method, whereas 26.7% of tested isolates produced MβL with EDTA-disc potentiation using ceftazidime out of which 65.0% were P. aeruginosa. Moreover, ESβL/MβL co-production was evident in 22.7% of the tested bacterial isolates with P. aeruginosa constituting 64.7%.
Conclusion: ESβL and MβL co-production among the studied isolates indicates a heightened resistance to β-lactam antibiotics, suggesting grave health consequences, especially in immunocompromised individuals with already limiting therapeutic options in the region. The study revealed higher ESβL production compared to MβL production in isolates, with the predominating producing specie being P. aeruginosa, and higher ESβL and MβL detection by the combination disc method and EDTA-disc potentiation using ceftazidime, respectively.
Keywords: HIV, Extended-spectrum β-lactamase, Metallo-β-lactamase, Double-disc synergy test, Gram-negative bacteria
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Adeyemi FM, Oyedara OO, Wahab AA, Akinde SB. ESβL and MβL production in gram-negative bacteria isolated from HIV seropositive individuals. Avicenna J Clin Microbiol Infect. 2023; 10(1):1-8. doi:10.34172/ajcmi.2023.3394
Introduction
Gram-negative organisms, especially the family of Enterobacteriaceae, generally cause both community and nosocomial infections. They are also implicated in secondary infections associated with high mortality in individuals infected with human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (1). The global occurrence of extended-spectrum β-lactamase (ESβL) and metallo-β-lactamase (MβL) production in Enterobacteriaceae is increasing steadily, especially among HIV patients (2) as the over-dependence on β-lactam antibiotics in the hospital setting has led to increased resistance to them in bacteria (3).
ESβLs are enzymes with hydrolytic capabilities to split open the beta-lactam unit of the penicillins, thereby deactivating them. ESβLs can hydrolyze many penicillins, cephalosporins (from first to the fourth generation), and monobactams (aztreonam), excluding the carbapenems or cephamycins (4,5). MβLs, on the other hand, are an Amber class B carbapenemase that hydrolyses all the above antimicrobials along with carbapenems (6) but is powerless against monobactams (7). Various gene clusters of MβLs such as blaIMP, blaNDM, and blaVIM are present on plasmids carried by a wide range of medically relevant bacteria (6).
ESβL and MβL production in bacteria is recurrently linked to co-resistance to non-β-lactam drugs, and thus, displays multidrug resistance to many other antibiotic classes along with β-lactam antibiotics (3,8-10). This ability may significantly impair the therapy of serious and life-threatening infections and also restrict the management options for infections, especially in immunocompromised individuals.
The prevalence of ESβL and MβL-producing bacteria is on the global rise as the etiology of AIDS, antibiotic resistance patterns, and antibiotic resistance mechanisms in gram-negative bacteria are constantly changing (11). There is evidence that the occurrence of carbapenemase-producing bacteria in Africa is common, and it has also been reported in Nigeria (12,13). Olaitan et al (12) reported the incidence of a multidrug-resistant, OXA-23-producing strain of Acinetobacter baumanniiamong clinical isolates in Ibadan, Southwest Nigeria. The spread of ESβL and MβL-producing bacteria poses a significant threat to health practitioners and the public. HIV infection predisposes infected individuals to opportunistic infections due to immunosuppression, and this is usually a principal cause of morbidity and even mortality (14,15).
With challenges for prevention and curative therapy, HIV-induced immune compromise significantly intensifies the menace of bacterial infections, with possibilities for re-occurrence. This study, therefore, aimed to investigate the production of ESβL and MβL in 75 gram-negative rods recovered from skin and rectal swabs of HIV seropositive patients.
Materials and Methods
Isolate Selection
The bacterial isolates for this study comprised 75 randomly selected and non-repeated gram-negative bacilli isolated from samples obtained from HIV seropositive individuals in an earlier study (16,17). Each strain was unique in terms of species identification or resistance pattern even when more than one isolate was recovered from the same patient.
Previously, the selected bacterial isolates were identified by conventional microbiology techniques, including Gram staining, growth patterns on MacConkey agar, ChromoBio TBX, and Hi Chrome agar, and rapid biochemical tests were conducted with the Analytical Profile Index API 20E and 20NE (bioMérieux, France). The preserved isolates stored at -20°C in Tryptone soy broth containing 15% glycerol were revived in nutrient broth and incubated at 35 ± 2°C overnight. Fresh cultures were then streaked onto sterile MacConkey agar, incubated at 35 ± 2°C overnight, and used for subsequent analyses.
Human Immunodeficiency Virus Viral Load and CD4 T Cell Count
HIV viral load and CD4 T cell count were determined as previously described (17). HIV viral load was evaluated by the Amplicor HIV-1 monitor which is an in vitro nucleic acid amplification test (Roche version 1.5, Switzerland), while CD4 T cell count was measured by flow cytometry using the CyFlow Counter SL-3 (Partec, Germany). Data gathered for age, weight, height, CD4, and viral load is presented using descriptive statistics.
Screening of Isolates for the Production of Extended-spectrum β-lactamase
The Double-Disc Synergy Test
This was done as a universal disc diffusion technique using Mueller-Hinton agar (MHA). The latter was inoculated with the test organism. The test isolate was suspended in Ringer solution and standardized to 0.5 McFarland turbidity standard, and inoculation was done using a sterile swab stick to create a lawn of the isolate. Antibiotic discs (Mast Diagnostics) of aztreonam, cefotaxime, ceftriaxone, and piperacillin (30µg each) were arranged at a distance of about 30mm from each other (center-to-center) on the surface of the agar around a ceftazidime/clavulanic-acid (20 µg/10 µg) and subsequently incubated overnight at 37°C (18).
A probable ESβL production was indicated by the expansion of the clear zone of inhibition towards the ceftazidime/clavulanic-acid disc, signifying a synergy between clavulanic acid and any one of the antibiotics employed in screening (18). A negative reaction was recorded when the inhibition zone did not expand around the ceftazidime/clavulanic-acid disc. For the control organisms, American Type Culture Collection isolatesKlebsiella pneumoniae ATCC 700603 (positive) and Escherichia coli ATCC 25922 (negative) strains were used.
The Combination Disc Method
This was done with pairs of discs containing piperacillin with/without tazobactam as well as cefotaxime with/without clavulanic acid (Mast Diagnostics). The preparation of each test organism and inoculation of MHA was performed as described above. The two discs were then placed on opposite sides of the plate inoculated with the test organism. The discs were arranged in such a way that the piperacillin was placed next to piperacillin/tazobactam, and the cefotaxime was placed next to cefotaxime/clavulanate, leaving a space of about 25mm between them. The plates were incubated at 37°C overnight, and the diameters of inhibition zones were then measured (19). ESβL production was implied if the clear zone of inhibition around each combination disc was not less than 5mm larger than that of the cephalosporin alone, or if the clearing around the combination disc was approximately 50% larger than that of the single disc due to the presence of the tazobactam or clavulanic acid (19).
Screening of Isolates for the Production of Metallo -β-Lactamase
Imipenem-Ethylene Diamine Tetra-acetic Acid Combination Disc Method
The isolates were screened phenotypically for the production of MβL using imipenem supplemented with ethylene diamine tetra-acetic acid (EDTA) (20,21). The antibiotics used were imipenem (10 µg) and imipenem-EDTA, (10 µg/750 µg). Antibiotic/EDTA combined discs were formulated locally by dispensing 20 µL of the 0.1M EDTA (Sigma Chemicals) solution onto 10 µg imipenem discs to attain the required concentration of 750 µg in each disc. These discs were then dried slowly in an oven at 70°C and stored in airtight containers devoid of desiccants at -20°C until required. The standardized broth culture of the test strain prepared as previously described was inoculated on a sterile MHA plate using sterile cotton-tipped applicators and was allowed to dry. One imipenem (10 µg) disc was then dispensed on the surface of the already inoculated MHA agar plate, with another EDTA/imipenem combination disc set at least 25 mm away from it. Incubation was done at 37°C for 18 to 24 hours. MβL-producing strains were confirmed by visually observing and measuring the clear zones of inhibition, which was indicated by an expansion of ≥ 7 mm around the combination disc as against the single imipenem disc (21).
Ethylene Diamine Tetra-acetic Acid-disc Potentiation Using Ceftazidime
For the EDTA-disc potentiation with ceftazidime, a modification of the methods by Behera et alwas used (22,23). Lawns of the test isolates were created on MHA plates, and a 6mm blank disc was placed on the agar surface along with ceftazidime (30 µg) no less than 25 mm from the blank disc. A 10 µL aliquot of EDTA solution (0.5M) was carefully dropped onto the blank disc, and the plates were covered and then incubated at 35°C overnight. Augmentation of the zone of inhibition in the space between the EDTA disc and the ceftazidime disc compared with the size of the clear zone on the other side of the ceftazidime disc was inferred to indicate MβL production.
Results
Gram-Negative Bacterial Distribution in the Human Immunodeficiency Virus Seropositive Patients
The selected isolates, as described in a previous study (16,17), were obtained from 46 HIV-positive patients, comprising 36 females and 10 males (78.3% and 21.7%, respectively), and their ages ranged from 7 to 60 years with a mean of 37.8 years. A total of 26 (56.5%) had commenced antiretroviral therapy, while 43.5% of them were antiretroviral therapy-naive. Moreover, the CD4 T cell count varied widely between 7 cells/µL as the lowest value and 1108 cells/µL as the highest value, with a mean of 483.4 cells/µL. Furthermore, the viral load values ranged between 54 and 4042 HIV-1 RNA copies/mL with a mean value of 553.9 copies/mL (Tables 1 and 2).
Table 1.
Distribution of HIV Seropositive Participants
|
Gender
|
Age Group (y)
|
CD4
(cells/µL), n=46
|
VL (copies/mL), n=46
|
|
≤200
|
201–350
|
351–500
|
>500
|
≤400
|
>400
|
ND
|
| Male |
≤ 19 |
0 |
1 |
0 |
1 |
1 |
0 |
1 |
| 20-39 |
0 |
1 |
0 |
1 |
0 |
1 |
1 |
| 40-59 |
3 |
2 |
0 |
1 |
4 |
0 |
2 |
| ≥ 60 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Female |
≤ 19 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| 20-39 |
4 |
5 |
2 |
12 |
12 |
3 |
8 |
| 40-59 |
1 |
2 |
3 |
6 |
6 |
3 |
3 |
| ≥ 60 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
Note. HIV: Human immunodeficiency virus; VL: Viral load; ND: Not determined.
Table 2.
Profile of CD4 T-Cell Counts and HIV-1 Plasma VL of the Participants
|
Variables
|
Male
|
Female
|
|
On ART
|
Not on ART
|
On ART
|
Not on ART
|
| Mean CD4 T-cell count (cells/µL) |
333.67 (n = 3) |
476.71 (n = 7) |
545.52 (n = 23) |
411.54 (n = 13) |
| Mean VL values (HIV-1 RNA copies/mL) |
131.5 (n = 2) |
205.50 (n = 4) |
767.42 (n = 19) |
190.2 (n = 5) |
Note. HIV: Human immunodeficiency virus; VL: Viral load; ART: Anti-retroviral therapy.
A total of 75 non-repetitive gram-negative bacterial isolates belonging to 8 different genera comprising of 10 bacterial species, namely, Pseudomonas aeruginosa (49), Salmonella typhi (15), Enterobacter aerogenes, Klebsiella pneumoniae, and Serratia liquefaciens(2 isolates each), as well as Chryseomonas luteola, Citrobacter freundii, Enterobacter cloacae, Serratia marcescens, and Proteus mirabilis (1 isolate each) were assessed phenotypically to detect ESβL and MβL production. All the isolates selected for ESβL and MβL screening were recovered from skin swabs (80%) except S. typhi isolates which were cultured from rectal swabs (20%).
Extended-spectrum β-lactamase Production in the Gram-Negative Isolates
Altogether, 57 (76.0%) out of 75 isolates were positive for ESβL production either with the double-disc synergy test (DDST), combination method, or both. Forty-nine of these isolates were positive with the combination method only and six organisms with both methods, while only 2 organisms were positive with the DDST method alone. The DDST for ESβL production detected the enzyme in 10.7% (8 out of 75) of the isolates (Figure 1). Six (75.0%) out of these were obtained with the synergy of ceftazidime/clavulanic acid with only one other disc, 4 with only piperacillin (50.0%), and 2 with only ceftriaxone (25.0%), as depicted in Table 3. The remaining 2 ESβL-positive isolates were obtained with synergy with 3 other discs, namely, piperacillin/ceftriaxone/cefotaxime and aztreonam/ceftriaxone/cefotaxime, respectively. It is noteworthy that all the ESβL-positive isolates detected with the DDST screening method were P. aeruginosa. Overall, 55 (73.3%) isolates tested positive for ESβL production using the combination disc method. ESβL production was detected in 47 (85.5%) of the isolates with piperacillin and piperacillin/tazobactam combination only, including P. aeruginosa (59.6%), S. typhi (23.4%), Enterobacter aerogenes and Serratia liquefaciens (4.3%), and each of Chryseomonas luteola, Citrobacter freundii, Klebsiella pneumonia, and Proteus mirabilis at 2.1%. ESβL production was detected in only 3 P. aeruginosa (5.4%) strains using cefotaxime/clavulanate (Table 3). However, 5 (9.1%) out of the ESβL producing strains were identified with the use of the two combinations employed in this study, including P. aeruginosa, Enterobacter cloacae, S. typhi, and S. marcescens.
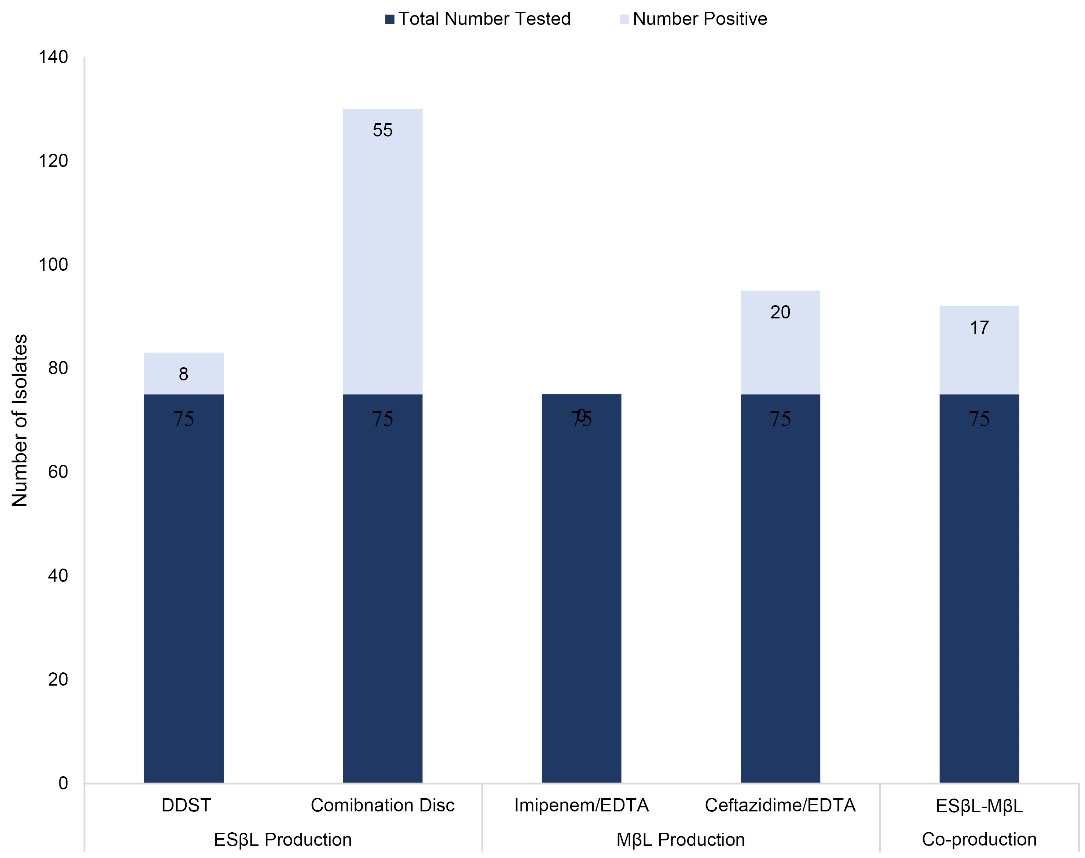
Figure 1.
Frequency of Occurrence of the ESβL and MβL Producers. Note. ESβL: Extended spectrum β-lactamase; MβL: Metallo-β-lactamase; DDST: Double-disc synergy test; EDTA: Ethylene diamine tetra acetic acid
.
Frequency of Occurrence of the ESβL and MβL Producers. Note. ESβL: Extended spectrum β-lactamase; MβL: Metallo-β-lactamase; DDST: Double-disc synergy test; EDTA: Ethylene diamine tetra acetic acid
Table 3.
Frequency of Occurrence of the ESβL and MβL Producing Gram-Negative Bacterial Isolates
|
Bacterial Specie
|
Total Number Tested
|
ESβL
|
MβL
|
|
DDST
|
Combination Disc
|
Combination Disc
|
EDTA-Disc Potentiation
|
|
1
|
2
|
3
|
4
|
Number Positive
|
PRL/ PTZ
|
CTX/ CTCV
|
Both
|
Number Positive
|
Imipenem/ EDTA
|
Ceftazidime/ EDTA
|
|
Chryseomonas luteola |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
|
Citrobacter freundii
|
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
|
Enterobacter aerogenes
|
2 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
2 |
0 |
0 |
|
Enterobacter cloacae
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
|
Klebsiella pneumoniae
|
2 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
|
Proteus mirabilis
|
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
|
Pseudomonas aeruginosa
|
49 |
6 |
0 |
2 |
0 |
8 |
28 |
3 |
2 |
33 |
0 |
13 |
|
Salmonella typhi
|
15 |
0 |
0 |
0 |
0 |
0 |
11 |
0 |
1 |
12 |
0 |
6 |
|
Serratia liquefaciens
|
2 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
2 |
0 |
0 |
|
Serratia marcescens
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
0 |
1 |
| Total |
75 |
6 |
0 |
2 |
0 |
8 |
47 |
3 |
5 |
55 |
0 |
20 |
Note. ESβL: Extended spectrum β-lactamase; MβL: Metallo-β-lactamase; DDST: Double-disc synergy test; PRL: Piperacillin; PTZ: Piperacillin with tazobactam; CTX: Cefotaxime; CTCV: Cefotaxime with clavulanic acid; EDTA: Ethylene diamine tetra acetic acid; “1-4”: Detection with 1, 2, 3, or 4 different discs.
Metallo -β-Lactamase Production in the Gram-Negative Isolates
MβL production was detected in none of the isolates when screened with imipenem/imipenem-EDTA, whereas, 20 (26.7%) of the isolates showed a positive result when screened with EDTA-disc potentiation using ceftazidime (Figure 1),13 (65.0%) of which were P. aeruginosa, 6 (30.0%) were S. typhi, and the last isolate was S. marcescens (5.0%).
Extended-spectrum β-Lactamase and Metallo -β-Lactamase Co-Production
ESβL and MβL co-production was observed in 22.7% (17/75) of the screened isolates, and 11 (14.7%) of these were P. aeruginosa. Other species were S. typhi (6.7%) and S. marcescens (1.3%).
Discussion
This study was designed to detect the production of ESβLs and MβLs in 75 gram-negative bacteria that were isolated from various samples obtained from HIV-positive individuals using phenotypic screening methods. The advent and spread of ESβL- and carbapenemase-producing gram-negative bacteria is regarded as a major public health issue, most especially in immunocompromised individuals as found in HIV seropositive patients.
The current study revealed an elevated number of ESβL producers amongst the screened isolates, with P. aeruginosa predominating. In total, 76.0% of the screened isolates produced ESβL, whereas only 26.7% of isolates produced MβL with EDTA-disc potentiation using ceftazidime. Of these ESβL producers, 65.0% were P. aeruginosa as ESβL production was detected in 67.3% (33/49) of tested P. aeruginosa strains and constituted 60.0% of all the isolates which tested positive with the combination disc method (33/55), while MβL production was evident in 26.5% (13/49) of screened P. aeruginosa strains and made up 65.0% of all MβL positive organisms using the EDTA-ceftazidime disc potentiation test. Different values have also been recorded by various authors from several studies. This corresponds with a study by Begum et al (24) in which a prevalence value of 37.8% was reported for ESβL-producing gram-negative bacteria, with 90.2% being P. aeruginosa, highlighting the high rate of ESβL production in this species. The rates reported in our study are, however, higher than those in Basak et al (25) at 40% and 11.2% for ESβL and MβL production, respectively.
Various reports of MβL production prevalence rates have been recorded ranging from 69.5% in imipenem-resistant P. aeruginosa isolates (26), 54.0% (27), and 43.6% in clinical isolates (28), and all values were much higher than those obtained in our study. However, these values were lower than those reported for MβL production from Pseudomonas species by Franco et al (29) who reported MβL production of 77.0%. More recently, phenotypic testing of gram-negative bacterial isolates suggested the presence of an ESβL and a carbapenemase in 14.0% of isolates (30), while Rani et al(31) detected ESβL production in 37.3% of the isolates in their study. At variance with these rates is the report of 0.2% MβL production by Pseudomonas species (32), while Thapa et al (7) reported MβL production in 5.8%, prevalently from Acinetobacter calcoaceticus baumanniicomplex followed by P. aeruginosa. More recently, another study (33) reported ESβL and carbapenemase prevalence rates of 61.0% and 28.0% from Ghana, respectively, a rate more in agreement with that recorded in the present study.
There is quite a lot of data available on the prevalence of ESβL enzyme production among members of the family Enterobacteriaceae, however, there is a dearth of literature on the P. aeruginosa family. The phenotypic analyses endorsed by European Committee on Antimicrobial Susceptibility Testing (EUCAST) for detecting ESβL production in Enterobacteriaceaeare not always appropriate for the detection of the same in P. aeruginosa. This could be a result of the variations in the families of ESβLs present in P. aeruginosa isolates and within the Enterobacteriaceae, possible co-production of MβLs, and the production and overexpression of natural AmpC cephalosporins. Further, carbapenem resistance in P. aeruginosa could be a result of the loss in permeability caused by the loss of the oprD porin, the improved regulation of a dynamic efflux system, or the production of MβLs (34). Carbapenem-hydrolysing MβLs have been widely studied and reported by various authors in numerous countries; hence, they are considered the most vital mechanism of resistance to carbapenems in P. aeruginosa (34,35).
No result was obtained for the detection of MβL production with the imipenem/EDTA combination even though this method has been reported to be more effective than the DDST or the EDTA-disc potentiation test (36). The reason for this is unknown but could be attributed to the existence of extra imipenem/EDTA combination resistance mechanisms in these isolates. If this is the case, this method may not detect MβL production as suggested previously (36).
A study from Tanzania reported that ESβL-producing bacterial strains were significantly more prevalent in HIV-infected children than in their HIV-negative counterparts as HIV-positive children were ten times more likely to be ESβL carriers than the control group of HIV-negative children (37). Few reports exist on the likelihood of HIV status being a risk factor for ESβL carriage; nevertheless, HIV-positive individuals are more susceptible to opportunistic infections, and are, therefore, expected to be more frequently on hospital admission, and tend to be administered more antimicrobials than HIV-negative persons.
The production of antibiotic-hydrolyzing enzymes from bacterial isolates detected in this study is a strong indication of possible multidrug resistance, probably due to the constant use of multiple drugs associated with the category of patients from which the isolates were obtained. It is also likely that frequent visits to the hospital or hospitalization may impact colonization by microorganisms as a result of constant exposure. This agrees with reports of other authors who reported that patients during hospitalization acquired microorganisms at rates of 47.5% and 94.0%, respectively (38,39)
A salient fact is a probability that many of these microorganisms might have already acquired resistance to certain antibiotics, which is a trait easily transferable between organisms by horizontal gene transfer and common in the healthcare setting. This trend is worrisome because such bacterial strains are implicated in infections attributed to more severe illness and even death. Furthermore, since MβLs have hydrolytic capabilities against ß-lactams of all classes, and research is still ongoing in the search for nontoxic alternative remedies for infectious ailments, the sustained rise in their prevalence would portend serious public health challenges and could be a clinical catastrophe.
The presence and persistence of an ESβL- or MβL- producer is not only problematic for treatment but also creates a major challenge for the management and control of infections. Since their detection is challenging, their roles in spread within clinical settings and beyond as well as horizontal MβL gene transfer between pathogens go largely unnoticed, thereby posing substantial risks to colonizing hosts (3). The continued local surveillance of organisms capable of producing antibiotic-hydrolyzing enzymes in hospitals may play a valuable role in tracking emerging resistant traits in such strains and will help track outbreaks of infections by such strains. It will also aid therapeutic options in severely affected patients, ultimately reducing ineffective prescriptions and shortening hospital stays. Clinicians and microbiologists should also be regularly updated with local surveillance information.
Conclusion
The global emergence of ESβLs and MβL production among clinically important gram-negative bacteria is a growing problem, especially in Africa. Nevertheless, the magnitude of the problem is not fully conveyed because of the inadequate number of studies highlighting the need for detection and pinpointing resistance mechanisms, especially in Nigeria. Recommendations to combat this scourge in Nigeria include the routine screening, characterization, and reporting of the presence of gram-negative organisms with these traits in the healthcare setting, instigating antimicrobial stewardship programs, and prohibiting the sale of antimicrobial agents without a prescription. Other measures of control may include the improvement and continued development of essential infection control measures such as hand hygiene, equipping microbiology laboratories to detect the emergence of new strains, and the expansion of regional surveillance on gram-negative bacteria with ESβL and MβL producing traits both in the hospital and in the community.
Authors’ Contribution
Conceptualization: Folasade Muibat Adeyemi.
Data curation: Folasade Muibat Adeyemi, Sunday Babatunde Akinde.
Formal analysis: Folasade Muibat Adeyemi, Sunday Babatunde Akinde.
Funding acquisition: Folasade Muibat Adeyemi, Sunday Babatunde Akinde.
Investigation: Folasade Muibat Adeyemi, Omotayo Opemipo Oyedara, Abideen Akinkunmi Wahab.
Methodology: Folasade Muibat Adeyemi, Omotayo Opemipo Oyedara.
Project administration: Omotayo Opemipo Oyedara, Abideen Akinkunmi Wahab.
Validation: Folasade Muibat Adeyemi, Abideen Akinkunmi Wahab, Sunday Babatunde Akinde.
Visualization: Folasade Muibat Adeyemi.
Resources: Folasade Muibat Adeyemi, Sunday Babatunde Akinde
Supervision: Folasade Muibat Adeyemi.
Writing–original draft: Folasade Muibat Adeyemi.
Writing–review & editing: Folasade Muibat Adeyemi, Sunday Babatunde Akinde.
Competing Interests
The authors declare that there is no conflict of interests.
Ethical Approval
The study was reviewed and approved by both the Ethical Review Board of the Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Osun State, Ile-Ife, as well as the Ondo State Specialist Hospital Management Board, Akure, Nigeria (Protocol Number ERC/2012/11/05).
Funding
No funding was obtained for this study.
References
- Pires J, Bernasconi OJ, Hauser C, Tinguely R, Atkinson A, Perreten V. Intestinal colonisation with extended-spectrum cephalosporin- and colistin-resistant Enterobacteriaceae in HIV-positive individuals in Switzerland: molecular features and risk factors. Int J Antimicrob Agents 2017; 49(4):519-21. doi: 10.1016/j.ijantimicag.2017.02.004 [Crossref] [ Google Scholar]
- Vasoo S, Barreto JN, Tosh PK. Emerging issues in gram-negative bacterial resistance: an update for the practicing clinician. Mayo Clin Proc 2015; 90(3):395-403. doi: 10.1016/j.mayocp.2014.12.002 [Crossref] [ Google Scholar]
- Tan X, Kim HS, Baugh K, Huang Y, Kadiyala N, Wences M. Therapeutic options for metallo-β-lactamase-producing enterobacterales. Infect Drug Resist 2021; 14:125-42. doi: 10.2147/idr.s246174 [Crossref] [ Google Scholar]
- Yusuf I, Haruna M. Detection of AmpC and ESBL producers among Enterobacteriaceae in a tertiary health care in, Kano-Nigeria. Int J Sci Technol 2013; 3(4):220-5. [ Google Scholar]
- Adeyemo AT, Adeyemo AT, Odetoyin BW, Onipede AO. Prevalence and risk factors for extended-spectrum β-lactamase producing gram-negative bacterial infections in hospitalized patients at a tertiary care hospital, southwest Nigeria. Afr J Clin Exp Microbiol 2022; 23(2):149-58. doi: 10.4314/ajcem.v23i2.5 [Crossref] [ Google Scholar]
- Ain NU, Iftikhar A, Bukhari SS, Abrar S, Hussain S, Haider MH. High frequency and molecular epidemiology of metallo-β-lactamase-producing gram-negative bacilli in a tertiary care hospital in Lahore, Pakistan. Antimicrob Resist Infect Control 2018; 7:128. doi: 10.1186/s13756-018-0417-y [Crossref] [ Google Scholar]
- Thapa P, Bhandari D, Shrestha D, Parajuli H, Chaudhary P, Amatya J. A hospital based surveillance of metallo-beta-lactamase producing gram negative bacteria in Nepal by imipenem-EDTA disk method. BMC Res Notes 2017; 10(1):322. doi: 10.1186/s13104-017-2640-7 [Crossref] [ Google Scholar]
- Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60(2):1067-78. doi: 10.1128/aac.02379-15 [Crossref] [ Google Scholar]
- El-Shouny WA, Ali SS, Sun J, Samy SM, Ali A. Drug resistance profile and molecular characterization of extended spectrum beta-lactamase (ESβL)-producing Pseudomonas aeruginosa isolated from burn wound infections Essential oils and their potential for utilization. Microb Pathog 2018; 116:301-12. doi: 10.1016/j.micpath.2018.02.005 [Crossref] [ Google Scholar]
- Bush K, Bradford PA. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev 2020; 33(2):e00047-19. doi: 10.1128/cmr.00047-19 [Crossref] [ Google Scholar]
- Potron A, Poirel L, Bussy F, Nordmann P. Occurrence of the carbapenem-hydrolyzing beta-lactamase gene blaOXA-48 in the environment in Morocco. Antimicrob Agents Chemother 2011; 55(11):5413-4. doi: 10.1128/aac.05120-11 [Crossref] [ Google Scholar]
- Olaitan AO, Berrazeg M, Fagade OE, Adelowo OO, Alli JA, Rolain JM. Emergence of multidrug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase, Nigeria. Int J Infect Dis 2013; 17(6):e469-70. doi: 10.1016/j.ijid.2012.12.008 [Crossref] [ Google Scholar]
- Manenzhe RI, Zar HJ, Nicol MP, Kaba M. The spread of carbapenemase-producing bacteria in Africa: a systematic review. J Antimicrob Chemother 2015; 70(1):23-40. doi: 10.1093/jac/dku356 [Crossref] [ Google Scholar]
- Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol 2016; 12(11):662-74. doi: 10.1038/nrneurol.2016.149 [Crossref] [ Google Scholar]
- GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3(8):e361-e87. doi: 10.1016/s2352-3018(16)30087-x [Crossref] [ Google Scholar]
- Adeyemi FM, Ako-Nai KA, Adejuyigbe E, Ebhodaghe BI, Osho PO, Oyeniyi TT. Molecular characterization and antibiotic resistance profiles of bacterial isolates cultured from HIV seropositive patients. Arch Clin Microbiol 2015; 6(1):1-11. [ Google Scholar]
- Ako-Nai KA, Adeyemi FM, Adejuyigbe E, Ebhodaghe BI, Osho PO, Kassim OO. The dynamics of bacteria population on the skin, throat, and gastrointestinal tract of HIV-seropositive patients. Ann Trop Med Public Health 2015; 5(8):164-76. [ Google Scholar]
- Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis 1988; 10(4):867-78. doi: 10.1093/clinids/10.4.867 [Crossref] [ Google Scholar]
- Carter MW, Oakton KJ, Warner M, Livermore DM. Detection of extended-spectrum β-lactamases in Klebsiellae with the Oxoid combination disk method. J Clin Microbiol 2000; 38(11):4228-32. doi: 10.1128/jcm.38.11.4228-4232.2000 [Crossref] [ Google Scholar]
- Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm?. Clin Microbiol Rev 2005; 18(2):306-25. doi: 10.1128/cmr.18.2.306-325.2005 [Crossref] [ Google Scholar]
- Bashir D, Thokar MA, Fomda BA, Bashir G, Zahoor D, Ahmad S. Detection of metallo-beta-lactamase (MBL) producing Pseudomonas aeruginosa at a tertiary care hospital in Kashmir. Afr J Microbiol Res 2011; 5(2):164-72. doi: 10.5897/ajmr10.694 [Crossref] [ Google Scholar]
- Behera B, Mathur P, Das A, Kapil A, Sharma V. An evaluation of four different phenotypic techniques for detection of metallo-beta-lactamase producing Pseudomonas aeruginosa. Indian J Med Microbiol 2008; 26(3):233-7. doi: 10.4103/0255-0857.39587 [Crossref] [ Google Scholar]
- Ruwali P, Pandey S, Rawat V, Ambwani T. Detection of metallo-β-lacatamase producing bacteria in clinical isolates of patients using EDTA & ceftazidime. Asian J Pharm Life Sci 2012; 2(4):453-6. [ Google Scholar]
- Begum S, Salam MA, Alam Kh F, Begum N, Hassan P, Haq JA. Detection of extended spectrum β-lactamase in Pseudomonas spp isolated from two tertiary care hospitals in Bangladesh. BMC Res Notes 2013; 6:7. doi: 10.1186/1756-0500-6-7 [Crossref] [ Google Scholar]
- Basak S, Attal RO, Rajurkar M. Pseudomonas aeruginosa and newer β-lactamases: an emerging resistance threat. In: Sudhakar C, ed. Infection Control. IntechOpen; 2012. 10.5772/34743.
- Sharma M, Yadav S, Chaudhary U. Metallo-beta-lactamase producing Pseudomonas aeruginosa in neonatal septicemia. J Lab Physicians 2010; 2(1):14-6. doi: 10.4103/0974-2727.66701 [Crossref] [ Google Scholar]
- Sepehriseresht S, Boroumand MA, Pourgholi L, Sotoudeh Anvari M, Habibi E, Sattarzadeh Tabrizi M. Detection of vim- and ipm-type metallo-beta-lactamases in Pseudomonas aeruginosa clinical isolates. Arch Iran Med 2012; 15(11):670-3. [ Google Scholar]
- Shah SR, Karanje NC. Study of metallo-beta-lactamase producing gram-negative bacteria in a tertiary care hospital. Saudi J Pathol Microbiol 2019; 4(7):550-4. doi: 10.21276/sjpm.2019.4.7.14 [Crossref] [ Google Scholar]
- Franco MR, Caiaffa-Filho HH, Burattini MN, Rossi F. Metallo-beta-lactamases among imipenem-resistant Pseudomonas aeruginosa in a Brazilian university hospital. Clinics (Sao Paulo) 2010; 65(9):825-9. doi: 10.1590/s1807-59322010000900002 [Crossref] [ Google Scholar]
- Myat TO, Hannaway RF, Zin KN, Htike WW, Win KK, Crump JA. ESBL- and carbapenemase-producing Enterobacteriaceae in patients with bacteremia, Yangon, Myanmar, 2014. Emerg Infect Dis 2017; 23(5):857-9. doi: 10.3201/eid2305.161100 [Crossref] [ Google Scholar]
- Rani VS, Rao RK, Ravinder S, Kanakadurga P. Prevalence of extended-spectrum beta-lactamase (ESBL) producing Pseudomonas aeruginosa isolates from burns patients. Int J Contemp Med Res 2016; 3(5):1297-300. [ Google Scholar]
- Berrazeg M, Diene S, Medjahed L, Parola P, Drissi M, Raoult D. New Delhi metallo-beta-lactamase around the world: an eReview using Google Maps. Euro Surveill 2014; 19(20):20809. doi: 10.2807/1560-7917.es2014.19.20.20809 [Crossref] [ Google Scholar]
- Acolatse JEE, Portal EAR, Boostrom I, Akafity G, Dakroah MP, Chalker VJ. Environmental surveillance of ESBL and carbapenemase-producing gram-negative bacteria in a Ghanaian Tertiary Hospital. Antimicrob Resist Infect Control 2022; 11(1):49. doi: 10.1186/s13756-022-01090-2 [Crossref] [ Google Scholar]
- Nordmann P, Poirel L. Emerging carbapenemases in gram-negative aerobes. Clin Microbiol Infect 2002; 8(6):321-31. doi: 10.1046/j.1469-0691.2002.00401.x [Crossref] [ Google Scholar]
- Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis 1998; 27(Suppl 1):S93-S9. doi: 10.1086/514909 [Crossref] [ Google Scholar]
- Behera B, Mathur P, Das A, Kapil A, Sharma V. An evaluation of four different phenotypic techniques for detection of metallo-beta-lactamase producing Pseudomonas aeruginosa. Indian J Med Microbiol 2008; 26(3):233-7. doi: 10.4103/0255-0857.39587 [Crossref] [ Google Scholar]
- Tellevik MG, Blomberg B, Kommedal Ø, Maselle SY, Langeland N, Moyo SJ. High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae among children in Dar es Salaam, Tanzania. PLoS One 2016; 11(12):e0168024. doi: 10.1371/journal.pone.0168024 [Crossref] [ Google Scholar]
- Andriatahina T, Randrianirina F, Hariniana ER, Talarmin A, Raobijaona H, Buisson Y. High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect Dis 2010; 10:204. doi: 10.1186/1471-2334-10-204 [Crossref] [ Google Scholar]
- Woerther PL, Angebault C, Jacquier H, Hugede HC, Janssens AC, Sayadi S. Massive increase, spread, and exchange of extended spectrum β-lactamase-encoding genes among intestinal Enterobacteriaceae in hospitalized children with severe acute malnutrition in Niger. Clin Infect Dis 2011; 53(7):677-85. doi: 10.1093/cid/cir522 [Crossref] [ Google Scholar]