Avicenna Journal of Clinical Microbiology and Infection. 9(3):124-134.
doi: 10.34172/ajcmi.2022.3389
Review Article
Viral Gastroenteritis Prevalence in Iranian Pediatric Population: A Systematic Review
Nooshin Mojahed 1  , Mohammad Ali Mohammadkhani 2, Masoumeh Pourasgari 1, Golnosh Gol-Jah Rad 1, Ashraf Mohamadkhani 1, *
, Mohammad Ali Mohammadkhani 2, Masoumeh Pourasgari 1, Golnosh Gol-Jah Rad 1, Ashraf Mohamadkhani 1, * 
Author information:
1Liver and Pancreatobiliary Diseases Research Center, Digestive Diseases Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
2Technical and Vocational University, Tehran, Iran
Abstract
Background: Viral gastroenteritis infection, a prevalent condition in adolescents and children, is still a rigid and serious problem among humans. This disease is responsible for up to three million fatalities nationwide. noroviruses, rotaviruses, astroviruses, adenoviruses, and sapoviruses are the most common and well-known pathogens associated with viral gastroenteritis agents. In this systematic review, we extracted all original articles and data on viral gastroenteritis that were performed on the Iranian pediatric population.
Methods: To investigate the viral agent pathogens of gastroenteritis in Iran, 48 articles on the identification of viral gastroenteritis were gathered from the existing data. Viral gastroenteritis was detected in fourteen provinces, including the southern and northern parts of Iran. The seasonal distribution in Iran was analyzed as well. Finally, all the data from 1978-2021, along with their detailed information, were summarized, including the number of patients, the number of positive cases, applied technics, and the region of the studied cases in Iran.
Results: Based on the results, most of the viral detection was associated with Rotavirus, the major pathogen responsible for gastroenteritis disease, followed by Adenovirus, Norovirus, Parechovirus, Bocavirus, Astrovirus, Aichivirus, Sapovirus, and three case reports of SARS-CoV-2 that were associated with viral gastroenteritis.
Conclusion: Different studies conducted over Iran, including the northern, southern, and central regions, were obtained based on the data. Most studies had been merely dedicated to rotavirus, which had the highest prevalence of all other viral gastroenteritis. Our review clearly demonstrated that Rotavirus genotype G1P [8] is the dominating sereotype among the other studied gastroenteritis viral agents in Iran in which the most frequency rate was during the winter (44.26%), while the least frequency rate was observed during summer (8.96%).
Keywords: Iranian child gastroenteritis, Viral gastroenteritis, Rotavirus, Adenovirus, Norovirus
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Mojahed N, Mohammadkhani MA, Pourasgari M, Gol-Jah Rad G, Mohamadkhani A. Viral gastroenteritis prevalence in iranian pediatric population: A systematic review. Avicenna J Clin Microbiol Infect. 2022; 9(3):124-134. doi:10.34172/ ajcmi.2022.3389
Introduction
Viral gastroenteritis infection has been recognized as a common illness among children and adolescents. Considering that the oral route is regularly the underlying region of the pathogen’s exposure, this infection is mainly observed in young children under the age of five and infants. Gastroenteritis infection outbreak happens more often in developing countries where children have no sufficient access to clean water and environmental sanitization (1,2). Studies have shown that the rate of mortality is higher in developing countries; however, the rate of infection cannot be ignored in developed countries either (3). Although gastroenteritis is normally a self-limited disease, it is reported that it accounts for up to three million deaths globally (4,5). Therefore, the investigation of this matter is highly imperative. Due to gastroenteritis symptoms, this disease is also known as infectious diarrhea (2). The symptoms are typically diarrhea, vomiting, fever, and abdominal pain. In acute gastroenteritis, it is possible to observe high dehydration and electrolyte imbalance which can lead to death and hospitalization (6,7).
In today’s world viruses are the initial pathogens that are responsible for 75%-90% of infectious diarrhea, and several viruses are involved in this disease (8). Although the discovery of gastrointestinal viruses is still an ongoing project for scientists, noroviruses, rotaviruses, astroviruses, adenoviruses, and sapoviruses are the main and well-known pathogens related to viral gastroenteritis agents (9). The recent coronavirus (coronavirus disease 19, COVID-19) has also been reported for some gastroenteritis symptoms, especially in young children, with the observation of diarrhea and vomiting throughout the phase of illness (10), and the continuous observation of viral RNA positivity in children’s stool samples is boosting great concerns regarding the control and inhibition of COVID-19. Human Bocavirus, Aichivirus, and human parechoviruses have also been reported as viral gastroenteritis agents (11).
As mentioned above, the prevalence of acute gastroenteritis in developing countries is higher in comparison with developed countries; therefore, it was aimed to provide a systematic review to make a broader view of this subject in the pediatric population of Iran.
Materials and Methods
Search Strategy and Selection Criteria
The present study was accompanied using the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist (12).
At the beginning of our study, PubMed, Medline, Google Scholar, and National databases were searched through August and September 2021. All of our searches included English and Persian languages without any date restriction. All the databases were searched using keywords and terms such as Acute Gastroenteritis, Viral Gastroenteritis, Child Gastroenteritis, acute Gastroenteritis ‘AND’ Iran, Viral Gastroenteritis ‘AND’ Norovirus, Viral Gastroenteritis ‘AND’ Rotavirus, Viral Gastroenteritis ‘AND’ Sapovirus Viral Gastroenteritis and Adenovirus, and Viral Gastroenteritis ‘AND’ Coronavirus. The other keywords were Viral Gastroenteritis ‘AND’ SAR-CoV-2’ Viral Gastroenteritis ‘AND’ Bocavirus, Viral Gastroenteritis AND Astrovirus, Viral Gastroenteritis AND Aichivirus, Viral Gastroenteritis ‘AND Iran, Rotavirus ‘AND’ Iran Norovirus ‘AND’ Iran Sapovirus ‘AND’ Iran Coronavirus ‘AND’ Iran, Adenovirus ‘AND’ Iran, and Bocavirus ‘AND’ Iran, In addition, the reference sections of the studies were reviewed and rechecked to consolidate and identify further relevant studies. Then, the applicable publications which were most relevant to epidemiological and clinical child viral gastroenteritis were selected through our collected data.
Screening and Data Extraction
First, all the publications were studied and screened based on their title and abstracts. The eligible abstracts were selected for the full-text version study. Then, the studies were selected whether they met the inclusion criteria; they included the detection of the viruses (Norovirus, Sapovirus, Rotavirus, Astrovirus, Adenovirus, Coronavirus, Bocavirus, and Aichivirus) in the stool samples of children with acute gastroenteritis, epidemiology of each mentioned virus, the prevalence of each viral infection in children among different cities in Iran, use of reported standardized laboratory techniques for viral detection. On the other hand, studies were excluded if they were applied to species other than human and environmental experiments. All the studies were determined based on their full text, and those which did not meet our criteria were excluded from further investigations. The flow of information in the systematic review is shown in Figure 1. Subsequently, data and information were extracted based on the detection rate of the mentioned viruses in Iranian pediatric patients.
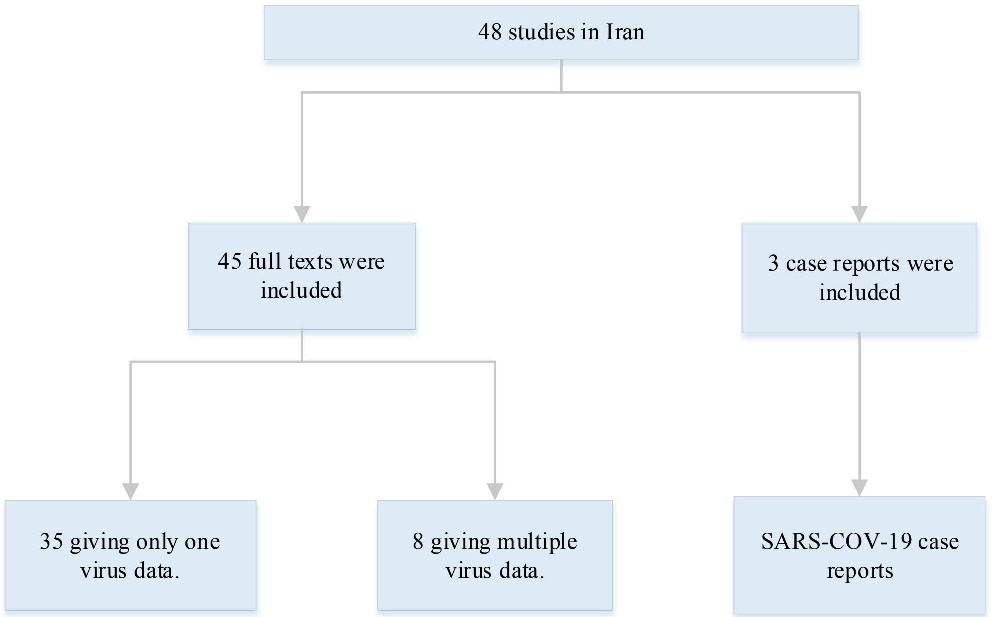
Figure 1.
Information Flow Through the Phase of the Systematic Review. Our search was performed using the key words Iran and viral gastroenteritis. Overall, 48 studies were published between 1987 and 2021, including case reports
.
Information Flow Through the Phase of the Systematic Review. Our search was performed using the key words Iran and viral gastroenteritis. Overall, 48 studies were published between 1987 and 2021, including case reports
Results
Viral Gastroenteritis Aspects
In this study, 48 articles related to viral gastroenteritis detection were extracted, which were performed on the Iranian population, 3 of which were case reports related to SARS-CoV-2. The features of main viruses associated with viral gastroenteritis among virologic characteristics and patterns are described in Table 1.
Table 1.
Virologic Attributes and Means of Main Viruses Associated With Viral Gastroenteritis (
13)
|
Virus
|
Family
|
Size (nm)
|
Genome Size (kb)
|
Appearance Using EM
|
Nucleic Acid
|
Detection Methods
|
| Norovirus (14) |
Caliciviridae
|
27-30 |
7.5 |
Small, round-structured |
Positive-single stranded RNA viruses |
RT-PCR, ELISA, & EM |
| Rotavirus (15) |
Reoviridae |
70 |
18.5 |
Wheel-shaped triple-layered capsid |
dsRNA |
EM, ELISA, & RT-PCR |
| Astrovirus (16) |
Astroviridae |
27-34 |
6.8-7.9 |
SRV- star shaped |
ss( + )RNA |
EM, ELISA, & RT-PCR |
| Adenovirus (17) |
Adenoviridae |
70-100 |
26-45 |
Icosahedral capsid |
dsDNA |
EM, EIA, RT-PCR, & culture |
| Bocavirus (18) |
Parvoviridae |
20 |
4-6 |
Icosahedral and non-enveloped |
Single- Stranded DNA |
PCR |
| Aichivirus (19) |
Picornaviridae
|
30 |
8.2 |
Icosahedral morphology |
ss( + ) RNA |
N-PCR & RT-PCR |
| Sapovirus (20) |
Caliciviridae |
30-38 |
7.4 |
Cup shaped |
Non enveloped ss( + ) RNA |
EM, ELISA, & RT-PCR |
| Parechovirus (21) |
Picornavirus |
30 nm |
7.3 |
Spherical and round geometries |
Ss( + ) RNA |
EM & RT-PCR |
| Coronavirus (22) |
Coronaviridae |
60-200 |
26.4-31.7 |
Pleomorphic with club-shaped projections |
ss( + ) RNA |
EM & RT-PCR |
Note. EM: Electron microscopy; SRV: Standard recovery vehicle; Reverse transcriptase polymerase chain reaction; ELISA: Enzyme-linked immunosorbent assay; EIA: enzyme immune assay.
All the patients that were recognized with gastroenteritis symptoms were gathered, and then positive cases with various virus detection were divided to bring a wider insight into the study of this disease. The numerical distribution of positive viral gastroenteritis cases among the studied population is illustrated in Figure 2. All the numbers of patients involved in gastroenteritis studies in the Iranian pediatric population from 1987 to 2021 are gathered in this diagram. A total of 17 789 patients were diagnosed to have acute gastroenteritis based on their clinical symptoms, approximately all of the patients had diarrhea, and other manifested clinical symptoms included vomiting, abdominal pain, fever, and in some cases, dehydration. Based on the diagram, the number of patients who were involved with Rotavirus was significantly higher compared to other viral agents. It should be noted that studies and investigations of other viral agents were not sufficient to have an accurate overview. There was an investigation over fourteen provinces, along with the northern and southern regions of Iran. Rotavirus, Adenovirus, Aichivirus, Astrovirus, Bocavirus, Sapovirus, and Norovirus were observed in children’s stool samples. Three case reports related to SARS-CoV-2 with gastroenteritis symptoms were observed and reported as well. Overall, 5900 positive cases were found to report Rotavirus. Co-infection between Rotavirus and Norovirus was reported in three cases. Except for three case reports related to SARS-CoV-2, the least detection of the viral agent was associated with Sapovirus (eight positive cases). Table 2 presents all the complication reports among viral gastroenteritis cases with the method, frequency, and genotyping if available.
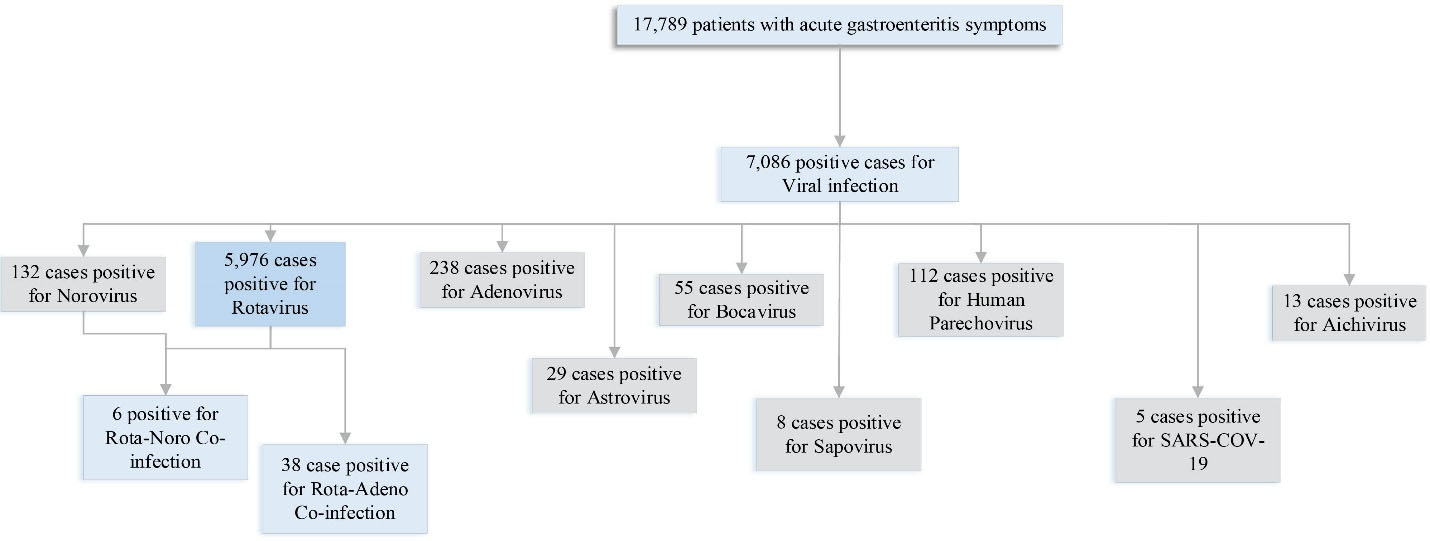
Figure 2.
Numerical Distribution of All Viral Gastroenteritis Studied in Iran
.
Numerical Distribution of All Viral Gastroenteritis Studied in Iran
Table 2.
Studies Performed in Iran Related to Viral Gastroenteritis
|
First Author (References)
|
Geographical Region and Frequency of Gastroenteritis
|
Viral Agent Occurrence (%)
|
Test Method
|
Genotyping
|
Gender
|
| Tariverdi (23) |
Tehran |
SARS-CoV-2 (1 patient) |
RT-PCR |
NA |
Female |
| Moradveisi (24) |
Sanandaj |
SARS-CoV-2 (1 patient) |
RT-PCR |
NA |
Female |
| Ekbatani (25) |
Tehran |
SARS-CoV-2 (3 patients) |
RT-PCR |
NA |
66.6% Male
33.3% Female |
| Taghinejad (26) |
Karaj, Tehran |
Aichivirus (8.1) |
RT-PCR |
NR |
30% Male
70% Female |
| Farsi (27) |
Tehran |
Rotavirus (17.1) |
RT-PCR |
GII 17.1% |
56.7% Male
43.3% Female |
| Shams (28) |
Qom |
Rotavirus (16.9) |
RT-PCR |
G1 27% G9 18% G2 9% G3 9% G4 9% G12 5% G non-typable 23% P[8] 50% P[6] 23% P[4] 14% P non-typable 13% |
60% Male
40% Female |
| Farahmand (29) |
Tehran |
Rotavirus (100) |
RT-PCR |
P[8] 94.4% P[4] 2.8%
P[6] 2.8% |
NR |
| Arashkia (30) |
Tehran |
HAdV (4.3) |
PCR |
(AdV41) 62.5% (AdV1,2,6,) 31.25% (AdV3) 6.25% |
56.7% Male
43.3% Female |
| Lorestani (31) |
Gorgan, Golestan |
Rotavirus (11.17) |
Latex agglutination PAGE (Semi-nested multiplex RT-PCR for G and P genotyping) |
G1 57% G2 18.70% G3 4.69% G4 3.13% G9 6.26% non-typable 6.26% P[8] 97.80% P[4] 2.20% |
61% Male
39% Female |
| Azaran (32,33) |
Ahvaz, Khuzestan |
Rotavirus (32) |
Latex agglutination RT-PCR |
G9 37.5% G2 21.9% G1 12.5% G12 9.4% G4 9.4% G2G9 6.9% G3 3.1% P[8] 62.5% P[4] 31.5% P[4]P[8] 3.1% |
55% Male
45% Female |
| Mousavi Nasab (34) |
Tehran |
HAdV (5) HAstV (6.7) SaV (2.5) |
RT-PCR |
NR |
18.2% Male
9.3% Female |
| Mousavi Nasab (35) |
Tehran |
Rotavirus (23.3) |
RT-PCR |
G1 75% G2 14.3% G9 7.14% Mixed G1/G2 3.58% P[8] 75% P[4] 25% |
NR |
| Mousavi Nasab (36) |
Tehran |
Rotavirus (28.8)
Norovirus (8.8) Rota-Noro Coinfection (3.5) |
RT-PCR |
Norovirus: GII 86.7% GI 13.3% GIV 0% |
54.1% Male
45.9% Female |
| Sharifi-Rad (37) |
Zabol |
Rotavirus (70.2)
Adenovirus (20.3)
Norovirus (9.5) |
Immunochromatography Test |
NR |
NR |
| Azaran (32) |
Ahvaz, Khuzestan |
Rotavirus (36.5) |
ELISA RT-PCR |
G and P 86.3% 13.7% non-typable |
61.6% Male
38.4% Female |
| Monavari (38) |
Tehran |
HBoV (8) |
RT-PCR |
NR |
72% Male
28% Female |
| Kargar (39) |
Yasuj |
Rotavirus (28.2) |
RT-PCR |
G1 1.92% G2 7.69% G4 1.92% G8 46.16% non-typeable 40.39% Mixed 1.92% |
12.5% Male
15.76% Female |
| Khoshdel (40) |
Shahrekord |
Rotavirus (30) |
RT-PCR |
G1 20% G9 20% G1G9 13.3% G1G4 6.7% G1G3 3.3% G1G8 3.3.% |
53% Male
47% Female |
| Jadali (41) |
Bandar Abbas Shiraz Mashhad Tabriz Tehran |
Rotavirus
(16.7) (14.2) (7.76) (7.56) (8.97) |
ELISA |
NR |
59.9% Male 40.1% Female |
| Shokrollahi (42) |
Tehran |
Rotavirus (48)
adenovirus (20)
HBoV (20)
HPeV (23.2)
Adeno-Rota co infection (6) |
"Rota and adeno: rapid
chromatographic tests
HBoV: RT-PCR
HPeV-1: nested-RT PCR" |
NR |
58% Male
42% Female |
| Kargar (43) |
Borazjan |
Rotavirus (27.85) |
ELISA Nested Multiplex PCR |
G1 52.27% non-typable 40.91% G9 4.54% G4 2.27% |
28.4% Male 21.77% Female |
| Najafi (44) |
Borazjan |
Rotavirus (24.27) Norovirus (12.35) Adenovirus (5.1) Astrovirus (2.4) |
ELISA |
NR |
NR |
| Motamedifar (45) |
Shiraz |
Rota (42) Adeno (9)
Rota-Adeno Coinfection (4) |
ELISA |
NR |
59.5% Male 40.5% Female |
| Romani (46) |
Tehran |
HBoV (9.18) |
PCR |
(HBoV1 )11.1% (HBoV2) 35.1% (HBov3) 3.7% |
48% Male
52% Female |
| Romani (47) |
Tehran |
Sapovirus (11.9) |
RT-PCR |
NR |
60% Male
40% Female |
| Rezaei (48) |
Tehran |
Adenovirus (8) |
PCR |
Adv 40,41 |
62.5% Male 37.5% Female |
| Hamedi (49) |
Mashhad |
Adenovirus (2) |
Lattex Agglutination |
NR |
NR |
| Romani (50) |
Tehran |
Norovirus (9.8) |
RT-PCR |
Noro I 35% Noro II 65% |
57% Male
43% Female |
| Ataei-Pirkooh (51) |
Tehran |
Rotavirus (43) |
Latex agglutination Electron microscopy |
NR |
NR |
| Ghazi (52) |
Tehran |
HPeVs (23.7) |
RT-PCR |
(HPeV-1)23.7% |
35.3% Male 16.0% Female |
| Ghorashi (53) |
Tabriz |
Rotavirus (55.6) |
ELISA |
NR |
60.2% Male 39.8% Female |
| Nadji (54) |
Tehran |
hBoV (12.8) |
PCR |
NR |
40.4% Male 59.6% Female |
| Esteghamati (55) |
Tabriz Mashhad Northern Region Shiraz Bandar Abbas Southern Region Tehran |
Rotavirus
(49) (49.4) (66.3) (63.3) (65) (44) |
RT-PCR |
G4 44.5% G non-typable 36.4% P[8] 64.5% P[4] 6.4% P[9] 0.9% P non-typable 28.2% |
NR |
| Emamghorashi (56) |
Jahrom |
Rotavirus (67.6) |
ELISA latex agglutination test |
NR |
52.1% Male 47.8% Female |
| Sadeghian (57) |
Mashhad |
Rotavirus (28.8) |
Latex agglutination test |
NR |
30.1% Male 27.4% Female |
| Nakhaei Sistani (58) |
Tehran |
Adenovirus (6.3) |
PCR PAGE |
Adv 40,41 |
40%Male 60%Female |
| Hamkar (59) |
Mazandaran |
Rotavirus (62) Astrovirus (3) Adenovirus (2.3) |
ELISA |
NR |
64% Male 58.5% Female |
| Modaress (60) |
Tehran |
Rotavirus (19) |
PAGE RT-PCR |
G1 76.3% G4 11.5% G8 0.8% Mixed types 3.1% P[8] 66.4% p[4] 9.2% |
NR |
| Barari Savadkoohi (61) |
Babol |
Rotavirus (61.6) Adenovirus (2.9) Astrovirus (2.4) |
ELISA |
NR |
61.5% Male 38.5% Female |
| Farahtaj (62) |
Tehran |
Rotavirus (24.6) |
ELISA RT-PCR |
G1 G2 G4 G9 G12 P[4] P[8] P[10] |
NR |
| Modarres (63) |
Tehran |
Rotavirus (32.3) |
ELISA |
NR |
NR |
| Kazemi (64) |
Isfahan |
Rotavirus (30.8) |
ELISA |
NR |
52% Male
48% Female |
| Zarnani (65) |
Tehran |
Rotavirus (15.3) |
ELISA |
NR |
62.2% Male 37.7% Female |
| Khalili (66) |
Shahrekord |
Rotavirus (72.6) |
RT-PCR |
combination reported |
Only male |
| Samarbafzadeh (67) |
Ahwaz, Khuzestan |
Rotavirus (29.5) |
PAGE |
NR |
NR |
| Saderi (68) |
Tehran |
Adenovirus (8.7) |
MEIA |
(AdV40) 3.3% (AdV41) 3.4% |
60.1% Male 39.9% Female |
| Amini (69) |
Tehran |
Rotavirus (25) |
Latex agglutination |
NR |
26% Male
24% Female |
Note. RT-PCR: Reverse transcriptase polymerase chain reaction; ELISA: Enzyme-linked immunosorbent assay; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; NR, not reported; NA, not available; HBoV, human bocavirus; HPeV human parechovirus.
Gastroenteritis was spread widely throughout the year with the most frequency rate during the winter (44.26%), followed by autumn (32.61%), spring (14.19%), and summer (8.96%), which is consistent with the result of other studies in different countries. Figure 3 depicts the spreading points of viral gastroenteritis in geographical regions of Iran (a) and its seasonal distribution (b). Further details of the studies that have been conducted in Iran are described in this section.
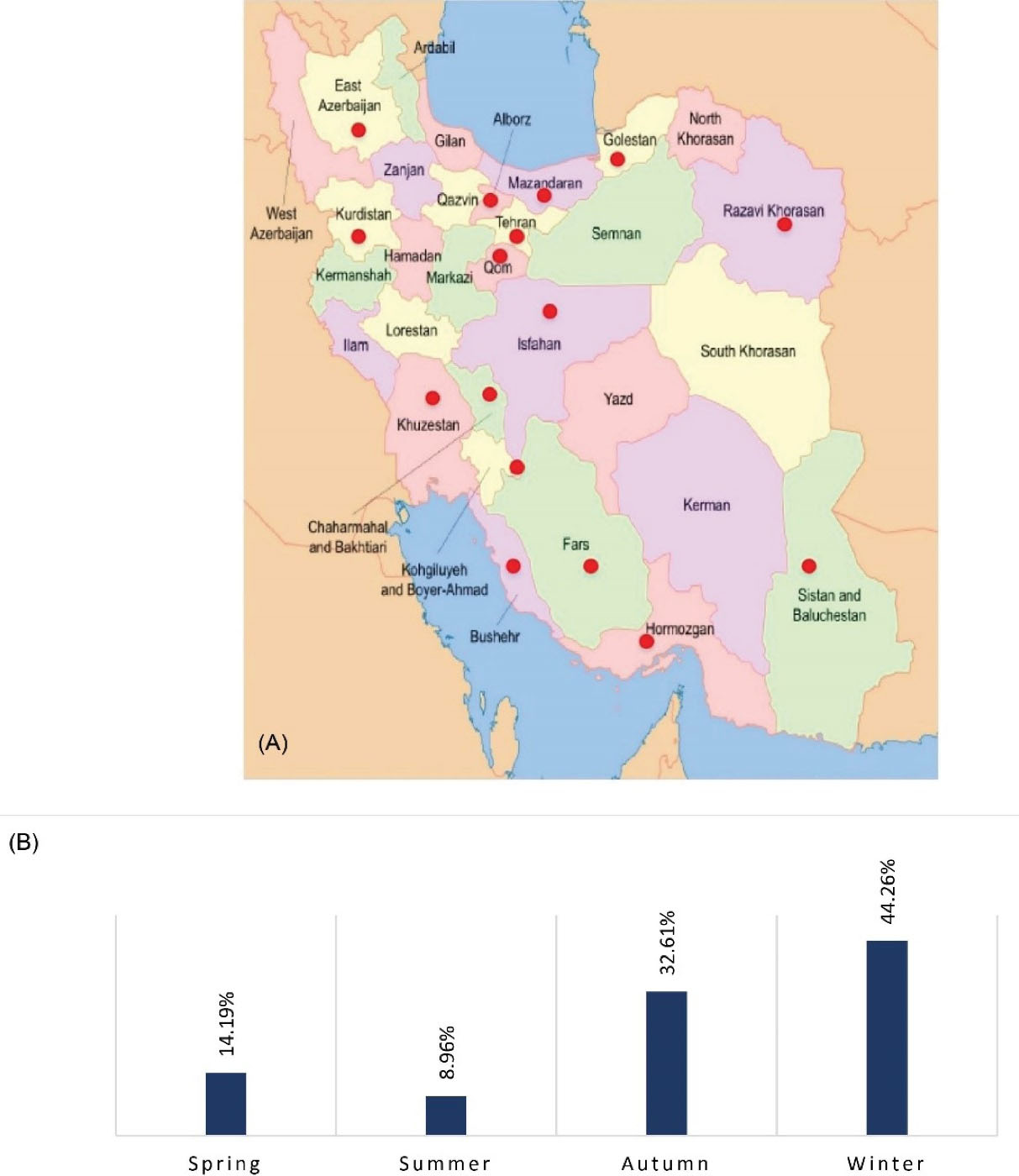
Figure 3.
(A) Viral Distribution of AGE in Different Geographical Regions. (B) Seasonal Distribution in Iran 1987-2021
.
(A) Viral Distribution of AGE in Different Geographical Regions. (B) Seasonal Distribution in Iran 1987-2021
Norovirus
Farsi et al studied children under 5 years old with acute gastroenteritis and found 36 positive samples for Norovirus genotype II from children stool’s samples out of a total of 210 samples (27).
Mousavi Nasab et al investigated over 170 patients presenting gastroenteritis symptoms and detected 15 Norovirus positive cases (8.8%). They also observed Rota-Noro co-infection which was 3.5%, including 6 samples. In their study, genotyping for Norovirus was also performed on the prevalence of GI (13.3%) and GII (86.7%), and there was no GIV (36). In a cross-sectional study by Najafi et al,the rate of Norovirus was 47 (12.35%) among 375 patients (44). Likewise, Romani et al detected 26 positive samples from a total of 293 (9.8%). Genotyping was performed and 9 (35%) and 17 (65%) samples were found to belong to GI and GII strains, respectively (50).
Rotavirus
In a study by Shams et al,22 out of 130 Rotavirus were found, and genotyping was conducted for 22 positive samples (for VP7 isolates). G1 was the most predominant sereotype accounting for 27%, no G8 strains were observed, and 23% of samples were non-typable. For VP4 genotyping, P [8] was the most predominant sereotype accounting for 50%, and 13% of the samples were P nontypables among G-P combinations, and G1P [8] 32% was the most frequent genotype (28). Farahmand et al found 108 positive cases out of 108 samples (100%). They evaluated the genetic pattern of VP8 protein from different P genotypes, and the phylogenetic tree showed that all P genotypes related to the Iranian population belonged to P(II) Geno groups, and the most prevalent among them was P [8] accounting for 94.4%, which was a significant result (29). In Gorgan, Lorestani et al conducted a study on children with gastroenteritis, and Rotavirus was detected in 46 out of 349 positive samples for Rotavirus genotype A. They also identified G and P genotypes in positive samples that G1 as the most predominant sereotype among G types [8]. The predominant G1P [8] 57.82% was the highest rate in G/P combinations in their study as well (31). Similarly, Azaran et al detected 32 positive Rotavirus among 100 stool samples in Ahvaz, Khuzestan. G and P genotyping was performed on positive cases, and G9 turned out to be the most predominant for VP7 genotyping accounting for 37.5%, and VP4 genotyping P [8] with 62.45% was the most common. Among G-P combinations G9P [8] accounting for 28.13% had the highest rate of infection, followed by G2P [4] at 18.75% (33). In another study, Mousavi Nasab et al detected 28 Rotavirus positive out of 130 stool samples, G1 and P [8], accounting for 75% and 75%, respectively, were the most predominant sereotypes (35).
Azaran et al found 73/200 positive for Rotavirus, reporting that G1 (55.6% ) and P [8] (83.3%) were the dominant sereotypes in this study (32). Another research in Yasuj during 2010-2011 was performed on Rotavirus genotyping out of 52 positive samples, and G8 was the most common circulating genotype accounting for (46.16%). In addition, the prevalence of the G1 sereotype, which was the most predominant one in the latest research, was only 1.92% (39). Khoshdel et al showed that from 30% positive Rotavirus cases, all under the age of five, G1 and G9 with a prevalence of 20% were the most common ones, mixed genotypes (G1 + G9) (40).
Jadali et al detected 1649 positive Rotavirus from 2988 stool samples; their study was conducted in 5 different cities with the most prevalence in Bandar Abbas (41). In a cross-sectional study by Shokrolahi et al, the prevalence of the Rotavirus in Tehran was 48% (42). In another study by Karegar et al,the prevalence of Rotavirus was 27.85%, and G1 was the most predominant circulating G genotype with a significant percentage of 52.27% (43). In a similar cross-sectional study by Najafi et al, the rate of Rotavirus was 24.27% among 375 patients in Borazjan (44).
Motamedifar et al also conducted a study in Shiraz and reported that 347/827 patients were infected with Rotavirus, which accounted for 42% of the total patients (45). Ataei-Pirkooh et al detected 43 positive Rotavirus among 100 patients with diarrhea (51). Ghorashi et al also found 284 positive cases in a total of 511 stool samples (53). Likewise, Esteghamati et al detected Rotavirus in 5 different cities (Tabriz, Mashhad, Shiraz, Bandar Abbas, and Tehran) plus northern and southern regions among 2198 patients in total. The average percentage of Rotavirus among the mentioned locations was 59.1%. Genotyping for G (VP7) and P (VP4) was performed for 110 samples. G4 (44.5%) and P [8] (64.6%) were the most common among G and P sereotypes, respectively. Among G-P combinations, G4P [8] manifesting at 30.9% was the most predominant strain, and G1P [8] was found at 10.9%. Further, the least combinations were observed among G2P [4] at 5.5%. Moreover, P [8] strains were detected with G non-typeable (21.8%) and G4 with P non-typeable (55).
Additionally, Emamghorashi et al studied 102 patients, and 69 of them (67.6%) were positive for Rotavirus (56). Sadeghian et al also investigated over 156 stool samples of patients in Mashhad among them, 45 samples were positive for Rotavirus (57). In their study, Hamkar et al evaluated 400 patients with diarrhea in Mashhad and detected three different viruses (Rotavirus, Adenovirus, and Astrovirus), and 248 of them were diagnosed to be positive for Rotavirus (59). Furthermore, Modaress et al detected 131 out of 700 stool samples in Tehran; the determination of G and P genotyping showed that G1 (76.3%) was the most common among VP4 genotyping, and P [8] was the most prevalent with the percentage of 66.4% among VP7 genotypes. Finally, G1P [8] turned out to be the highest G-P combination (53.4%) in the detected samples (60).
In a study conducted in one main children’s hospital in Amirkola, Babol North of Iran, Barari Savadkoohi et al analyzed 208 samples for the detection of three viral agents (Rotavirus, Adenovirus, and Astrovirus), and the prevalence of Rotavirus was 61.6% among 127 total samples (61). Farahtaj et al also detected 92 positive Rotavirus among 374 patients (24. 6%); in their report, G1P [8] and G9P [8] with a prevalence of 59.2% and 15.5%, respectively, were the predominant genotype combinations (62).
Similarly, Modaress et al detected 404 among 1250 (32.3%). In their study, 341/404 were yielded for electrophoretic patterns. Based on their results, 90% and 8.8% of the strains belonged to long electrophoretic and short electrophoretic patterns, respectively (63). Kazemi et al also detected 57 out of 185 patients with gastroenteritis (64). Likewise, Zarnani et al detected 108 (15.3%) out of 704 patients (65). Khalili et al also detected 146 out of 186 stool samples. It is worth mentioning that in their study, Coronavirus was the second viral agent that was recognized through their research. electrophoretypes were performed for 46 Rotavirus positive samples, and G2P [4] belonged to all short electrophoretype strains, while G1 [P8] and G2P [8] were related to long electrophoretypes (66). In their study, Samarbafzadeh et al found 59 out of 200 samples (67). In another study, Amini et al investigated 229 (25%) positive Rotavirus samples out of 915 patients under 5 years with AGE symptoms in seven hospitals in Tehran (69).
Human Astrovirus
Astrovirus was observed in eight out of 120 patients in a study conducted by Mousavi Nasab et al with a prevalence of 6.7% (32,33). Najafi et al detected 2.4% in nine out of 375 patients (44). Hamkaret al reported Astrovirus among 12 out of 400 patients. The prevalence of this viral agent turned out to be 3% (59). In another study, Barari Savadkoohi et al detected only 5 positive samples out of 208 patients (61).
Human Adenovirus
Based on our investigation, ten studies were found regarding Adenovirus. Arashkia et al detected 16 positive samples for Adenovirus among 376 patients. In their research, they reported the genetic characterization of the human adenovirus as well. HAdv-41 was the most common genotype; interestingly, human adenovirus types C and B were observed as well. HAdV-C, which includes types 1, 2, and 6, was detected in 5 samples (31.25%), and HAdV-B, which represents type 3, was observed in 1 (6.25%) sample (30). Mousavi Nasab et al observed 120 stool samples from children for three viral agents HAdv, Human Sapovirus, and HAstV, among them, 6 positive samples (5%) were found to be positive for Adenovirus (34). In a cross-sectional study by Shokrolahi et al, the rate of Adenovirus was 20%, which included 16 samples out of 80 in their report, and the co-infection of Rota-Adeno was also reported, including 6% of samples (42).
Najafi et al detected 19 (5.1%) positive Adenovirus samples out of 375 patients (44). Moatmedifar et al also found 76 positive Adenovirus samples out of 827 patients, among them, 34 samples were Rota-Adeno coinfection (45). Likewise, Rezaei et al reported only 8 samples out of 100 patients, all eight samples were positive for AdV40-AdV1 (48). In another study, Hamedi et al observed 4 samples positive for adenovirus among 200 patients with diarrhea (49). In the study by Nakhaei Sistani et al, only 5 samples from a total of 80 samples of children were positive for human adenovirus (58). Similarly, Hamkar et al found 9 positive samples for Adenovirus from a total of 400 stool samples. Moreover, Barari Savadkoohi et al reported 6 positive samples among a total of 208 samples (59). Eventually, Saderi et al found 76 positive samples out of 872 samples (68).
Human Bocavirus
Bocavirus was detected in a total of 200 patient samples, and the prevalence of Bocavirus was observed in 16 (8%) samples (38). Bocavirus infection has been investigated through blood, saliva, feces, urine, sewage, and river water. It was reported that this virus is mainly detected in newborns aged 6-24 months (70). Bocavirus case reports have shown a high rate of co-infections such as adenovirus, norovirus, and rotavirus (71).
Shokrolahi et al found 8 positive samples for Bocavirus out of 80 stool samples (42). Romani et al also detected 27 positive samples from a total of 294 patients. In their study, NP1 (10 samples) products of PCR and NS1 (17 samples) were then performed for direct sequencing. The results revealed that 3 and 13 patients were infected by HBoV-1 and HBoV-2, respectively, and only 1 patient was found with HBoV-3 (46). In another study, Najdi et al detected only 6 positive samples out of 47 total samples (54).
Aichivirus
Taghinejad et al first detected the Aichivirus in Karaj, Alborz in the stool samples of Iranian children patients presenting acute gastroenteritis symptoms, the most common clinical symptoms were diarrhea and fever. In addition, 13 out of 160 samples were positive for this virus. In this study, the co-infection of Aichivirus with other common viruses was not detected (Norovirus, human adenovirus, and rotavirus), but 12 and 11 samples were also positive for Salivirus and Saffold virus, respectively, and in one patient, triple infections were observed with the participation of both Salivirus and Saffold virus. The peak season of this viral agent was observed in winter (26).
Human Sapovirus
Sapovirus was detected in the study by Mousavi Nasab et al.Based on their results, 2.5% of Sapovirus belonged to patients in Tehran (34). Romani et al found five positive Sapoviruses among 42 samples (47).
SARS-CoV-2
Tariverdi et al reported diarrhea as the only demonstration of COVID-19 in a 27-month child with fever and bloody diarrhea, and the patient was diagnosed with COVID-19. Her stool and pharyngeal sample were both positive for SARS-CoV-2 (23). Moradveisi et al also reported a 16-month female patient presenting diarrhea, vomiting, and lethargy, and she tested positive for SARS-CoV-2. It is highly important to note that other symptoms related to coronavirus were absent (24). These studies demonstrate that the presentation of COVID-19 in pediatric patients may be various from the typical clinical symptoms of the infection, specifically in comparison with adults, respiratory involvement, and most probably, diarrhea is the highlighted presentation, and long-term isolation must be considered due to the viral shedding.
Human Parechovirus
Shokrolahi et al detected 19 positive HPeV-1 stool samples among 80 patients (42). In another study by Ghazi et al, 472 samples were detected for HPeV-1, and 112 of them were positive (52).
Discussion
Although the viral agent condition regarding gastroenteritis is well determined in many countries, narrowed and limited available data led us to provide a summary of the current situation and studies that have been performed in our country in order to bring insight into this disease.
Based on our gathered data, studies have been conducted in different regions of Iran, including the northern, southern, and central parts of the country. Rotavirus had the most prevalence among other viral gastroenteritis, and the highest number of studies had been exclusively assigned to rotavirus. Our review vividly represents that Rotavirus genotype G1P [8] is the dominant sereotype although other sereotypes and non-typeable strains were observed as well. Given that this disease has a high prevalence among the Iranian children population, vaccination could be a helpful factor to be taken into consideration. Before the advent of the Rotavirus vaccine, this virus has been the main cause of children’s gastroenteritis illness, but after the vaccination in some developed countries such as the USA, the rate of morbidity and acute gastroenteritis demonstrated a significant decline. Right now, two vaccines related to Rotavirus, namely, RotaTeq (RV5) and Rotarix (RV1), are recommended by the World Health Organization for the routine immunization of infants (72). Rotavirus vaccines have been introduced into the national immunization program of 82 countries; it is essential to mention that these vaccines do not cover all the strains of Rotavirus; therefore, further investigations on vaccination and this viral agent are necessary (72).
In Iran, not many experiments and studies have been performed on other viral agents such as human Parechovirus (2 studies), Norovirus (5 studies), Bocavirus (4 studies), Astrovirus (4 studies), Sapovirus (2 studies), and Aichivirus (1 study). Therefore, further investigation over other pathogenic agents associated with other viral gastroenteritis is far essential (73).
The very first record of SARS-CoV-2 associated with the gastrointestinal tract was reported in China (73). Significantly, the detection and proof of SARS-CoV-2 in patient fecal samples have increased great concerns toward COVID-19 disease control (74). In a review article studied by Gupta and his team, it was pointed out that COVID-19 diarrhea has mostly been observed 1-8 days after the disease onset, with a medium period of 3.3 days (10,75). It also has been reported that a number of patients had diarrhea as the initial symptom, and one-third of diarrhea has been watery, and it lasted for 1-14 days (76).
In addition, the viral receptor angiotensin-converting enzyme 2 was found to be expressed in gastrointestinal epithelial cells (77). In one study, 39 out of 73 COVID-19-positive patients (53%) had positive SARS-CoV-2 RNA in their stool samples, and the duration was reported as 1-12 days. What is highly essential in this case is that 17 patients still had positive RNA stool samples after receiving the negative test result from the respiratory tract (78). In one research, 8 out of 10 children tested positive for rectal swabs regardless of the negative nasopharyngeal tract. Another study verified viral shedding in children with COVID-19 and reported diarrhea in three out of ten infected children (11). The evident continuous updates have provided noteworthy information related to the feasible transmission of COVID-19 through the oral route. Studies suggest that the gastroenteritis tract may shed the virus, and oral-fecal transition might be feasible (79). The majority of public health initiatives to manage and prevent viral gastroenteritis infections have been concentrated on identifying and managing outbreaks (80). Considering that foodborne exposures generate a substantial share of illnesses, efforts have been made to develop techniques for identifying and eradicating virus infection from food sources (81). Furthermore, regular hand washing, avoiding contact with virus-infected individuals, and disinfecting contaminated environmental surfaces are all recommended in this regard (82). It is important to note that gastroenteritis in young children, particularly in infancy, decreased after the rotavirus vaccine was introduced in Taiwan (83). Therefore, more studies on vaccination and the effects of vaccines and different strains of various gastroenteritis agents are needed to have more sufficient control over the illness.
Conclusion
In general, considering that the prevalence of this disease is still high, current data collection indicates that research into viral gastroenteritis agents, and more especially, their nucleic acid genotypes, will have a significant impact on efforts to prevent viral gastroenteritis agents in humans, particularly newborns, children, and vulnerable adults.
Acknowledgments
This work was supported by the Digestive Disease Research Institute, Tehran University of Medical Sciences.
Authors’ Contribution
NM contributed for bibliographic review and written paper, MAM guaranteed for statistical and graphic notes, MP and GGJR added for bibliographic review and AM contributed for discussion and final manuscript correction.
Conflict of Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Not applicable.
References
- Elliott EJ. Acute gastroenteritis in children. BMJ 2007; 334(7583):35-40. doi: 10.1136/bmj.39036.406169.80 [Crossref] [ Google Scholar]
- Florez ID, Niño-Serna LF, Beltrán-Arroyave CP. Acute infectious diarrhea and gastroenteritis in children. Curr Infect Dis Rep 2020; 22(2):4. doi: 10.1007/s11908-020-0713-6 [Crossref] [ Google Scholar]
- Zbinden A. [Acute viral gastroenteritis: viruses other than norovirus]. Praxis (Bern 1994) 2019; 108(5):335-9. doi: 10.1024/1661-8157/a003182 [Crossref] [ Google Scholar]
- Shah MP, Hall AJ. Norovirus illnesses in children and adolescents. Infect Dis Clin North Am 2018; 32(1):103-18. doi: 10.1016/j.idc.2017.11.004 [Crossref] [ Google Scholar]
- Mohamadkhani A, Pourasgari M, Saveh M, Fazli H, Shahnazari P, Poustchi H. Association of delta-aminolevulinic acid dehydratase gene variant with serum level of alanine aminotransferase. Hepat Mon 2019; 19(8):e94664. doi: 10.5812/hepatmon.94664 [Crossref] [ Google Scholar]
- Fletcher SM, McLaws ML, Ellis JT. Prevalence of gastrointestinal pathogens in developed and developing countries: systematic review and meta-analysis. J Public Health Res 2013; 2(1):42-53. doi: 10.4081/jphr.2013.e9 [Crossref] [ Google Scholar]
- Mohamadkhani A MS, Fazli H R, Mirzaei S, Sharafkhah M, Besharat S, Montazeri G, Poustchi H. 8-Hydroxy-2’-Deoxyguanosin in Peripheral Leukocyte Associated With HBsAg in Patients with Chronic Hepatitis B. Jundishapur J Microbiol 2017; 10(5):e42609. [ Google Scholar]
- Glass RI, Bresee J, Jiang B, Gentsch J, Ando T, Fankhauser R. Gastroenteritis viruses: an overview. Novartis Found Symp 2001; 238:5-19. doi: 10.1002/0470846534.ch2 [Crossref] [ Google Scholar]
- Chen CJ, Wu FT, Huang YC, Chang WC, Wu HS, Wu CY. Clinical and epidemiologic features of severe viral gastroenteritis in children: a 3-year surveillance, multicentered study in Taiwan with partial rotavirus immunization. Medicine (Baltimore) 2015; 94(33):e1372. doi: 10.1097/md.0000000000001372 [Crossref] [ Google Scholar]
- Gupta R, Beg S, Jain A, Bhatnagar S. Paediatric COVID-19 and the GUT. Indian J Med Microbiol 2020; 38(3 & 4):261-4. doi: 10.4103/ijmm.IJMM_20_331 [Crossref] [ Google Scholar]
- Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020; 26(4):502-5. doi: 10.1038/s41591-020-0817-4 [Crossref] [ Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136 [Crossref] [ Google Scholar]
- Wilhelmi I, Roman E, Sánchez-Fauquier A. Viruses causing gastroenteritis. Clin Microbiol Infect 2003; 9(4):247-62. doi: 10.1046/j.1469-0691.2003.00560.x [Crossref] [ Google Scholar]
- Capece G, Gignac E. Norovirus. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022.
- Estes MK, Cohen J. Rotavirus gene structure and function. Microbiol Rev 1989; 53(4):410-49. doi: 10.1128/mr.53.4.410-449.1989 [Crossref] [ Google Scholar]
- Cortez V, Meliopoulos VA, Karlsson EA, Hargest V, Johnson C, Schultz-Cherry S. Astrovirus Biology and Pathogenesis. Annu Rev Virol 2017; 4(1):327-48. doi: 10.1146/annurev-virology-101416-041742 [Crossref] [ Google Scholar]
- Cupelli K, Stehle T. Viral attachment strategies: the many faces of adenoviruses. Curr Opin Virol 2011; 1(2):84-91. doi: 10.1016/j.coviro.2011.05.024 [Crossref] [ Google Scholar]
- Calvo C, García-García ML, Pozo F, Carvajal O, Pérez-Breña P, Casas I. Clinical characteristics of human bocavirus infections compared with other respiratory viruses in Spanish children. Pediatr Infect Dis J 2008; 27(8):677-80. doi: 10.1097/INF.0b013e31816be052 [Crossref] [ Google Scholar]
- Sasaki J, Taniguchi K. Aichi virus 2A protein is involved in viral RNA replication. J Virol 2008; 82(19):9765-9. doi: 10.1128/jvi.01051-08 [Crossref] [ Google Scholar]
- Oka T, Wang Q, Katayama K, Saif LJ. Comprehensive review of human sapoviruses. Clin Microbiol Rev 2015; 28(1):32-53. doi: 10.1128/cmr.00011-14 [Crossref] [ Google Scholar]
- Sano K, Hamada H, Hirose S, Sugiura K, Harada S, Koizumi M. Prevalence and characteristics of human parechovirus and enterovirus infection in febrile infants. Pediatr Int 2018; 60(2):142-7. doi: 10.1111/ped.13467 [Crossref] [ Google Scholar]
- Malik YA. Properties of coronavirus and SARS-CoV-2. Malays J Pathol 2020; 42(1):3-11. [ Google Scholar]
- Tariverdi M, Farahbakhsh N, Gouklani H, Khosravifar F, Tamaddondar M. Dysentery as the only presentation of COVID-19 in a child: a case report. J Med Case Rep 2021; 15(1):65. doi: 10.1186/s13256-021-02672-1 [Crossref] [ Google Scholar]
- Moradveisi B, Ataee P, Ghaffarieh A, Karimi A, Fattahi N, Nasseri K. Diarrhea as a presenting symptom of coronavirus disease 2019 in children. Adv Biomed Res 2020; 9:35. doi: 10.4103/abr.abr_90_20 [Crossref] [ Google Scholar]
- Ekbatani MS, Hassani SA, Tahernia L, Yaghmaei B, Mahmoudi S, Navaeian A. Atypical and novel presentations of coronavirus disease 2019: a case series of three children. Br J Biomed Sci 2021; 78(1):47-52. doi: 10.1080/09674845.2020.1785102 [Crossref] [ Google Scholar]
- Taghinejad M, Ghaderi M, Mousavi Nasab SD. High frequency of aichivirus in children with acute gastroenteritis in Iran. Pediatr Infect Dis J 2020; 39(7):576-9. doi: 10.1097/inf.0000000000002638 [Crossref] [ Google Scholar]
- Farsi M, Roodbari F, Nejati B, Arashkia A, Jalilvand S, Nateghian A. Prevalence and genetic diversity of norovirus genogroup II in children less than 5 years of age with acute gastroenteritis in Tehran, Iran. Med Microbiol Immunol 2018; 207(3-4):201-10. doi: 10.1007/s00430-018-0541-6 [Crossref] [ Google Scholar]
- Shams S, Mousavi Nasab SD, Heydari H, Tafaroji J, Ahmadi N, Shams Afzali E. Detection and characterization of rotavirus G and P types from children with acute gastroenteritis in Qom, central Iran. Gastroenterol Hepatol Bed Bench 2020; 13(Suppl1):S128-S33. [ Google Scholar]
- Farahmand M, Jalilvand S, Arashkia A, Shahmahmoodi S, Afchangi A, Mollaei-Kandelous Y. Association between circulating rotavirus genotypes and histo-blood group antigens in the children hospitalized with acute gastroenteritis in Iran. J Med Virol 2021; 93(8):4817-23. doi: 10.1002/jmv.26808 [Crossref] [ Google Scholar]
- Arashkia A, Bahrami F, Farsi M, Nejati B, Jalilvand S, Nateghian A. Molecular analysis of human adenoviruses in hospitalized children < 5 years old with acute gastroenteritis in Tehran, Iran. J Med Virol 2019; 91(11):1930-6. doi: 10.1002/jmv.25539 [Crossref] [ Google Scholar]
- Lorestani N, Moradi A, Teimoori A, Masodi M, Khanizadeh S, Hassanpour M. Molecular and serologic characterization of rotavirus from children with acute gastroenteritis in northern Iran, Gorgan. BMC Gastroenterol 2019; 19(1):100. doi: 10.1186/s12876-019-1025-x [Crossref] [ Google Scholar]
- Azaran A, Makvandi M, Samarbafzadeh A, Neisi N, Hoseinzadeh M, Rasti M. Study on rotavirus infection and its genotyping in children below 5 years in south west Iran. Iran J Pediatr 2016; 26(2):e2080. doi: 10.5812/ijp.2080 [Crossref] [ Google Scholar]
- Azaran A, Makvandi M, Teimoori A, Ebrahimi S, Heydari F, Nikfar R. Distribution of rotavirus genotypes ccirculating in Ahvaz, Iran in 2016. Iran Biomed J 2018; 22(2):107-16. doi: 10.22034/ibj.22.2.107 [Crossref] [ Google Scholar]
- Mousavi Nasab SD, Zali F, Kaghazian H, Aghasadeghi MR, Mardani R, Gachkar L. Prevalence of astrovirus, adenovirus, and sapovirus infections among Iranian children with acute gastroenteritis. Gastroenterol Hepatol Bed Bench 2020; 13(Suppl1):S122-S7. [ Google Scholar]
- Mousavi Nasab SD, Sabahi F, Kaghazian H, Paryan M, Mirab Samiee S, Ghaderi M. A real-time RT-PCR assay for genotyping of rotavirus. Iran Biomed J 2020; 24(6):399-404. doi: 10.29252/ibj.24.6.394 [Crossref] [ Google Scholar]
- Mousavi Nasab SD, Sabahi F, Makvandi M, Mirab Samiee S, Nadji SA, Ravanshad M. Epidemiology of Rotavirus-Norovirus Co-Infection and Determination of Norovirus Genogrouping among Children with Acute Gastroenteritis in Tehran, Iran. Iran Biomed J 2016; 20(5):280-6. doi: 10.22045/ibj.2016.05 [Crossref] [ Google Scholar]
- Sharifi-Rad J, Hoseini Alfatemi SM, Sharifi-Rad M, Miri A. Frequency of adenoviruses, rotaviruses and noroviruses among diarrhea samples collected from infants of Zabol, southeastern Iran. Jundishapur J Microbiol 2015; 8(3):e15440. doi: 10.5812/jjm.15440 [Crossref] [ Google Scholar]
- Monavari SH, Noorbakhsh S, Mollaie H, Fazlalipour M, Abedi Kiasari B. Human bocavirus in Iranian children with acute gastroenteritis. Med J Islam Repub Iran 2013; 27(3):127-31. [ Google Scholar]
- Kargar M, Khodadadi P, Najafi A, Ansari H. Predominance of rotavirus G8 genotype in hospitalized children with acute gastroenteritis in Yasuj, Iran. Eur Rev Med Pharmacol Sci 2014; 18(5):699-702. [ Google Scholar]
- Khoshdel A, Parvin N, Doosti A, Eshraghi A. Prevalence and molecular characterization of rotaviruses as causes of nosocomial diarrhea in children. Turk J Pediatr 2014; 56(5):469-74. [ Google Scholar]
- Jadali F, Karimi A, Fallah F, Zahraei M, Esteghamati A, Navidinia M. A survey on rotavirus associated diarrhea in 5 main cities of Iran. Arch Pediatr Infect Dis 2013; 1(1):23-6. doi: 10.5812/pedinfect.6431 [Crossref] [ Google Scholar]
- Shokrollahi MR, Noorbakhsh S, Monavari HR, Ghavidel Darestani S, Vosoughi Motlagh A, Javadi Nia S. Acute nonbacterial gastroenteritis in hospitalized children: a cross sectional study. Jundishapur J Microbiol 2014; 7(12):e11840. doi: 10.5812/jjm.11840 [Crossref] [ Google Scholar]
- Kargar M, Najafi A, Zandi K, Hashemizadeh Z. Genotypic distribution of rotavirus strains causing severe gastroenteritis in children under 5 years old in Borazjan, Iran. Afr J Microbiol Res 2011; 5(19):2936-41. [ Google Scholar]
- Najafi A, Najafi S, Vahdat K, Kargar M, Javdani N. Importance of viral pathogens in children with acute gastroenteritis in the south of Iran. Ann Saudi Med 2013; 33(2):124-9. doi: 10.5144/0256-4947.2013.124 [Crossref] [ Google Scholar]
- Motamedifar M, Amini E, Talezadeh Shirazi P. Frequency of rotavirus and adenovirus gastroenteritis among children in Shiraz, Iran. Iran Red Crescent Med J 2013; 15(8):729-33. doi: 10.5812/ircmj.4415 [Crossref] [ Google Scholar]
- Romani S, Mohebbi SR, Khanyaghma M, Azimzadeh P, Bozorgi SM, Damavand B. Detection of human bocavirus 1, 2 and 3 from patients with acute gastroenteritis. Gastroenterol Hepatol Bed Bench 2013; 6(Suppl 1):S77-81. [ Google Scholar]
- Romani S, Azimzadeh P, Mohebbi SR, Bozorgi SM, Zali N, Jadali F. Prevalence of sapovirus infection among infant and adult patients with acute gastroenteritis in Tehran, Iran. Gastroenterol Hepatol Bed Bench 2012; 5(1):43-8. [ Google Scholar]
- Rezaei M, Sohrabi A, Edalat R, Siadat SD, Gomari H, Rezaei M. Molecular epidemiology of acute gastroenteritis caused by subgenus F (40, 41) enteric adenoviruses in inpatient children. Lab Med 2012 Jan 1; 43(1):10-5. [ Google Scholar]
- Hamedi A, Sadeghian A, Syedi J. Incidence of Adenovirus diarrhea in children under 6 years referred to the pediatric emergency and clinic of Ghaem hospital, Mashhad, Iran. Iran J Pediatr Soc 2010; 2(2).
- Romani S, Mohebbi SR, Hosseini SM, Azimzadeh P, Vahedi M, Derakhshan F. Prevalence of norovirus infection in children and adults with acute gastroenteritis, Tehran, Iran, 2008-2009. Food Environ Virol 2012; 4(1):1-5. doi: 10.1007/s12560-011-9071-8 [Crossref] [ Google Scholar]
- Ataei-Pirkooh A, Shahrabadi M, Haghi-Ashtiani M. Incidence of coinfection between rotavirus and some enteropathogenic agents in children referred to Children Medical Center Hospital, Tehran, 2009. Iran J Virol 2011; 5(1):23-7. doi: 10.21859/isv.5.1.23 [Crossref] [ Google Scholar]
- Ghazi F, Ataei Z, Dabirmanesh B. Molecular detection of human parechovirus type 1 in stool samples from children with diarrhea. Int J Infect Dis 2012; 16(9):e673-6. doi: 10.1016/j.ijid.2012.05.1020 [Crossref] [ Google Scholar]
- Ghorashi Z, Ghalehgolab Behbahan A, Abdoli Oskouei S. Rotavirus enteric infection in children of northwest Iran. Pediatr Infect Dis J 2011; 30(7):616-8. doi: 10.1097/INF.0b013e31820a45cb [Crossref] [ Google Scholar]
- Nadji SA, Poos-Ashkan L, Khalilzadeh S, Baghaie N, Shiraghaei MJ, Hassanzad M. Phylogenetic analysis of human bocavirus isolated from children with acute respiratory illnesses and gastroenteritis in Iran. Scand J Infect Dis 2010; 42(8):598-603. doi: 10.3109/00365540903582442 [Crossref] [ Google Scholar]
- Esteghamati A, Gouya M, Keshtkar A, Najafi L, Zali MR, Sanaei M. Sentinel hospital-based surveillance of rotavirus diarrhea in iran. J Infect Dis 2009; 200 Suppl 1:S244-7. doi: 10.1086/605050 [Crossref] [ Google Scholar]
- Emamghorashi F, Rajabi S, Shadman A. Frequency of rotavirus infection in children with acute gastroenteritis in Jahrom, south of Iran. Iran J Med Sci 2008; 33(2):84-7. [ Google Scholar]
- Sadeghian A, Hamedi A, Sadeghian M, Sadeghian H. Incidence of rotavirus diarrhea in children under 6 years referred to the Pediatric Emergency and Clinic of Ghaem Hospital, Mashhad, Iran. Acta Med Iran 2010; 48(4):263-5. [ Google Scholar]
- Nakhaei Sistani R, Sadeghizadeh M, Saderi H, Kharazani Tafreshi N, Behmanesh M, Shirzad H. Detection of Types 40 and 41 Adenoviruses in Stool Samples of Diarrheal Children by Solid Phase PCR. Iran J Biotechnol 2007; 5(1):42-7. [ Google Scholar]
- Hamkar R, Yahyapour Y, Noroozi M, Nourijelyani K, Jalilvand S, Adibi L. Prevalence of rotavirus, adenovirus, and astrovirus infections among patients with acute gastroenteritis in, Northern Iran. Iran J Public Health 2010; 39(2):45-51. [ Google Scholar]
- Modaress S, Rahbarimanesh AA, Edalat R, Sohrabi A, Modarres S, Gomari H. Human rotavirus genotypes detection among hospitalized children, a study in Tehran, Iran. Arch Iran Med 2011; 14(1):39-45. [ Google Scholar]
- Barari Savadkoohi R, Ahmadpour-Kacho M, Yahyapour Y. Prevalence of viral gastroenteritis in children with acute gastroenteritis in Babol, Iran. J Pediatr Infect Dis 2007; 2(4):211-4. doi: 10.1055/s-0035-1557051 [Crossref] [ Google Scholar]
- Farahtaj F, Gallimore CI, Iturriza-Gomara M, Taremi M, Zali MR, Edalatkhah H. Rotavirus VP7, VP4 and VP6 genotypes co-circulating in Tehran, Iran, between 2003 and 2004. Epidemiol Infect 2007; 135(5):834-8. doi: 10.1017/s0950268806007485 [Crossref] [ Google Scholar]
- Modarres S, Rahbarimanesh AA, Karimi M, Modarres S, Motamedi-Rad M, Sohrabi A. Electrophoretic RNA genomic profiles of rotavirus strains prevailing among hospitalized children with acute gastroenteritis in Tehran, Iran. Arch Iran Med 2008; 11(5):526-31. [ Google Scholar]
- Kazemi A, Tabatabaie F, Agha-Ghazvini MR, Kelishadi R. The role of rotavirus in acute pediatric diarrhea in Isfahan, Iran. Pak J Med Sci 2006; 22(3):282-5. [ Google Scholar]
- Zarnani AH, Modarres S, Jadali F, Sabahi F, Moazzeni SM, Vazirian F. Role of rotaviruses in children with acute diarrhea in Tehran, Iran. J Clin Virol 2004; 29(3):189-93. doi: 10.1016/s1386-6532(03)00123-9 [Crossref] [ Google Scholar]
- Khalili B, Cuevas LE, Reisi N, Dove W, Cunliffe NA, Hart CA. Epidemiology of rotavirus diarrhoea in Iranian children. J Med Virol 2004; 73(2):309-12. doi: 10.1002/jmv.20092 [Crossref] [ Google Scholar]
- Samarbafzadeh A, Tehrani EM, Makvandi M, Taremi M. Epidemiological aspects of rotavirus infection in Ahwaz, Iran. J Health Popul Nutr 2005; 23(3):245-9. [ Google Scholar]
- Saderi H, Roustai MH, Sabahi F, Sadeghizadeh M, Owlia P, De Jong JC. Incidence of enteric adenovirus gastroenteritis in Iranian children. J Clin Virol 2002; 24(1-2):1-5. doi: 10.1016/s1386-6532(01)00206-2 [Crossref] [ Google Scholar]
- Amini S, Solati AA, Fayaz A, Mahmoodi M. Rotavirus infection in children with acute diarrhea in Tehran. Med J Islam Repub Iran 1990; 4(1):25-8. [ Google Scholar]
- De R, Liu L, Qian Y, Zhu R, Deng J, Wang F. Risk of acute gastroenteritis associated with human bocavirus infection in children: a systematic review and meta-analysis. PLoS One 2017; 12(9):e0184833. doi: 10.1371/journal.pone.0184833 [Crossref] [ Google Scholar]
- Foulongne V, Segondy M. [Human bocavirus (HBoV)]. Pathol Biol (Paris) 2009; 57(2):197-202. doi: 10.1016/j.patbio.2008.01.001 [Crossref] [ Google Scholar]
- Kirkwood CD, Ma LF, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine 2019; 37(50):7328-35. doi: 10.1016/j.vaccine.2017.03.076 [Crossref] [ Google Scholar]
- Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5(7):667-78. doi: 10.1016/s2468-1253(20)30126-6 [Crossref] [ Google Scholar]
- Amirian ES. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int J Infect Dis 2020; 95:363-70. doi: 10.1016/j.ijid.2020.04.057 [Crossref] [ Google Scholar]
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J. SARS-CoV-2 infection in children. N Engl J Med 2020; 382(17):1663-5. doi: 10.1056/NEJMc2005073 [Crossref] [ Google Scholar]
- D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol 2020; 18(8):1663-72. doi: 10.1016/j.cgh.2020.04.001 [Crossref] [ Google Scholar]
- Patel KP, Vunnam SR, Patel PA, Krill KL, Korbitz PM, Gallagher JP. Transmission of SARS-CoV-2: an update of current literature. Eur J Clin Microbiol Infect Dis 2020; 39(11):2005-11. doi: 10.1007/s10096-020-03961-1 [Crossref] [ Google Scholar]
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020; 158(6):1831-3. doi: 10.1053/j.gastro.2020.02.055 [Crossref] [ Google Scholar]
- Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible?. Lancet Gastroenterol Hepatol 2020; 5(4):335-7. doi: 10.1016/s2468-1253(20)30048-0 [Crossref] [ Google Scholar]
- Monroe SS. Control and prevention of viral gastroenteritis. Emerg Infect Dis 2011; 17(8):1347-8. doi: 10.3201/eid1708.110824 [Crossref] [ Google Scholar]
- Maalouf H, Schaeffer J, Parnaudeau S, Le Pendu J, Atmar RL, Crawford SE. Strain-dependent norovirus bioaccumulation in oysters. Appl Environ Microbiol 2011; 77(10):3189-96. doi: 10.1128/aem.03010-10 [Crossref] [ Google Scholar]
- Bányai K, Estes MK, Martella V, Parashar UD. Viral gastroenteritis. Lancet 2018; 392(10142):175-86. doi: 10.1016/s0140-6736(18)31128-0 [Crossref] [ Google Scholar]
- Lu MC, Shia BC, Kao YW, Lin SC, Wang CY, Lin WC. The impact of rotavirus vaccination in the prevalence of gastroenteritis and comorbidities among children after suboptimal rotavirus vaccines implementation in Taiwan: a population-based study. Medicine (Baltimore) 2021; 100(25):e25925. doi: 10.1097/md.0000000000025925 [Crossref] [ Google Scholar]