Avicenna Journal of Clinical Microbiology and Infection. 9(2):77-80.
doi: 10.34172/ajcmi.2022.12
Original Article
MicroRNA155 May Be an Important Part of Human Papilloma Virus-Related Cancers
Banafsheh Douzandeh-Mobarrez 1  , Ashraf Kariminik 1, *
, Ashraf Kariminik 1, *  , Babak Kheirkhah 2
, Babak Kheirkhah 2 
Author information:
1Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran
2Department of Veterinary Medicine, Baft Branch, Islamic Azad University, Baft, Iran
*
Corresponding author: Ashraf Kariminik, Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran, Tel: 00983431321376, Cell phone: 00989133413556 Email:
a.kariminik@iauk.ac.ir
Abstract
Background: Micro-RNAs (miRs) play several roles during infections with viruses. Therefore, the roles of miR21 and mir155 in the induction of the viral-related cancers have been the focus of attention in several studies. High risk human papilloma viruses (HPVs) are the main factors negatively contributing to the induction of HPV-related cancers. This study aimed to evaluate the expression of miR21 and mir155 in the patients with HPV-high risk genotypes in order to explore the roles of the miRs in the induction of HPV-related cancers.
Methods: In this study, 40 women infected with the high-risk HPV genotypes as well as 40 healthy controls were examined regarding the relative expression of miR21 and mir155 by adopting real-time polymerase chain reaction (PCR) technique. U6 was used for data normalization of miRNAs.
Results: Relative expressions of both miR21 and miR155 were significantly higher in the HPV-infected patients compared to those in non-infected women.
Conclusions: It was concluded that miR21 and miR155 may have played key roles in the induction of HPV-related cancers among Iranian patients.
Keywords: Human papilloma virus, MicroRNA, Cervical cancer
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Douzandeh-Mobarrez B, Kariminik A, Kheirkhah B. MicroRNA155 may be an important part of human papilloma virus-related cancers. Avicenna J Clin Microbiol Infect. 2022; 9(2):77-80. doi:10.34172/ajcmi.2022.12
Introduction
Human papilloma viruses (HPVs) are presently the most prevalent viruses in the world and are considered as important inducers of the cervical cancers (1). Furthermore, HPVs participate in the pathogenesis of anal, head, and neck cancers (2). Recent investigations have revealed that more than 200 HPV genotypes are prevalent among the women and approximately ~80% of HPV-related cervical cancers are associated with infections with HPV genotype-16 (HPV16), 18, 31, 33 and 35 (3). Since the viruses use the internal factor to induce cancers, it is generally argued that micro-RNAs (miRs) may be the targets of the viruses (4). It has been confirmed that cervical cancers are the multifactorial disorders and miRs are the crucial factors responsible for determining the HPV infection outcome. It has been also reported that miRs are the small RNAs that modulate the stabilities of the mRNAs and regulate the translation process (5). Although these are the physiological functions of the miRs, they play pivotal roles in the induction and development of the human cancers via up- and down-regulation of oncogenes and suppressor genes, respectively. Accordingly, it has been determined that miR21 and miR155 are the important miRs to induce several tumors, including breast and cervical cancers (6-8). For example, miR21 has been documented to target the tumor inhibitor proteins and be capable of inducing resistance to radiation and chemotherapy (9). Mir155 also employs several mechanisms to manipulate cancer cells. For instance, over-expression of miR155 can inhibit the expression of caspase 3, an important molecule for inducing apoptosis (10). Since HPV is a main risk factor for the induction of cervical cancers, its association with the increased expression of miRs may also be a main risk factor for cervical cancers. This study, therefore, aimed to evaluate the vagina epithelial cells miR21 and miR155 expression levels of the healthy women in comparison to those of the infected women with HPV16, 18, 31, 33 and 35 genotypes.
Materials and Methods
This study was performed from July to December, 2021 to investigate the outpatients HPV-infected and non-infected women referring to the Niknafs Maternity Ward Center for annually checkup. The participants were examined regarding the infection by HPV16, 18, 31, 33, and 35 genotypes, as the most prevalent and high-risk genotype in the Rafsanjan city, Iran. Accordingly, 40 women infected with HPV16, 18, 31, 33, and 35 genotypes, as well as 40 non-infected women were selected and confirmed by real-time polymerase chain reaction (PCR). The infected patients and healthy controls aged 40 ± 10 and 42 ± 13, respectively. The control group was selected from the non-infected participants with the same nationality (i.e., Iranian), and their age ranged between 30-55 years. The controls were not infected previously by HPV or other sexual transmitted microbes. Therefore, the participants were all Iranian females without history of cancers, sexually transmitted diseases, and autoimmunity. They were not also in the pregnancy and lactating time. The wet pap smear samples were taken by an obstetrician and transferred immediately to the laboratory in transferring media.
HPV-DNA Purification
HPV-DNA was purified using a commercial kit (Karmania Pars Gene Company, Kerman, Iran). The samples (200 µL) from the transferring media were mixed with the 500 µL lysis solution, and the precipitation solution (500 µL) was added and mixed gently after 5 minutes incubation at 65ºC. The mixtures were transferred to the high absorbance column and centrifuged (16 000 g for 1 minute) and followed by washing buffer. Finally, the purified HPV-DNA was separated from the columns via adding 50 µL pre-warmed DNase/RNase free water and centrifuging it at 16 000 g for 1 minute.
HPV Detection
A commercial kit from Karmania Pars Gene Company, Kerman, Iran (KPG-HPVHR), capable of detecting HPV16, 18, 31, 33 and 35 high risk genotypes, was used to confirm the HPV infection in the participants. Accordingly, the positive and negative cases were approved as HPV-infected and non-infected women, respectively.
MicroRNA Extraction and Specific cDNA Synthesize
MicroRNAs were purified using the microRNA-extraction kit from Karmania Pars Gene Company, Kerman, Iran. The protocol of microRNA extraction was similar to that of HPV-DNA extraction, except for using the specific column for microRNAs. To synthesize microRNA cDNA, the commercial kits from Karmania Pars Gene Company, Kerman, Iran were used, including miR21 and miR155 specific cDNA synthesize kits. The kit operates using a specific primer for cDNA synthesizing the microRNAs. The temperature program was as follows: 70ºC for 5 minutes, and 40ºC for 70 minutes followed by 90ºC for 5 minutes.
Relative Expression of MiR21 and MiR155
Relative expressions of miR21 and miR155, in parallel with U6 as housekeeping gene, were examined using the real-time PCR kits from Karmania Pars Gene Company, Kerman, Iran. A master mix, containing specific primers, was used to amplify the microRNAs in a Rotor-Gene instrument following the program of: 95ºC for 3 minutes and, then, 40 cycles with 95ºC for 15 seconds/60ºC for 20 seconds. Taking into account the efficacy more than 96 and 97% for miR21 and miR155, respectively, 2-∆∆Ct formula was finally used to analyze the raw data (11). All real-time PCR reactions were performed in duplicates.
Statistical Analysis
SPSS version 18 was used to analyze the miR21 and miR155 relative expressions, and the student t test was used for comparing the HPV-positive patients with the negative participants.
Results
According to the results of real-time PCR test, all patients were positive for HPV-DNA. The results also showed that expressions of miR21 and miR155 were elevated 8.43- and 8.83-folds, respectively, in the HPV positive patients compared to those in the healthy controls. In addition, the relative expressions of miR21 were 8.43 ± 3.48 and 1 ± 0.22 in the HPV positive and HPV negative women, respectively; while relative expressions of miR155 were 8.83 ± 3.02 and 1 ± 0.19 in the HPV positive and negative women, respectively. The results from t test revealed that the decreased expressions of both miR21 (P = 0.044) and miR155 (P = 0.015) were significant. Figure 1 illustrates the relative expressions of miR21 and miR155 in both HPV infected and non-infected women.
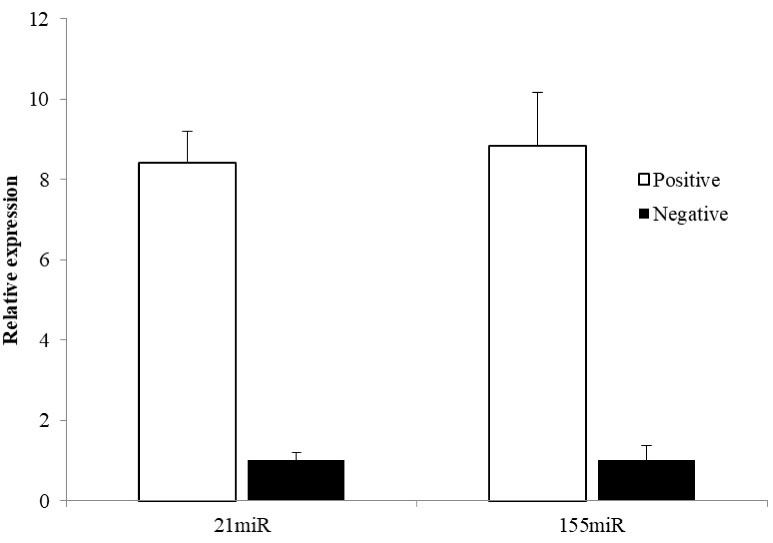
Figure 1.
Relative Expression of MiR21 and MiR155 in the HPV Infected in Comparison to Healthy Women. The statistical analysis showed that the relative expression of both miR21 (P = 0.044) and miR155 (P = 0.015) were significantly increased in the HPV infected women.
.
Relative Expression of MiR21 and MiR155 in the HPV Infected in Comparison to Healthy Women. The statistical analysis showed that the relative expression of both miR21 (P = 0.044) and miR155 (P = 0.015) were significantly increased in the HPV infected women.
Discussion
HPV detection for cervical cancer screening is commonly used as an early diagnostic guide to prevent the progression of cervical cancer (12). The wet pap smear (cytology) is a revolutionary method with a major public health impact that has changed the incidence of cervical cancer since its general introduction in screening programs (13). The miRNAs have been the subjects of some studied due to their association with HPV infection as the potential diagnostic and prognostic indicators (14). Some oncogenic miRNAs are the main responsible molecules for cervical cancers (15,16). The results confirmed that all HPV positive patients were infected with the high-risk HPVs, which had been already discovered to be the inducers of ~80% of cervical cancers (3). Therefore, the increased expression of the molecules associated with the infections may have been considered as the key candidate in the pathogenesis of HPV-related complications. The current results showed that the expression of either miR21 or miR155 was significantly increased in the women with the high-risk HPVs. Due to the important roles played by the HPV genotypes in the pathogenesis of ~80% of cervical cancers (3), it was suggested that both miR21 and miR155 may have participated in the pathogenesis of the HPV infections and its related complications among the Iranian population. Our study results were in line with the findings from the study by Park et al reporting that the concentrations of both miR21 and miR155 were increased in the cervical cancer compared to those in the non-cancerous tissue (17). Interestingly, they demonstrated that miR-155 increased the risk of the cancer to 27.9-folds for the HPV infected patients (17). In addition, several investigations have indicated that miR21 and miR155 play key roles in the induction of cervical cancers (18-21). Wang et al revealed that the expressions of miR-92a and miR-378 were associated with cancer progressions in HPV positive tissue samples (22). Furthermore, Hoelzle et al demonstrated that miR21 was upregulated in the cervical cancer tissue in comparison to healthy one, and in the HPV infected patients compared to the non-infected ones (23). Interestingly, the elevated expressions of the microRNA have been reported to be the key mechanisms for the escape from immunosurveillance (20). Therefore, the increased expression of miR21 and miR155 may downregulate the immune responses and, as the result, may lead to either acceleration in the process of tumor induction in the infected individuals, or replication and production of E6 and E7 oncoproteins by HPV. As mentioned previously, HPV uses both E6 and E7 oncoproteins to induce the HPV-related cancers. Therefore, it was argued that miR21 and miR155 may have been the key factors responsible for inducing the HPV-related cancers in direct and indirect formats.
Conclusions
It was concluded that HPV may have induced the expression of miR21 and miR155 to overcome the immune responses and, as a result, increased the HPV proliferation, caused downregulation of anti-cancerous molecules, and facilitated the proliferation of the cancer cells. Therefore, it was argued that targeting the miRs may have been considered as a key molecular therapy against HPV-related cancers. Since there was insufficient information about the relationship between the expressions of the molecules among Iranian population, especially among Kerman province population, it was recommended that further studies with larger sample sizes should be carried out to explore the given issue. Taking into account the fact that the induction of the HPV-related cancers is multifactorial, moreover, it was strongly suggested that other aspects of the immune system should be investigated.
Acknowledgments
The authors are grateful to the staff of the Niknafs Maternity Ward Center who helped them to collect the required samples, as well as to Islamic Azad University of Kerman for providing them with financial support partially.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships influencing the results reported in this paper.
Ethical Approval
All participants completed the required written informed consent form prior to enter the study, and the Ethical Committee of Islamic Azad University (IAU), Kerman Branch, approved the study protocol under IR.IAU.Kerman.REC.1400.009 code.
References
- Chen L, Riaz N, Lee N, McBride S. Current considerations for radiotherapy in HPV-associated head and neck cancer. J Surg Oncol 2021; 124(6):945-51. doi: 10.1002/jso.26689 [Crossref] [ Google Scholar]
- Alhamlan FS, Alfageeh MB, Al Mushait MA, Al-Badawi IA, Al-Ahdal MN. Human papillomavirus-associated cancers. Adv Exp Med Biol 2021; 1313:1-14. doi: 10.1007/978-3-030-67452-6_1 [Crossref] [ Google Scholar]
- Quinlan JD. Human papillomavirus: screening, testing, and prevention. Am Fam Physician 2021; 104(2):152-9. [ Google Scholar]
- Whiteside MA, Siegel EM, Unger ER. Human papillomavirus and molecular considerations for cancer risk. Cancer 2008; 113(10 Suppl):2981-94. doi: 10.1002/cncr.23750 [Crossref] [ Google Scholar]
- Bhaskaran M, Mohan M. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Vet Pathol 2014; 51(4):759-74. doi: 10.1177/0300985813502820 [Crossref] [ Google Scholar]
- Li N, Cui T, Guo W, Wang D, Mao L. MiR-155-5p accelerates the metastasis of cervical cancer cell via targeting TP53INP1. Onco Targets Ther 2019; 12:3181-96. doi: 10.2147/ott.s193097 [Crossref] [ Google Scholar]
- Lao G, Liu P, Wu Q, Zhang W, Liu Y, Yang L. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol 2014; 35(12):11933-8. doi: 10.1007/s13277-014-2479-7 [Crossref] [ Google Scholar]
- Lei C, Wang Y, Huang Y, Yu H, Huang Y, Wu L. Up-regulated miR155 reverses the epithelial-mesenchymal transition induced by EGF and increases chemo-sensitivity to cisplatin in human Caski cervical cancer cells. PLoS One 2012; 7(12):e52310. doi: 10.1371/journal.pone.0052310 [Crossref] [ Google Scholar]
- Javanmardi S, Aghamaali MR, Abolmaali SS, Mohammadi S, Tamaddon AM. miR-21, an oncogenic target miRNA for cancer therapy: molecular mechanisms and recent advancements in chemo and radio-resistance. Curr Gene Ther 2017; 16(6):375-89. doi: 10.2174/1566523217666170102105119 [Crossref] [ Google Scholar]
- Zheng SR, Guo GL, Zhai Q, Zou ZY, Zhang W. Effects of miR-155 antisense oligonucleotide on breast carcinoma cell line MDA-MB-157 and implanted tumors. Asian Pac J Cancer Prev 2013; 14(4):2361-6. doi: 10.7314/apjcp.2013.14.4.2361 [Crossref] [ Google Scholar]
- Nasiri E, Kariminik A. Up-regulation of AIM2 and TLR4 and down-regulation of NLRC4 are associated with septicemia. Indian J Med Microbiol 2021; 39(3):334-8. doi: 10.1016/j.ijmmb.2021.05.002 [Crossref] [ Google Scholar]
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141(4):664-70. doi: 10.1002/ijc.30716 [Crossref] [ Google Scholar]
- G Gocze K, Gombos K, Juhasz K, Kovacs K, Kajtar B, Benczik M. Unique microRNA expression profiles in cervical cancer. Anticancer research 2013; 33(6):2561-7. [ Google Scholar]
- Kim JY, Park S, Nam BH, Roh JW, Lee CH, Kim YH. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. Journal of clinical oncology 2009; 27(30):5088-93. [ Google Scholar]
- Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of Known and Novel Small RNAs in Human Cervical Cancer. Google Patents; 2010.
- Gocze K, Gombos K, Juhasz K, Kovacs K, Kajtar B, Benczik M. Unique microRNA expression profiles in cervical cancer. Anticancer Res 2013; 33(6):2561-7. [ Google Scholar]
- Park S, Eom K, Kim J, Bang H, Wang HY, Ahn S. MiR-9, miR-21, and miR-155 as potential biomarkers for HPV positive and negative cervical cancer. BMC Cancer 2017; 17(1):658. doi: 10.1186/s12885-017-3642-5 [Crossref] [ Google Scholar]
- Gocze K, Gombos K, Kovacs K, Juhasz K, Gocze P, Kiss I. MicroRNA expressions in HPV-induced cervical dysplasia and cancer. Anticancer Res 2015; 35(1):523-30. [ Google Scholar]
- Tornesello ML, Faraonio R, Buonaguro L, Annunziata C, Starita N, Cerasuolo A. The role of microRNAs, long non-coding RNAs, and circular RNAs in cervical cancer. Front Oncol 2020; 10:150. doi: 10.3389/fonc.2020.00150 [Crossref] [ Google Scholar]
- He G, Ding J, Zhang Y, Cai M, Yang J, Cho WC. microRNA-21: a key modulator in oncogenic viral infections. RNA Biol 2021; 18(5):809-17. doi: 10.1080/15476286.2021.1880756 [Crossref] [ Google Scholar]
- Zamani S, Sohrabi A, Hosseini SM, Rahnamaye-Farzami M, Akbari A. Deregulation of miR-21 and miR-29a in cervical cancer related to HPV infection. Microrna 2019; 8(2):110-5. doi: 10.2174/2211536607666181017124349 [Crossref] [ Google Scholar]
- Wang X, Wang HK, Li Y, Hafner M, Banerjee NS, Tang S. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc Natl Acad Sci U S A 2014; 111(11):4262-7. doi: 10.1073/pnas.1401430111 [Crossref] [ Google Scholar]
- Hoelzle CR, Arnoult S, Borém CRM, Ottone M, de Magalhães K, da Silva IL. MicroRNA levels in cervical cancer samples and relationship with lesion grade and HPV infection. Microrna 2021; 10(2):139-45. doi: 10.2174/2211536610666210604123534 [Crossref] [ Google Scholar]