Avicenna Journal of Clinical Microbiology and Infection. 9(2):70-76.
doi: 10.34172/ajcmi.2022.11
Original Article
The Relationship Between Positive Cultures With Multidrug-Resistant Microorganisms and Mortality in a General Intensive Care Unit
Bahareh Marghoob 1  , Malihe Khosravi-Khezri 2, *
, Malihe Khosravi-Khezri 2, * 
Author information:
1Department of Nephrology, Hasheminejad Kidney Center, Iran University of Medical Sciences, Tehran, Iran
2Department of Clinical Pharmacy, Hasheminejad Kidney Center, Iran University of Medical Sciences, Tehran, Iran
*
Corresponding author: Malihe Khosravi-Khezri, Hasheminejd Kidney Center, Vali-Nejad Street, Vanak Square Vali-e-Asr Boulevard, Tehran, Iran. Tel: +982181161 Fax: 88644497 Email:
malihe1989khosravi@gmail.com
Abstract
Aim: The patients in the intensive care unit (ICU) are more susceptible to healthcare-associated infections (HAI). Higher rates of nosocomial infections in ICU patients are associated with higher morbidity, mortality, and costs. The primary outcome of our study was to investigate the relationship between antibiotic resistance and mortality in ICU patients, and the secondary outcome was to evaluate the relationship between antibiotic resistance and the length of ICU or hospital stay.
Methods: A 5-year observational retrospective study was conducted on patients in the ICU of Hasheminejad Kidney Center affiliated with Iran University of Medical Sciences, Tehran, Iran from January 1, 2015, to January 1, 2020. The data related to age, gender, admission type, comorbidities, length of ICU stay, length of hospital stay, infection source, microorganism type, and resistance pattern of all isolates and outcomes were collected based on the study purpose.
Results: During the 5-year study, 2899 patients were admitted to the ICU, but only 747 patients were enrolled in the study, including 426 males (57%). The median age was 65 years (19-97 years). The mean length of ICU stay was greater in culture-positive patients (8.42 vs. 3.5 days, P>0.001). Culture-positive patients had significantly higher mortality compared to culture-negative patients (63.8% vs. 36.2%, P>0.001). In our study, it was found that resistant microorganisms have increased mortality by 2.6 times in the ICU in the crude model (OR: 2.6, P>0.001).
Conclusion: The findings of our study suggest that multidrug-resistant pathogens increase ICU stay and mortality.
Keywords: Intensive Care Unit, Mortality, Resistant microorganisms
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Marghoob B, Khosravi Khezri M. The relationship between positive cultures with multidrug-resistant microorganisms and mortality in a general intensive care unit. Avicenna J Clin Microbiol Infect. 2022; 9(2):70-76. doi:10.34172/ajcmi.2022.11
Introduction
The patients in the intensive care unit (ICU) are more susceptible to healthcare-associated infections (HAI) because of the dysregulation of the immune response and host defense reduction, invasive procedures (mechanical ventilation, vascular access, and urinary catheter), more severe disease, and broad-spectrum antibiotics administration. Higher rates of nosocomial infections in ICU patients are related to higher morbidity, mortality, and costs (1-3).
The occurrence of resistant infections such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae, and carbapenem-resistant Enterobacteriaceae, especially in the ICU has increased in recent years. These resistant infections have negative effects on patient survival (1-3).
Microorganisms such as MRSA,methicillin-resistant Staphylococcus epidermidis (MRSE),VRE,andESBL-producing Enterobacteriaceae are considered resistant.
Multi-drug resistance (MDR) organism is determined by resistance to at least one agent in three or more antimicrobial categories. Extensive drug resistance is defined as resistance to at least one agent in all but two or fewer antimicrobial categories (i.e., bacterial isolates remain susceptible to only one or two categories), and pan-drug resistance (PDR) is referred to as non-susceptibility to all agents in all antimicrobial categories (4).
It is estimated that about 650 000 people in the United States develop nosocomial infections each year, of which 20% are caused by resistant microorganisms (5,6).
Several studies demonstrated that patients admitted to the ICU who are undergoing invasive procedures are more infected with resistant microorganisms (1,7). This can increase mortality by up to 70% in ICU patients (2,8,9).
In recent years, overuse and misuse of antibiotics have led to antibiotic resistance in various countries, which is a major threat to the world (3). It is estimated that approximately 20%-50% of all antibiotics and 30% of antibiotic use in United States acute care hospitals are unnecessary or ineffective (10,11). Every year, at least 2.8 million people become infected with antibiotic-resistant infections in the United States, and more than 35 000 people die accordingly (12). The treatment of resistant infections is difficult and costly.
Infections caused by resistant microorganisms are associated with increased mortality and hospitalization (13,14).
Optimizing antibiotic prescription using antibiotic stewardship programs can reduce antibiotic resistance and improve the cure rate.
In Iran, antimicrobial resistance is a serious problem and there is no complete antimicrobial monitoring system for data gathering, analysis, and decision-making in this regard. It is important to determine the local antibiotic resistance pattern in each center because it helps prescribe more appropriate antibiotics.
The aim of our study was to investigate the association between resistant microorganisms and length of ICU stay and mortality. In addition, we determined the pattern of antibiotic resistance of more frequent infections at our ICU center. It was hypothesized that ICU stay and mortality rates were higher in patients with resistant infections.
Methods
We performed a 5-year observational retrospective study. All adult patients (≥18 years) admitted to the general ICU (6 beds) of Hasheminejad Kidney Center affiliated to Iran University of Medical Sciences, Tehran, Iran from January 1, 2015 to January 1, 2020 enrolled in the study. The inclusion criterion was to have at least one microbial culture during their stay in the ICU. On the other hand, patients with incomplete or missing data were excluded from the study.
Patient characteristics were retrospectively collected, including age, gender, admission type, comorbidities, length of ICU stay, length of hospital stay, infection source, microorganism type, and resistance pattern of all isolates and outcomes.
The samples of blood, urine, trachea/sputum, catheter, wound, and other bodily fluids and tissues with a clinical suspicion of infection were taken for testing. If the patients had more than one positive culture from different sites, it was considered a separate infection.
Statistical analyses were performed using SPSS-21 software. Data were reported as the mean or median, as well as frequencies or percentages for quantitative and qualitative variables, respectively.
Bivariate analyses were performed to indicate the associations between infection by resistant pathogens and death. Then, multivariate logistic regression models were developed to identify multiple variables associated with our outcome. Moreover, the association between length of ICU stay and death was assessed using a bivariate model. Ultimately, multiple covariates in multivariate analyses were tested to assess whether there were any associations between these variables and the outcome.
The odds ratios (ORs) with 95% confidence intervals estimated from the beta coefficients, were calculated, and P > 0.05 was considered statistically significant.
Results
During the 5-year study, 2899 patients were admitted to the ICU, but only 747 patients were enrolled in the study. They included 426 males (57%). The median age was 65 years (19-97 years, Table 1).
Table 1.
Demographic Data and Clinical Features of ICU Patients
|
Characteristic, No. (%)
|
Patients (N=747)
|
| Age (y) |
65 (19-97) |
| Gender |
|
| Male |
426 (57%) |
| Female |
321 (43%) |
| Admission category |
|
| Nephrology |
492 (66) |
| Urology |
200 (26.8) |
| Surgery |
14 (1.9) |
| Missing |
40 (5.4) |
| Comorbidities |
|
| Hypertension |
168 (22.5) |
| Diabetes mellitus |
162 (21.7) |
| End-stage kidney disease |
218 (29.2) |
| Heart disease |
132 (17.7) |
| Chronic kidney disease |
122 (16.3) |
| Malignancy |
102 (13.7) |
| Transplantation |
55 (7.4) |
| Obstructive uropathy |
127 (17) |
| Neurologic disorders |
22 (3) |
| Glomerulonephritis |
23 (3.1) |
| Other |
126 (16.9) |
Note. ICU: Intensive care unit.
The mean patient age in culture-positive and culture-negative groups was 65 and 62 years, respectively (Table 1). Two hundred and twenty-seven (60.85%) and 198 (53.22%) patients in the culture-positive and culture-negative groups were males, respectively (Table 2).
Table 2.
Major Clinical and Demographic Characteristic of Patients With Negative and Positive Culture Results
|
|
Culture Status
|
P
Value
|
|
Culture +
|
Culture -
|
|
No. (%)
|
No. (%)
|
| Age (mean) |
65 |
62 |
0.017 |
| Gender |
|
|
|
| Male |
227 (60.69) |
198 (53.08) |
0.035 |
| Female |
147 (39.30) |
175 (46.91) |
| Hospital stay (day) |
17.04 |
10.54 |
> 0.001 |
| ICU stay (day) |
8.42 |
3.50 |
> 0.001 |
| Admission type |
|
|
|
| Nephrology |
237 (48.2) |
255 (51.8) |
0.15 |
| Urology |
110 (55) |
90 (45) |
| Surgery |
9 (64.3) |
5 (35.7) |
| Outcome |
|
|
|
| Live |
205 (42.5) |
277 (57.5) |
> 0.001 |
| Dead |
169 (63.8) |
96 (36.2) |
| Year |
|
|
|
| 2015 |
88 (49.2) |
91 (50.8) |
0.22 |
| 2016 |
68 (48.6) |
72 (51.4) |
| 2017 |
75 (47.2) |
84 (52.8) |
| 2018 |
82 (49.4) |
84 (50.6) |
| 2019 |
61 (59.2) |
42 (40.8) |
Note. ICU: Intensive care unit.
During the study period, 374 patients (50.1%) developed at least one culture positive. Of them, 138 (18.45%), 116 (15.5%), 167 (22.3%), 49 (6.5%), and 20 (2.6%) cases had positive blood culture, positive urine culture, positive culture related to the respiratory tract, positive catheter culture, and positive wound culture, respectively.
Two hundred and sixty-five patients (35.5%) died during hospital admission. The overall mortality in different years is indicated in Table 3.
Table 3.
Overall Mortality, ICU Stay, Hospital Stay, and Infection Site by Years
|
|
Year
|
|
2015
|
2016
|
2017
|
2018
|
2019
|
| Patients in the ICU (N) |
179 |
140 |
159 |
166 |
103 |
| In-hospital mortality (%) |
36.9 |
43.6 |
32.1 |
31.3 |
34 |
| ICU stay, days (median) |
3 (0.5-60) |
2 (0.5-88) |
3 (0.5-119) |
3 (0.5-63) |
4 (0.5-63) |
| Hospital stay, days (median) |
8.9 (2-60.8) |
8.9 (2-89.17) |
9.4 (2-122.3) |
8.9 (2-72.75) |
11.38 (2-82.88) |
| Infection site, No. (%) |
|
|
|
|
|
| Blood |
28 (15.6) |
21 (15) |
24 (15.1) |
24 (14.5) |
15 (14.6) |
| Urine |
21 (11.7) |
24 (17.1) |
19 (11.9) |
18 (10.8) |
15 (14.6) |
| Trachea/sputum |
35 (19.6) |
32 (22.9) |
29 (18.2) |
24 (14.5) |
24 (23.3) |
| Catheter |
7 (3.9) |
8 (5.7) |
6 (3.8) |
10 (6) |
6 (5.8) |
| Wound |
3 (1.7) |
3 (2.1) |
0 (0) |
5 (3) |
5 (4.9) |
| Others |
15 (8.4) |
13 (9.3) |
20 (12.6) |
12 (7.2) |
10 (9.7) |
Note. ICU: Intensive care unit.
Five hundred and seventy-one microorganisms were isolated of whom 458 (80.2%) cases were considered MDR. The most frequently isolated organisms were E. coli (24%), Acinetobacter baumannii (19%), Klebsiella pneumonia (16%), Staphylococcus epidermidis (13%), and Staphylococcus aureus (11%). The distribution of various microorganisms is illustrated in Figure 1.
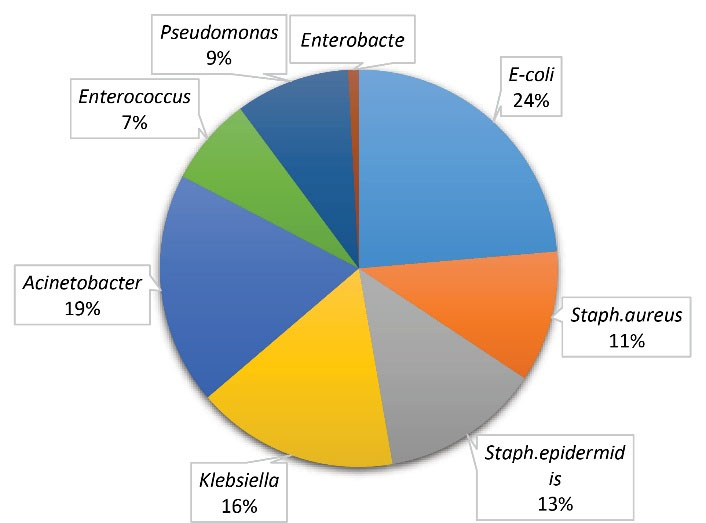
Figure 1.
Microorganisms Isolated From ICU Patients. Note. ICU: Intensive care unit.
.
Microorganisms Isolated From ICU Patients. Note. ICU: Intensive care unit.
The mean length of ICU stay was greater in culture-positive patients compared to culture-negative patients (8.42 vs. 3.5 days, P > 0.001). In addition, culture-positive patients had significantly higher mortality in comparison to culture-negative patients (63.8% vs. 36.2%, P > 0.001).
Microbial isolates collected from any sources are presented in Table 4. We evaluated the variations in the frequency of microorganisms and MDR isolates for 5 years. Figures 2 and 3 display the trend of microorganism frequencies and MDR pathogens over 5 years. The incidence of MDR isolates had variations across the study period. For example, the incidence of A. baumannii PDR decreased over years, but that of VRE incidence represented an increase. The incidence of other MDR pathogens did not have any significant changes.
Table 4.
Most Frequent Microorganisms Obtained in ICU Patients From Different Samples
|
|
Blood
|
Urine
|
Tracheal/Sputum
|
Catheter
|
Wound
|
Other
|
Total
|
|
Escherichia coli
|
|
|
|
|
|
|
|
| Sensitive |
4 |
7 |
2 |
0 |
0 |
7 |
20 |
| ESBL |
27 |
36 |
18 |
5 |
2 |
27 |
115 |
|
Staphylococcus aureus
|
|
|
|
|
|
|
|
| MSSA |
5 |
0 |
4 |
7 |
1 |
3 |
20 |
| MRSA |
11 |
6 |
16 |
4 |
1 |
3 |
41 |
|
Staphylococcus epidermidis
|
|
|
|
|
|
|
|
| MSSE |
20 |
3 |
6 |
8 |
1 |
4 |
42 |
| MRSE |
11 |
6 |
8 |
5 |
0 |
2 |
32 |
|
Klebsiella pneumoniae
|
|
|
|
|
|
|
|
| Sensitive |
0 |
1 |
0 |
1 |
0 |
1 |
3 |
| KPC |
23 |
19 |
27 |
3 |
5 |
13 |
90 |
| PDR |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
|
Acinetobacter baumannii
|
|
|
|
|
|
|
|
| Sensitive |
0 |
0 |
2 |
0 |
0 |
0 |
2 |
| ESBL |
1 |
1 |
5 |
3 |
1 |
0 |
11 |
| PDR |
16 |
9 |
50 |
7 |
4 |
9 |
95 |
|
Enterococcus
|
|
|
|
|
|
|
|
| Sensitive |
2 |
9 |
5 |
1 |
0 |
3 |
20 |
| VRE |
6 |
7 |
3 |
1 |
3 |
1 |
21 |
|
Pseudomonas aeruginosa
|
|
|
|
|
|
|
|
| Sensitive |
1 |
2 |
2 |
1 |
0 |
0 |
6 |
| ESBL |
5 |
6 |
7 |
0 |
1 |
3 |
22 |
| PDR |
5 |
3 |
9 |
3 |
1 |
4 |
25 |
|
Enterobacter
|
|
|
|
|
|
|
|
| Sensitive |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| ESBL |
1 |
1 |
2 |
0 |
0 |
1 |
5 |
| Total |
138 |
116 |
167 |
49 |
20 |
81 |
571 |
Note. ICU: Intensive care unit. ESBL: Extended-spectrum beta-lactamase; MSSA: Methicillin-sensitive Staphylococcus aureus; MRSA: Methicillin-resistant Staphylococcus aureus; SE: Staphylococcus epidermidis; MSSE: Methicillin-sensitive Staphylococcus epidermidis; MRSE: Methicillin-resistant Staphylococcus epidermidis; KPC: Klebsiella pneumoniae carbapenemase, PDR: Pandrug-resistance; VRE: Vancomycin-resistant enterococcus.
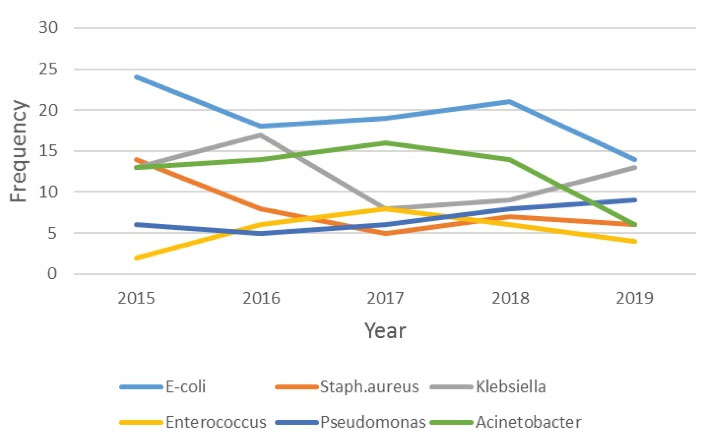
Figure 2.
Annual Changing of Microorganisms Frequencies in ICU. Note. ICU: Intensive care unit.
.
Annual Changing of Microorganisms Frequencies in ICU. Note. ICU: Intensive care unit.
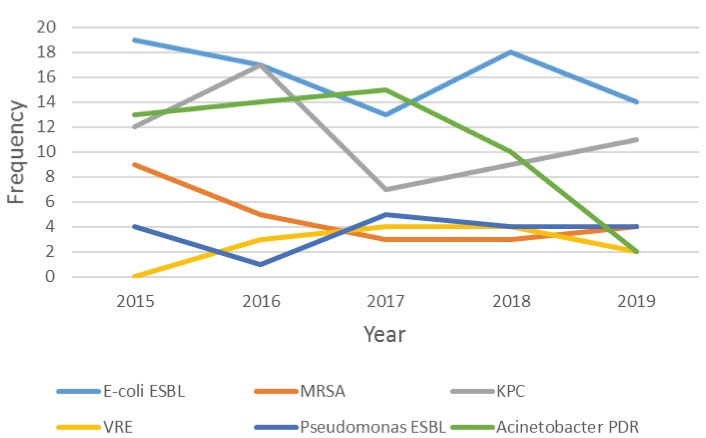
Figure 3.
Annual Changing of MDR Microorganisms Frequencies in ICU. Note. MDR: Multidrug-resistant; ICU: Intensive care unit.
.
Annual Changing of MDR Microorganisms Frequencies in ICU. Note. MDR: Multidrug-resistant; ICU: Intensive care unit.
In our center’s laboratory, the criteria of the Clinical and Laboratory Standards Institute (CLSI) are used to assess the susceptibility to antimicrobial agents.
In the current study, it was demonstrated that resistant microorganisms have increased mortality by 2.6 times in the ICU in the crude model (OR: 2.6, P > 0.001). In the multivariate regression analysis, the initial OR has changed in the presence of urinary tract infection and pneumonia (OR: 2.92, OR: 1.6, respectively).
In the final multivariate regression analysis, in the presence of urinary tract infection and pneumonia, resistant microorganisms have increased mortality by 1.7 times in the ICU (Table 5).
Table 5.
Multivariate Analysis to Identify Variables Associated With Mortality Caused by Resistance Microorganisms Using Logistic Regression
|
|
B
|
SE
|
Wald
|
df
|
P
Value
|
OR
|
| Step 1a |
Resistance |
0.571 |
0.202 |
8.026 |
1 |
0.005 |
1.770 |
| Respiratory tract |
1.401 |
0.214 |
42.816 |
1 |
0.000 |
4.059 |
| Urine |
-0.188 |
0.257 |
.539 |
1 |
0.463 |
0.828 |
| Constant |
-1.040 |
0.102 |
104.477 |
1 |
0.000 |
0.353 |
Note. OR: Odds ratio, SE, standard error.
a Variable(s) entered on step 1: Resistance, respiratory tract, and urine.
The β of the length of ICU stay on death in a crude model was 0.074 and changed in the multivariate regression analysis model in the presence of pneumonia (β = 0.041, Table 6).
Table 6.
Multivariate Analysis to Identify Variables Associated With Mortality Caused by ICU Stay Using Logistic Regression
|
|
B
|
SE
|
Wald
|
df
|
P
Value
|
Exp(B)
|
| Step 1a |
ICU stay (day) |
.041 |
.013 |
10.438 |
1 |
.001 |
1.042 |
| Respiratory tract |
1.259 |
.223 |
31.889 |
1 |
.000 |
3.521 |
| Constant |
-1.129 |
.108 |
109.827 |
1 |
.000 |
.323 |
Note. ICU: Intensive care unit, SE, standard error.
aVariable(s) entered on step 1: ICU stay (day) and respiratory tract.
Discussion
Antibiotic resistance is an important problem throughout the world, especially in the healthcare settings such as hospitals. Knowing the local resistance pattern is of great value in guiding empiric antimicrobial therapy and infection control. Infections caused by resistant microorganisms are difficult to treat because there are limited effective antimicrobial alternatives. Furthermore, it is associated with more hospital stays and mortality (15,16).
In agreement with other studies (5,17,18), the most prevalent positive culture in our study was culture related to the respiratory tract.
In our study, it was found that more than 50% of ICU patients had at least one positive culture. The most prevalent and resistant microorganisms were E. coli and A. baumannii, respectively. The results of our study conform to those of several studies in Iran (19,20) and other countries (21,22), confirming that E. coli was the most common pathogen. Our findings are in concordance with the results of Saxena et al, indicating that most of the Acinetobacter and Klebsiella species were MDR (2).
In our survey, 80.2% of isolates were MDR, which is more than the MDR pathogen rate reported by Cornejo-Juárez et al (1). This can be due to excessive and irrational administration of antibiotics and delays in the initiation of effective and appropriate antimicrobial therapy in our community.
In the present study, the net number of resistant isolates (E. coli ESBL, MRSA, MDR Acinetobacter, and Klebsiella pneumoniae carbapenemase) reduced slightly over 5 years, which is probably due to the more organized antibiotic prescription and curbing inappropriate use of broad-spectrum antibiotics.
The ICU mortality is high and had been reported from 9% to 38% (23). This mortality rate can increase up to 70% in patients with infections caused by resistant pathogens (24).
In our study, in-hospital mortality was 35.5%. The ICU mortality was reported at 40.3% in patients with antimicrobial resistance infections in the study of Lakbar et al (25), which is compatible with the results of Colpan et al (24).
In another study on patients with hematological malignancy admitted to the ICU, in-hospital mortality was reported at 46% (26). The higher mortality rate can be due to the underlying disease of patients. Furthermore, in the study performed on patients admitted to surgical ICU, Saxena et al indicated that the overall mortality was 28%, and only 4 patients died due to infectious causes (2).
According to our results, mortality was significantly more frequent in culture-positive patients (63.8%) compared to culture-negative patients (36.2%, P > 0.001), which is in line with the findings of a multicenter study in Brazil (27) and the study of Toufen et al (28). Moreover, the ICU stay (8.42 vs. 3.5 days) and hospital stay (17.04 vs. 10.54 days) were significantly longer in the culture-positive group (P > 0.001). Similarly, Vincent et al and Blot et al identified a strong relationship between infection with ICU stay and hospital stay (27,29).
In the present study, it was observed that positive culture related to the respiratory tract increases mortality, which corroborates with the results of previous studies (25,30). Bonnet et al (16) demonstrated that 51.7% of patients with lung infections died and pulmonary infections increased the length of stay (P > 0.001). However, the reason is not clear.
The relationship between resistant pathogens and mortality is debated in various studies. We found that MDR microorganisms increased mortality by 2.6 folds. Some studies have revealed an association between resistant isolates and mortality (1,16,24,31-37), whereas other studies did not report such an association (29,38-41). Although the reason is unclear, it might be partly due to the low sample size of studies and differences in the study population. The higher mortality in the resistant group could be due to more virulence of resistant microorganisms or inappropriate empiric antibiotic treatment.
Conclusion
In general, the findings of our study suggest that MDR pathogens increase ICU stay and mortality. As a result, prevention and treatment strategies and rational administration of antibiotics to prevent the development of resistant microorganisms are highly important.
Acknowledgments
We would like to thank Dr. Taghvaye-Masoumi for his guidance and Dr. Mohsen Shatti for data analysis. We also appreciate the nurses of the general ICU of Hasheminejad Kidney Center for their support.
Authors’ Contribution
BM contributed to data gathering. MKK participated in idea, study design, data collection, and manuscript drafting and finalizing. All authors read and approved the final manuscript.
Conflict of Interests
There is no conflict of interests.
Ethical Approval
This study has been approved by local committee of Hasheminejad Kidney Center, Iran University of Medical Sciences.
Funding
Nil.
References
- Cornejo-Juárez P, Vilar-Compte D, Pérez-Jiménez C, Ñamendys-Silva SA, Sandoval-Hernández S, Volkow-Fernández P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int J Infect Dis 2015; 31:31-4. doi: 10.1016/j.ijid.2014.12.022 [Crossref] [ Google Scholar]
- Saxena S, Priyadarshi M, Saxena A, Singh R. Antimicrobial consumption and bacterial resistance pattern in patients admitted in ICU at a tertiary care center. J Infect Public Health 2019; 12(5):695-9. doi: 10.1016/j.jiph.2019.03.014 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Prevention of Hospital-Acquired Infections: A Practical Guide. Geneva, Switzerland: WHO; 2002.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3):268-81. doi: 10.1111/j.1469-0691.2011.03570.x [Crossref] [ Google Scholar]
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370(13):1198-208. doi: 10.1056/NEJMoa1306801 [Crossref] [ Google Scholar]
- Roberts RR, Scott RD 2nd, Hota B, Kampe LM, Abbasi F, Schabowski S. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care 2010; 48(11):1026-35. doi: 10.1097/MLR.0b013e3181ef60a2 [Crossref] [ Google Scholar]
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) StudyEPIC International Advisory Committee. JAMA 1995; 274(8):639-44. [ Google Scholar]
- Albrich WC, Angstwurm M, Bader L, Gärtner R. Drug resistance in intensive care units. Infection 1999; 27 Suppl 2:S19-23. doi: 10.1007/bf02561665 [Crossref] [ Google Scholar]
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118(1):146-55. doi: 10.1378/chest.118.1.146 [Crossref] [ Google Scholar]
- Frattari A, Savini V, Polilli E, Di Marco G, Lucisano G, Corridoni S. Control of Gram-negative multi-drug resistant microorganisms in an Italian ICU: rapid decline as a result of a multifaceted intervention, including conservative use of antibiotics. Int J Infect Dis 2019; 84:153-62. doi: 10.1016/j.ijid.2019.04.002 [Crossref] [ Google Scholar]
- Dhillon R, Clark J. Infection in the intensive care unit (ICU). Curr Anaesth Crit Care 2009; 20(4):175-82. doi: 10.1016/j.cacc.2009.01.003 [Crossref] [ Google Scholar]
-
https://www.cdc.gov/drugresistance/index.html.
- MacVane SH. Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J Intensive Care Med 2017; 32(1):25-37. doi: 10.1177/0885066615619895 [Crossref] [ Google Scholar]
- Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis 2004; 39(1):31-7. doi: 10.1086/420816 [Crossref] [ Google Scholar]
- Lin MF, Lan CY. Antimicrobial resistance in Acinetobacter baumannii: from bench to bedside. World J Clin Cases 2014; 2(12):787-814. doi: 10.12998/wjcc.v2.i12.787 [Crossref] [ Google Scholar]
- Bonnet V, Dupont H, Glorion S, Aupée M, Kipnis E, Gérard JL. Influence of bacterial resistance on mortality in intensive care units: a registry study from 2000 to 2013 (IICU Study). J Hosp Infect 2019; 102(3):317-24. doi: 10.1016/j.jhin.2019.01.011 [Crossref] [ Google Scholar]
- Luna CM, Rodriguez-Noriega E, Bavestrello L, Guzmán-Blanco M. Gram-negative infections in adult intensive care units of Latin America and the Caribbean. Crit Care Res Pract 2014; 2014:480463. doi: 10.1155/2014/480463 [Crossref] [ Google Scholar]
- Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180(10):1345-55. doi: 10.1001/jamainternmed.2020.3539 [Crossref] [ Google Scholar]
- Khalili H, Dashti-Khavidaki S, Shahidi MR, Abdollahi A, Jafari S, Jahangard-Rafsanjani Z. Changes in gram negative microorganisms’ resistance pattern during 4 years period in a referral teaching hospital; a surveillance study. Daru 2012; 20(1):28. doi: 10.1186/2008-2231-20-28 [Crossref] [ Google Scholar]
- Samanipour A, Dashti-Khavidaki S, Abbasi MR, Abdollahi A. Antibiotic resistance patterns of microorganisms isolated from nephrology and kidney transplant wards of a referral academic hospital. J Res Pharm Pract 2016; 5(1):43-51. doi: 10.4103/2279-042x.176559 [Crossref] [ Google Scholar]
- Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP. Emerging resistance among bacterial pathogens in the intensive care unit--a European and North American Surveillance study (2000-2002). Ann Clin Microbiol Antimicrob 2004; 3:14. doi: 10.1186/1476-0711-3-14 [Crossref] [ Google Scholar]
- Bouchillon SK, Badal RE, Hoban DJ, Hawser SP. Antimicrobial susceptibility of inpatient urinary tract isolates of gram-negative bacilli in the United States: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009-2011. Clin Ther 2013; 35(6):872-7. doi: 10.1016/j.clinthera.2013.03.022 [Crossref] [ Google Scholar]
- de Oliveira AC, Silva RS, Díaz ME, Iquiapaza RA. Bacterial resistance and mortality in an intensive care unit. Rev Lat Am Enfermagem 2010; 18(6):1152-60. doi: 10.1590/s0104-11692010000600016 [Crossref] [ Google Scholar]
- Colpan A, Akinci E, Erbay A, Balaban N, Bodur H. Evaluation of risk factors for mortality in intensive care units: a prospective study from a referral hospital in Turkey. Am J Infect Control 2005; 33(1):42-7. doi: 10.1016/j.ajic.2004.09.005 [Crossref] [ Google Scholar]
- Lakbar I, Medam S, Ronflé R, Cassir N, Delamarre L, Hammad E. Association between mortality and highly antimicrobial-resistant bacteria in intensive care unit-acquired pneumonia. Sci Rep 2021; 11(1):16497. doi: 10.1038/s41598-021-95852-4 [Crossref] [ Google Scholar]
- Exner M, Bhattacharya S, Christiansen B, Gebel J, Goroncy-Bermes P, Hartemann P. Antibiotic resistance: what is so special about multidrug-resistant gram-negative bacteria?. GMS Hyg Infect Control 2017; 12:Doc05. doi: 10.3205/dgkh000290 [Crossref] [ Google Scholar]
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302(21):2323-9. doi: 10.1001/jama.2009.1754 [Crossref] [ Google Scholar]
- Toufen C Jr, Franca SA, Okamoto VN, Salge JM, Carvalho CR. Infection as an independent risk factor for mortality in the surgical intensive care unit. Clinics (Sao Paulo) 2013; 68(8):1103-8. doi: 10.6061/clinics/2013(08)07 [Crossref] [ Google Scholar]
- Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis 2002; 34(12):1600-6. doi: 10.1086/340616 [Crossref] [ Google Scholar]
- Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011; 11(1):30-8. doi: 10.1016/s1473-3099(10)70258-9 [Crossref] [ Google Scholar]
- Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 1998; 157(2):531-9. doi: 10.1164/ajrccm.157.2.9705064 [Crossref] [ Google Scholar]
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002; 165(7):867-903. doi: 10.1164/ajrccm.165.7.2105078 [Crossref] [ Google Scholar]
- Kwa AL, Low JG, Lee E, Kurup A, Chee HL, Tam VH. The impact of multidrug resistance on the outcomes of critically ill patients with gram-negative bacterial pneumonia. Diagn Microbiol Infect Dis 2007; 58(1):99-104. doi: 10.1016/j.diagmicrobio.2006.11.014 [Crossref] [ Google Scholar]
- Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 2007; 65(3):204-11. doi: 10.1016/j.jhin.2006.11.010 [Crossref] [ Google Scholar]
- Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 2010; 31(12):1250-6. doi: 10.1086/657135 [Crossref] [ Google Scholar]
- Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 2012; 38(12):1930-45. doi: 10.1007/s00134-012-2695-9 [Crossref] [ Google Scholar]
- Dabar G, Harmouche C, Salameh P, Jaber BL, Jamaleddine G, Waked M. Community- and healthcare-associated infections in critically ill patients: a multicenter cohort study. Int J Infect Dis 2015; 37:80-5. doi: 10.1016/j.ijid.2015.05.024 [Crossref] [ Google Scholar]
- Peres-Bota D, Rodriguez H, Dimopoulos G, DaRos A, Mélot C, Struelens MJ. Are infections due to resistant pathogens associated with a worse outcome in critically ill patients?. J Infect 2003; 47(4):307-16. doi: 10.1016/s0163-4453(03)00100-2 [Crossref] [ Google Scholar]
- Ortega B, Groeneveld AB, Schultsz C. Endemic multidrug-resistant Pseudomonas aeruginosa in critically ill patients. Infect Control Hosp Epidemiol 2004; 25(10):825-31. doi: 10.1086/502303 [Crossref] [ Google Scholar]
- Daniels TL, Deppen S, Arbogast PG, Griffin MR, Schaffner W, Talbot TR. Mortality rates associated with multidrug-resistant Acinetobacter baumannii infection in surgical intensive care units. Infect Control Hosp Epidemiol 2008; 29(11):1080-3. doi: 10.1086/591456 [Crossref] [ Google Scholar]
- Pinheiro MR, Lacerda HR, Melo RG, Maciel MA. Pseudomonas aeruginosa infections: factors relating to mortality with emphasis on resistance pattern and antimicrobial treatment. Braz J Infect Dis 2008; 12(6):509-15. doi: 10.1590/s1413-86702008000600013 [Crossref] [ Google Scholar]