Avicenna Journal of Clinical Microbiology and Infection. 9(2):49-54.
doi: 10.34172/ajcmi.2022.08
Original Article
Role of Multidrug-resistant Pathogens in Ventilator-Associated Pneumonia in a Tertiary Care Hospital in India
Sadiya Fatima 1, *  , Mustafeed Uddin 1, P.L. Tapasya Rao 1, S.Rajeshwar Rao 1
, Mustafeed Uddin 1, P.L. Tapasya Rao 1, S.Rajeshwar Rao 1
Author information:
1Department of Microbiology, Gandhi Medical College and Hospital, Secunderabad, Telangana, India
*
Corresponding author: Sadiya Fatima, Department of Microbiology, Gandhi Medical College and Hospital, Secunderabad, Telangana, India. Tel: 7842404119, Email:
gdrsadiya1991@gmail.com
Abstract
Aim: Ventilator-associated pneumonia (VAP) is the second most common infection acquired in the intensive care unit (ICU). Bacteriological profiles cause VAP and their susceptibility patterns vary in different institutions.
Methods: A prospective study was conducted from June 2017 to May 2018 in a tertiary care hospital as per the recent NHSN guidelines in finding the incidence of VAP and further determining the etiological agents by both conventional and automated methods. The combination disk method (Phenotypic confirmatory test), ampicillin C (AmpC) disk test, modified carbapenem inactivation method, imipenem/ethylenediamine tetraacetic acid combined disc test, and cefoxitin disk test were performed for the detection of extended-spectrum beta-lactamases (ESBL), AmpC β-lactamases, carbapenemases, metallo-beta-lactamases (MBL), and methicillin-resistant Staphylococcus aureus, respectively.
Results: Among 104 patients, 31 cases developed PVAP (possible VAP) during their ICU stay; of these cases, two patients had two episodes of VAP each, and the incidence of VAP was 32%. The most common isolate was Acinetobacter baumannii (38%), followed by Pseudomonas aeruginosa (22%), Klebsiella pneumoniae (16%), and Escherichia coli (13.51%). Twenty (54%) of the 37 VAP pathogens were multidrug resistant. ESBL was produced by 40% and 67% of E. coli and K. pneumoniae, respectively. MBL was produced by 25% of P. aeruginosa. In addition, AmpC beta-lactamases were produced by 18% each of the Enterobacteriaceae and non-fermenters, respectively. One of the two S. aureus isolates was methicillin-resistant.
Conclusion: The majority of VAP cases in our setting were caused by highly resistant strains. The frequency of specific multidrug resistance pathogens causing VAP may vary due to hospital, patient population, exposure to antibiotics, type of ICU patients, and changes over time, emphasizing the need for timely local surveillance data.
Keywords: Ventilator-associated pneumonia, Extended-spectrum beta-lactamase, Modified carbapenem inactivation method, Intensive care unit, Metallo-beta-lactamase, Multidrug resistance
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Fatima S, Uddin M, Rao PLT, Rao SR. Role of multidrug-resistant pathogens in ventilator-associated pneumonia in a tertiary care hospital in india. Avicenna J Clin Microbiol Infect. 2022; 9(2):49-54. doi:10.34172/ajcmi.2022.08
Introduction
Ventilator-associated pneumonia (VAP) refers to bacterial pneumonia developed in patients who have been mechanically ventilated for more than 48 hours (1). Although mechanical ventilation is a life-saving intervention, it has its own potential complications. Newer antibiotics in the past decade have not decreased VAP-associated mortality in critical care facilities across the world (2).
Gram-negative organisms such as Pseudomonas spp., Acinetobacter spp., Escherichia coli (E. coli), and Klebsiella pneumoniae,along with Gram-positive organism Staphylococcus aureus (S. aureus) were identified as the common VAP pathogens with varying prevalence rates. Pseudomonas spp., Acinetobacter spp., and Enterobacteriaceae are often multidrug resistant due to the production of extended-spectrum beta-lactamases (ESBL), ampicillin C (AmpC) β-lactamases, or metallo-β-lactamases (MBL) (3). This nosocomial infection increases morbidity and likely mortality, as well as the cost of health care (4). The initial empirical therapy can be modified based on the knowledge of local microbiological data, patient characteristics, and sensitivity patterns of expected pathogens in the institution (5).
This study was conducted to elucidate the bacteriological profile and antimicrobial resistance pattern of VAP among mechanically ventilated patients attending the respiratory intensive care unit (RICU) Department of Gandhi Hospital. The objectives of this study were to identify the pathogens and determine their antibiotic susceptibility patterns in addition to identifying multidrug resistance (MDR) by the presence of ESBL, AmpC β-lactamases, carbapenemases, and MBL in these VAP pathogens.
Materials and Methods
Study Design
This prospective study was conducted in the RICU of a tertiary care hospital in India from June 2017 to May 2018.
Setting
The Departments of Microbiology, Anesthesiology, and Critical Care were involved in this study. The study population included patients requiring ventilation who were admitted to the RICU.
Subject and Sample Size
Overall, 204 patients admitted to the RICU were prospectively evaluated during the study period. Among them, 28 patients (13.72%) were not intubated and thus were excluded from the study. Among those requiring mechanical ventilation, 72 (35.29%) patients were mechanically ventilated for less than 48 hours; therefore, they were excluded from the study. In general, 104 (50.98%) patients, who were mechanically ventilated for more than 48 hours, were monitored daily.
Data Collection Procedure
All the included patients were monitored using the recent clinical and microbiological criteria of the Center for Disease Control and Prevention and National Healthcare Safety Network (NHSN) at frequent intervals for the ventilator events until either discharge or death.
Criteria for VAP Diagnosis
Oxygen demand on the ventilator was measured by the fraction of inspired oxygen or positive end-expiratory pressure. Criteria for defining ventilator-associated conditions (VAC), infection-related ventilator-associated complications (IVAC), and possible VAP were considered by recent NHSN guidelines as described in our previous article (6).
Microbiological Techniques
Specimen Collection
Endotracheal aspirate, which is a non-invasive method, was chosen as a sample in the patient qualifying IVAC criteria.
Methods
The organisms isolated by quantitative culture were identified based on standard bacteriological procedures, including colony morphology and biochemical reactions such as oxidase, catalase, triple sugar iron, citrate, urease, and motility (7). The susceptibility of the clinical isolates to routinely used antibiotics was determined by the Kirby-Bauer disk diffusion method (8). Ampicillin (10 mcg), gentamicin (10 mcg), amikacin (30 mcg), ceftazidime (30 mcg), ceftriaxone (30 mcg), ciprofloxacin (5 mcg), meropenem (10 mcg), ticarcillin (75 mcg), and trimethoprim-sulphamethoxazole (25 mcg) were tested for Enterobacteriaceae. Further, amikacin (30 mcg), gentamicin (10 mcg), ciprofloxacin (5 mcg), piperacillin-tazobactam (100/10 mcg), ceftriaxone (30 mcg), ceftazidime (30 mcg), meropenem (10 mcg), ticarcillin (75 mcg), and trimethoprim-sulphamethoxazole (25 mcg) were tested for Pseudomonas and Acinetobacter species. Moreover, penicillin (10 units), cefoxitin (30 mcg), tetracycline (30 mcg), ciprofloxacin (5 mcg), erythromycin (15 mcg), clindamycin (2 mcg), vancomycin (30 mcg), linezolid (30 mcg), and trimethoprim-sulphamethoxazole (25 mcg) were tested for S. aureus. All the antibiotics were purchased from HiMedia (India). All these antibiotics were chosen for a particular organism as per recent Clinical and Laboratory Standards Institute (CLSI) guidelines from CLSI document M100S (9). Quality control for antibiogram was taken as S. aureus ATCC 25923 and E. coli ATCC 25922 for gram-positive and gram-negative organisms, respectively. Identification and AST were also performed by the VITEK® 2 (bioMerieux) automated method.
ESBL production among the members of Enterobacteriaceae was tested with the CLSI phenotypic disk diffusion confirmatory test using both cefotaxime (30 µg) and ceftazidime (30 µg) disks alone and in combination with clavulanic acid (10 µg). Five mm or more increase in the zone of inhibition for either cefotaxime-clavulanic acid (30/10 µg)) or ceftazidime-clavulanic acid (30/10 µg) disk, compared to the cefotaxime or ceftazidime disk alone was taken as the confirmatory evidence of ESBL production, respectively (10). K. pneumoniae ATCC700603 and E. coli ATCC 25922 were used as QC as per CLSI guidelines. Phenotypic methods for MDR detection is summarised in Table 1 and each one has been described below.
Table 1.
MDR Detection by Phenotypic Methods
|
For Gram-negative Organisms
|
For Gram-positive Organisms
|
| 1. ESBL phenotypic disk diffusion confirmatory test |
1. Cefoxitin (30 µg) disk diffusion method |
| 2. AmpC disk test |
| 3. mCIM test |
| 4. Imipenem/EDTA combined disc test |
Note. MRD: Multidrug resistance; ESBL: Extended-spectrum beta-lactamases; AmpC: Ampicillin C; mCIM: Modified carbapenem inactivation method; EDTA: Ethylenediamine tetraacetic acid.
AmpC disk test was performed for the detection of AmpC lactamase (11). A flattening or indentation of the cefoxitin (30 µg) inhibition zone in the vicinity of the disk with the test strain was interpreted as positive for the production of AmpC β-lactamase, while an undistorted zone was considered negative.
Modified carbapenem inactivation method (mCIM)was performed to detect carbapenemase (9). The diameter of the zone of inhibition around each MEM disk was measured, and a zone diameter of 6-15 mm or the presence of pinpoint colonies within a 16-18-mm zone was considered positive (carbapenemase production). On the other hand, zone diameters of 16-18 and ≥19 mm were considered indeterminate and negative non- carbapenemase producing, respectively.
Imipenem (IMP)/ethylenediamine tetraacetic acid (EDTA) combined disc test (CDT) was conducted using IMP and IMP with EDTA for the detection of MBL (12). The presence of an expanded growth inhibition zone of IPM and EDTA of >7 mm than IPM was interpreted as positive for MBL production.
Methicillin-resistant S. aureus (MRSA)was detected by the cefoxitin (30 µg) disk diffusion method (13). A ≤ 21 mm growth inhibition zone was considered positive, while ≥ 22 mm was considered negative.
Results
The incidence of VAP by recent NHSN guidelines and its bacteriological profile have been thoroughly described in our previously published article (6). Generally, 31 (15.19%) patients developed VAP during their ICU stay. Two patients had two episodes of VAP each. Acinetobacter was the most common organism (37.83%), followed by Pseudomonas and Klebsiella species and E. coli,whileElizabethkingiaand Enterobacter were the least common organisms. The two isolates of S. aureus were the only identified gram-positive organisms.
It was observed that among non-fermenters, colistin and tigecycline were highly active against A. baumannii, whereas Tigecycline was active against Acinetobacter lwoffii. Piperacillin-tazobactam, gentamicin, and meropenem had good activity against Pseudomonas spp. Elizabethkingia meningosepticawas only sensitive to ciprofloxacin and tigecycline; All the remaining tested antibiotics were resistant.
Among Enterobacteriaceae, all the isolates of E. coli were sensitive to meropenem, and most of them were resistant to beta-lactams; Klebsiella was 100% resistant to beta-lactams, and half of the isolates were resistant to meropenem, while Enterobacter was completely sensitive to all the tested drugs as reported in Table 2.
Table 2.
Etiological Agents of VAP and Their Antibiotic Resistance Patterns (%)
|
Etiological Agents (No. of Isolates)
|
Antibiotic Resistance in % (No. of Isolates)
|
|
1. Non-fermenters
|
PTZ
|
GEN
|
CL
|
CIP
|
CPM
|
CAZ
|
MEM
|
TGC
|
SXT
|
|
Acinetobacter baumannii (13) |
100 (13) |
100 (13) |
7.69 (1) |
100 (13) |
100 (13) |
76.9 (10) |
100 (13) |
15.38 (2) |
92.3 |
|
Acinetobacter lwoffii (1) |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
0 |
100 |
|
Pseudomonas aeruginosa (8) |
50 (4) |
25 (2) |
37.5 (3) |
25 (2) |
50 (4) |
37.5 (3) |
25 (2) |
100 (8) |
50 (4) |
|
Elizabethkingia meningoseptica(1) |
100 |
100 |
100 |
0 |
100 |
0 |
100 |
0 |
100 |
|
2. Enterobacteriaceae
|
AMP
|
GEN
|
AMK
|
CIP
|
CTR
|
CAZ
|
MEM
|
PTZ
|
SXT
|
|
Escherichia coli (5) |
100 |
80 |
0 |
100 |
100 |
60 |
0 |
60 |
40 |
|
Klebsiella pneumoniae (6) |
- |
100 |
0 |
50 |
100 |
100 |
50 |
100 |
50 |
|
Enterobacter cloacae (1) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Note. PTZ: Piperacillin-tazobactam; GEN: Gentamicin; CL: Colistin; CIP: Ciprofloxacin; CPM: Cefepime; CAZ: Ceftazidime; MEM: Meropenem; TGC: Tigecycline; SXT: Trimethoprim sulfamethoxazole; AMP: Ampicillin; AMK: Amikacin; CTR: Ceftriaxone.
All the isolates of Klebsiella and E. coli representing the ceftazidime zone of ≤22 mm were tested for ESBL production by the phenotypic confirmatory disc diffusion test as shown in the Figures 1 and 2 respectively.
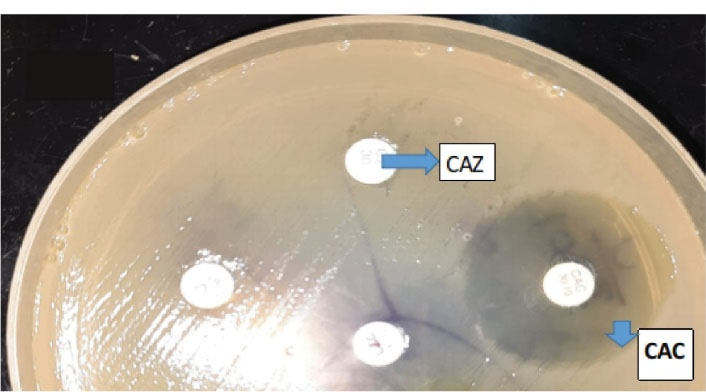
Figure 1.
Phenotypic Confirmatory Disc Diffusion Test. Note. Klebsiella species showing ESBL production was confirmed by an increase in the zone of ≥ 5 mm for ceftazidime/clavulanic acid (CAC) and cefotaxime/clavulanic acid (CEC) vs. ceftazidime (CAZ) and cefotaxime (CTX) alone, respectively.
.
Phenotypic Confirmatory Disc Diffusion Test. Note. Klebsiella species showing ESBL production was confirmed by an increase in the zone of ≥ 5 mm for ceftazidime/clavulanic acid (CAC) and cefotaxime/clavulanic acid (CEC) vs. ceftazidime (CAZ) and cefotaxime (CTX) alone, respectively.
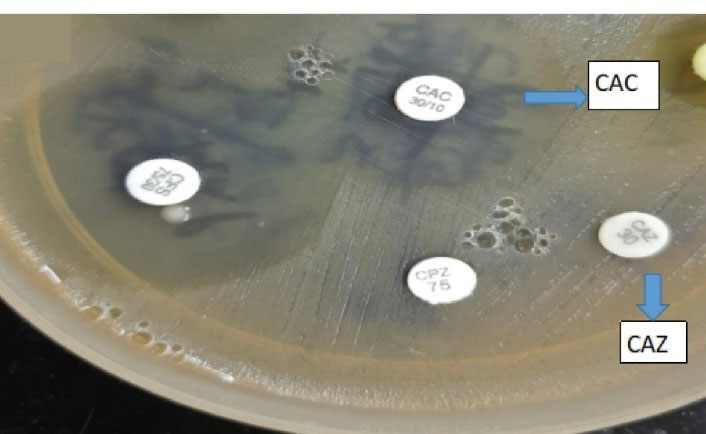
Figure 2.
Phenotypic Confirmatory Disc Diffusion Test. Note. ESBL: Extended-spectrum beta-lactamases; E. coli: Escherichia coli. E. coli indicating ESBL production was confirmed by an increase in the zone of ≥ 5 mm for ceftazidime/clavulanic acid (CAC) vs. ceftazidime (CAZ) alone. A combination of cefoperazone (CPZ) and cefoperazone sulbactam (CFS) was used as well.
.
Phenotypic Confirmatory Disc Diffusion Test. Note. ESBL: Extended-spectrum beta-lactamases; E. coli: Escherichia coli. E. coli indicating ESBL production was confirmed by an increase in the zone of ≥ 5 mm for ceftazidime/clavulanic acid (CAC) vs. ceftazidime (CAZ) alone. A combination of cefoperazone (CPZ) and cefoperazone sulbactam (CFS) was used as well.
Isolates that yielded a cefoxitin zone diameter of > 18 mm and were resistant to third-generation cephalosporins were tested for AmpC enzyme production by the popular AmpC disk test. Likewise, isolates that were not susceptible to Carbapenems were tested for Carbapenemase production by the mCIM test.
ESBL was confirmed in 40% and 67% of E. coli and K. pneumoniae, respectively. Out of 3 Carbapenem-resistant Klebsiella species, only one was a Carbapenemase producer as depicted in the Figure 3.

Figure 3.
mCIM Results. (A) Negative mCIM - Carbapenemase not detected (Zone diameter>19 mm). (B) Positive mCIM - Carbapenemase detected (Zone diameter- 6 mm). (C) Negative mCIM - Carbapenemase not detected (Zone diameter>19 mm).
.
mCIM Results. (A) Negative mCIM - Carbapenemase not detected (Zone diameter>19 mm). (B) Positive mCIM - Carbapenemase detected (Zone diameter- 6 mm). (C) Negative mCIM - Carbapenemase not detected (Zone diameter>19 mm).
Isolates demonstrating resistance to carbapenems were selected for the detection of MBL enzymes by IPM EDTA CDT.
Only two of the eight Pseudomonas species were tested for MBL (Figure 4) and both were positive, while AmpC β-lactamases were produced by 18% of each of the non-fermenters and Enterobacteriaceae members, respectively.
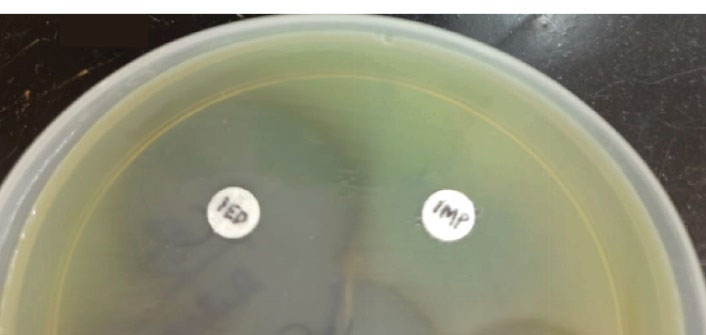
Figure 4.
MBL-producing Pseudomonas aeruginosa: IMP–EDTA Combined Disc Test Showing an Increased Zone With IMP-EDTA (IED) of 22 mm Compared to Only IMP. Note. MBL: Metallo-beta-lactamases; EDTA: Ethylenediamine tetraacetic acid; IMP: Imipenem.
.
MBL-producing Pseudomonas aeruginosa: IMP–EDTA Combined Disc Test Showing an Increased Zone With IMP-EDTA (IED) of 22 mm Compared to Only IMP. Note. MBL: Metallo-beta-lactamases; EDTA: Ethylenediamine tetraacetic acid; IMP: Imipenem.
Based on the results, 50% of the S. aureus causing VAP were MRSA as depicted in the Table 3, which was detected by the Cefoxitin 30 microgram disk diffusion method as illustrated in the Figure 5.
Table 3.
Etiological Agents (GPC) of VAP the Antibiotic Resistance Pattern (%)
|
|
PEN
|
CFX
|
TET
|
ERY
|
CIP
|
CL
|
VA
|
LZ
|
SXT
|
|
Staphylococcus aureus (2) |
50 |
50 |
0 |
100 |
100 |
0 |
0 |
0 |
100 |
Note. PEN: Penicillin; CFX: Cefoxitin; TET: Tetracycline; ERY: Erythromycin; CIP: Ciprofloxacin; CL: Clindamycin; VAN: Vancomycin; LZ: Linezolid; SXT: Trimethoprim sulfamethoxazole.
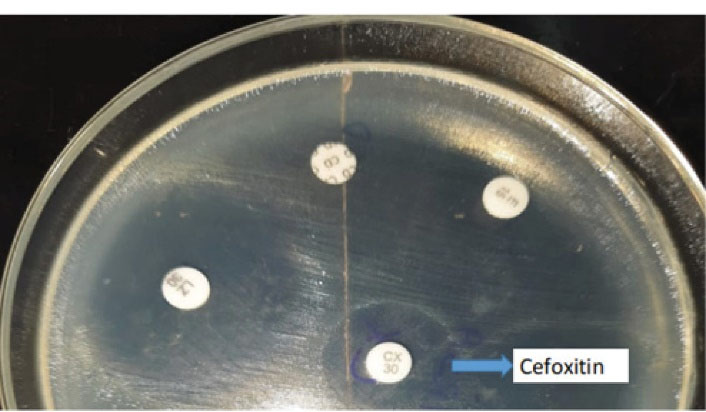
Figure 5.
Cefoxitin Disk Diffusion Test: MRSA Positive Staphylococcus aureus Species Showing CX (Cefoxitin) Disk Zone > 21 mm
.
Cefoxitin Disk Diffusion Test: MRSA Positive Staphylococcus aureus Species Showing CX (Cefoxitin) Disk Zone > 21 mm
Twenty (54%) of the 37 VAP pathogens were MDR in our study as reported in the Table 4, including gram-negative bacteria (Enterobacteriaceae and non-fermenters) producing ESBL, AmpC β lactamases, and MBL (Tables 5 and 6) and gram-positive MRSA.
Table 4.
MDR Pathogens
|
Organism
|
Total Isolates
|
MDR Isolates
|
Percentage
|
| Non-fermenters |
23 |
10 |
43.47% |
|
Enterobacteriaceae
|
12 |
09 |
75% |
| GPC |
02 |
01 |
50% |
| Total |
37 |
20 |
54% |
Note. MDR: Multidrug resistance; GPC: Gram-positive cocci.
Table 5.
ESBL, AmpC β-Lactamase, and Carbapenemase Production Among Enterobacteriaceae
|
Bacterial Isolates
|
ESBL
|
AmpCβ-Lactamase
|
Cp-CRE
|
|
Escherichia coli (5) |
2 |
1 |
0 |
|
Klebsiella pneumoniae (6) |
4 |
1 |
1 |
Note. ESBL: Extended-spectrum beta-lactamases; AmpC: Ampicillin C; Cp-CRE: Carbapenemase-producing carbapenem-resistant Enterobacteriaceae.
Table 6.
AmpC β Lactamase and MBL Production Among Non-fermenters
|
Bacterial Isolates
|
AmpCβ Lactamase
|
MBL
|
|
Pseudomonas aeruginosa (8) |
1 |
2 |
|
Acinetobacter baumannii (13) |
4 |
3 |
|
Acinetobacter lwoffii (1) |
0 |
0 |
Note. MBL: Metallo-beta-lactamase.
Discussion
To the best of our knowledge, this is the first study to identify VAP events according to newer NHSN guidelines, including clinical, radiological, and microbiological results rather than only CPIS scoring.
VAP accounts for 1/4th of the infections in critically ill patients and half of the antibiotic prescriptions in mechanically ventilated patients. Several countries have reported mortality rates ranging from 24% to 76%.
A patient seeks medical help only when it is absolutely inevitable owing to limited resources, and by the time, he/she is referred to the tertiary care center, his/her underlying condition is well advanced; this may necessitate a longer duration of mechanical ventilation, which is directly proportional to the development of VAP.
In this study, the most commonly isolated organism was Acinetobacter, followed by P. aeruginosa, E. coli, and K. pneumonia. The organisms implicated in VAP were similar in other studies such as Dube et al (14), Maqbool et al (15), Mathai et al (16), and Ranjan et al (17). Acinetobacter indeed has a wonderful ability to grow in various inserted catheters in patients in ICU, particularly the endotracheal tube (18).
In our study, 31% of Acinetobacter spp. were AmpC β-lactamase producers, which is less compared to other studies such as Kaur et al. In our study, 66% of Klebsiella isolates were ESBL producers, which is similar to the result of Joseph et al (19), whereas it is more when compared to studies by Kaur et al (20)and Sangale et al (21).
MBL producing Pseudomonas was 25% in our study, which is less compared to that of Krishnamurthy et al (22), which was 50%, while it is slightly more when compared to the result of Joseph et al (19),which was 20%. Most of the studies showed a similar incidence of MRSA, including Joseph et al (19), Balkhy et al (23), and Sangale et al (21).
MDR Acinetobacter, klebsiella, and Pseudomonas were the common organisms associated with greater mortality, among which Acinetobacter (57.14%) had the highest rate in our study, which is correlated with the result of a study from Odisa, where Acinetobacter was associated with 80% of mortality. This highlights the need for detecting MDR organisms and treatment rather than giving empirical treatment.
In this study, it was observed that Colistin is highly active against Acinetobacter spp., and Piperacillin/tazobactam combination has good activity against Pseudomonas spp. These findings need to be further confirmed by large clinical trials since we have only studied less number of isolates in a single tertiary care hospital.
ESBL and AmpC β-lactamases were produced by a large number of the Enterobacteriaceae similar to other studies (19).Therefore, the prophylactic use of antibiotics is not recommended, and exposure to antibiotics is a significant risk factor for colonization and infection with nosocomial multidrug-resistant pathogens as observed by other authors (24).
Conclusion
The notable strength of our study was that it was prospectively conducted with the diagnosis of VAP based on new NHSN guidelines rather than CPIS scoring, which was used earlier. VAP is highly associated with MDR pathogens.
As per our study revealed colistin was good for Acinetobacter, Piptaz against Pseudomonas species, and E. coli to carbapenems compared to Klebsiella.
There is a need for a multidisciplinary approach, proper planning, and infection control to combat VAP events, including continuous education and increased awareness of MDR, to reduce the duration of ventilation, to use proper antibiotics only after using susceptibility testing, and to follow all VAP bundles.
Acknowledgements
We thank the patients since this study would not have been completed without their consent. We also thank the Ethics Committee of Gandhi Medical College and Hospital for approving our study. Last but not least, we appreciate all the anesthesia and critical care nursing staff who have been extremely helpful during the whole study period.
Conflict of Interests
The authors declare that they have no conflict of interests.
Ethical Approval
This study was approved by the Research and Ethics Committees of Gandhi Medical College and Hospital, and informed consent was obtained from each patient.
References
- Davis KA. Ventilator-associated pneumonia: a review. J Intensive Care Med 2006; 21(4):211-26. doi: 10.1177/0885066606288837 [Crossref] [ Google Scholar]
- Patil HV, Patil VC. Incidence, bacteriology, and clinical outcome of ventilator-associated pneumonia at tertiary care hospital. J Nat Sci Biol Med 2017; 8(1):46-55. doi: 10.4103/0976-9668.198360 [Crossref] [ Google Scholar]
- Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 2001; 14(4):933-51, table of contents. doi: 10.1128/cmr.14.4.933-951.2001 [Crossref] [ Google Scholar]
- Ali HS, Khan FY, George S, Shaikh N, Al-Ajmi J. Epidemiology and outcome of ventilator-associated pneumonia in a heterogeneous ICU population in Qatar. Biomed Res Int 2016; 2016:8231787. doi: 10.1155/2016/8231787 [Crossref] [ Google Scholar]
- Chastre J, Combes A, Luyt CE. The invasive (quantitative) diagnosis of ventilator-associated pneumonia. Respir Care 2005; 50(6):797-807. [ Google Scholar]
- Fatima S, Rajeshwar Rao S, Shailaja VV, Nagamani K. Finding the incidence of ventilator associated pneumonia by recent NHSN guidelines and its bacteriological profile: a study conducted in a tertiary care hospital in Southern India. Int J Curr Microbiol Appl Sci 2019; 8(10):2080-9. doi: 10.20546/ijcmas.2019.810.242 [Crossref] [ Google Scholar]
- Mackie TJ, McCartney JE. Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. p. 978.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard, 9th ed. CLSI Document M2-A9. Wayne, PA: CLSI; 2006.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI Document M100S. Wayne, PA: CLSI; 2017.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Twenty Second Informational Supplement Update. CLSI Document M100-S22 U. Wayne, PA: CLSI; 2012.
- Singhal S, Mathur T, Khan S, Upadhyay DJ, Chugh S, Gaind R. Evaluation of methods for AmpC beta-lactamase in gram negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol 2005; 23(2):120-4. doi: 10.4103/0255-0857.16053 [Crossref] [ Google Scholar]
- Das NK, Grover N, Sriram R, Kumar M, Dudhat VL, Prasanna S. Prevalence of carbapenem resistance and comparison between different phenotypic methods for detection of metallo-β-lactamases in gram-negative non-fermentative bacteria in the acute wards of a tertiary care centre. Int J Curr Microbiol Appl Sci 2016; 5(5):109-19. doi: 10.20546/ijcmas.2016.505.012 [Crossref] [ Google Scholar]
- Cauwelier B, Gordts B, Descheemaecker P, Van Landuyt H. Evaluation of a disk diffusion method with cefoxitin (30 microg) for detection of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 2004; 23(5):389-92. doi: 10.1007/s10096-004-1130-8 [Crossref] [ Google Scholar]
- Dube M, Goswami S, Singh A, Raju BM, Dube P, Bhatia GC. Pattern and incidence of ventilator associated pneumonia among mechanically ventilated patients. Int J Adv Med 2018; 5(2):442-5. doi: 10.18203/2349-3933.ijam20181086 [Crossref] [ Google Scholar]
- Maqbool M, Shabir A, Naqash H, Amin A, Koul RK, Shah PA. Ventilator associated pneumonia-incidence and outcome in adults in medical intensive care unit of a tertiary care hospital of North India. Int J Sci Stud 2017; 4(10):73-6. doi: 10.17354/ijss/2017/14 [Crossref] [ Google Scholar]
- Mathai AS, Phillips A, Isaac R. Ventilator-associated pneumonia: a persistent healthcare problem in Indian Intensive Care Units!. Lung India 2016; 33(5):512-6. doi: 10.4103/0970-2113.188971 [Crossref] [ Google Scholar]
- Ranjan N, Chaudhary U, Chaudhry D, Ranjan KP. Ventilator-associated pneumonia in a tertiary care intensive care unit: Analysis of incidence, risk factors and mortality. Indian J Crit Care Med 2014; 18(4):200-4. doi: 10.4103/0972-5229.130570 [Crossref] [ Google Scholar]
- Coppadoro A, Berra L, Bigatello LM. Modifying endotracheal tubes to prevent ventilator-associated pneumonia. Curr Opin Infect Dis 2011; 24(2):157-62. doi: 10.1097/QCO.0b013e328343b733 [Crossref] [ Google Scholar]
- Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multi-drug resistant pathogens. J Infect Dev Ctries 2010; 4(4):218-25. doi: 10.3855/jidc.634 [Crossref] [ Google Scholar]
- Kaur M, Singla B, Malhotra R. Study of multidrug resistant (MDR) isolates in patients with ventilator associated pneumonia (VAP) in tertiary care hospital. Int J Res Dev Pharm Life Sci 2015; 4(2):1394-9. [ Google Scholar]
- Sangale A, Vivek B, Kelkar R, Biswas S. Microbiology of ventilator-associated pneumonia in a tertiary care cancer hospital. Indian J Crit Care Med 2021; 25(4):421-8. doi: 10.5005/jp-journals-10071-23790 [Crossref] [ Google Scholar]
- Krishnamurthy V, Kumar V, Prashanth HV, Nagaraj ER, Ramakrishna P. A study of ventilator associated pneumonia caused by multidrug resistant pathogens with special reference to ESBL, AMPC and MBL. Res J Pharm Biol Chem Sci 2013; 4(3):261-9. [ Google Scholar]
- Balkhy HH, El-Saed A, Maghraby R, Al-Dorzi HM, Khan R, Rishu AH. Drug-resistant ventilator associated pneumonia in a tertiary care hospital in Saudi Arabia. Ann Thorac Med 2014; 9(2):104-11. doi: 10.4103/1817-1737.128858 [Crossref] [ Google Scholar]
- Park DR. The microbiology of ventilator-associated pneumonia. Respir Care 2005; 50(6):742-63. [ Google Scholar]