Avicenna Journal of Clinical Microbiology and Infection. 8(4):139-144.
doi: 10.34172/ajcmi.2021.26
Original Article
Antimicrobial Properties and Shear Bond Strength of Composite Used in Orthodontics Following the Addition of Curcumin-Reduced Nanographene Oxide
Roghayeh Ghorbanzadeh 1  , Abbas Salehi Vaziri 1, *
, Abbas Salehi Vaziri 1, *  , Abbas Bahador 2
, Abbas Bahador 2
Author information:
1Department of Orthodontics, School of Dentistry, Shahed University, Tehran, Iran
2Fellowship in Clinical Laboratory Sciences, BioHealth Laboratory, Professor in Microbiology, Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
*
Corresponding author: Abbas Salehi Vaziri, Department of Orthodontics, School of Dentistry, Shahed University, Tehran, Iran, Email:
a.salehi@shahed.ac.ir
Abstract
Background: Composites used in the direct bonding of brackets in fixed orthodontics are considered a suitable environment for the multiplication of cariogenic bacteria and the establishment of microbial plaques by creating a distance between the bracket, tooth surface, and surface properties such as porosity. In the present study, we decided to combine curcumin-reduced nano-graphene oxide (rGO-NCUR) with a composite resin used in orthodontics to achieve a composite with optimal shear bond strength (SBS) and antimicrobial properties against Streptococcus mutans as a main cariogenic bacterium.
Methods: In the present study, nanoparticle of graphene oxide (NGO) was synthesized by the modified Hummers’ method using graphite powder and reacted by NCUR to regenerate reduced-NGO (rGO-NCUR). Scanning electron microscopy (SEM) was used to confirm the synthesis of NGO, NCUR, and rGO-NCUR. SBS and antimicrobial activity of Transbond XT composite containing 5% rGO-NCUR were performed against S. mutans biofilms. SPSS software, one-way ANOVA test, and Tukey post hoc test with a significance level equal to or less than 0.05 were used to analyze the data.
Results: The synthesis of NGO, NCUR, and rGO-NCUR in nanoscale was confirmed by SEM. The amount of SBS in the composite sample with 5% w/w rGO-NCUR was 12.30 ± 0.65 MPa. The optical density of S. mutans in the biofilm structure formed on composite containing 5% w/w rGO-NCUR was significantly reduced (62.10%) compared with the control group, namely, original composites used in orthodontics (P<0.05).
Conclusions: Based on the data of the present study, it can be concluded that composite containing 5% w/w rGO-CUR, without adverse effect on the physical-mechanical properties of composites, can be used as an antimicrobial additive of composites used in orthodontics to control biofilm formation and caries around orthodontic brackets.
Keywords: Nanoparticles, Curcumin, Graphene oxide, SBS, Streptococcus mutans
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
In fixed orthodontics, increased food retention and difficulty in maintaining oral hygiene by the patient lead to an increased risk of accumulation of microorganisms and microbial biofilms with cariogenic bacteria including Streptococcus mutans, formation and spread of microbial plaques, enamel decalcification, white spot lesions (WSLs), and caries around the brackets (1).
Studies evidence that most patients undergoing fixed orthodontic treatment have at least one mild WSL on their enamel. Mild to severe WSLs on enamel was found in 38% and 46% of patients who underwent fixed orthodontic treatment for 6 and 12 months, respectively (2).
The composites, adhesives, and bonds used to attach the brackets to the enamel, act as a suitable environment for the accumulation of cariogenic bacteria due to the presence of the porosity. Thus, recent studies have focused on the production of new dental materials with the potential to inhibiting microbial plaques, WSLs, and dental caries. Nano-resin composite is one of the most important recent achievements of nanotechnology in the field of dental materials which is used in orthodontics (3,4).
Studies demonstrated that the use of fluoride-releasing composites containing nano-fillers, orthodontics cement, and primers with silver nanoparticles can play an effective role in preventing the formation of microbial biofilms around fixed orthodontic appliances. Nano-filled composites have been found to have a minimal tendency to adhere to cariogenic bacteria such as S. mutans. The dental composite containing chlorhexidine displays antimicrobial effects, but its antimicrobial properties are short. Composites containing polyethyleneimine nanoparticles along with biocompatibility properties are effective in reducing the proliferation of cariogenic bacteria (5,6). Further, achieving a successful orthodontic treatment requires reducing the risk of caries, bonding the bracket to the tooth properly, and creating sufficient bond strength in the treatment course.
One nanotechnology achievement is nanoparticles of graphene oxide (NGO). The NGO, similar to graphene, has a flat two-dimensional structure. In addition, it is widely applied in biomedicine due to its increased water solubility, high mechanical and chemical stability, textile compatibility, biological compatibility, non-toxicity, biocompatibility, as well as easy and cost-effective synthesis (1,6-8). NGO is active owing to multiple chemical groups and can form several covalent and non-covalent interactions (9). NGO also contains some negative charges that activate oxygen functional groups on its surface, resulting in a high solubility (approximately 0.5 mg/mL) in water or other polar solvents such as ethylene glycol (10). The large specific surface area of NGO, with a large number of carboxylic acid, hydroxide, and epoxide groups on its surface, can bind to a variety of molecules through hydrophobic interactions, hydrogen bonds, and electrostatic interactions (11).
Curcumin [CUR, 1,7-bis (4-hydroxy-3-methoxyphenyl) -1,6-heptadiene-3,5, -dione] is the major yellow pigment extracted from turmeric with several medicinal properties such as anti-inflammatory, antibacterial, antifungal, and anti-cancer, which are mainly obtained by high doses of CUR. As assessment of clinical trials in humans indicate, due to the extensive antimicrobial activity of CUR and its biosafety, it has been modified to design new antimicrobial agents with antimicrobial activity and to enhance antimicrobial activity through the synthesis of various CUR-related derivatives. Nano-CUR (NCUR), unlike CUR, has been reported to have good solubility in water, thus having wide range of therapeutic applications and being biologically safe (12).
Moreover, previous studies indicated that the poor antimicrobial activities of NGO can be significantly increased by a reduction in CUR (i.e., reduced GO) (13,14). The synergistic effect between NGO and CUR in the format of reduced GO (rGO) may introduce new strategies for developing highly effective antimicrobial methods against cariogenic bacteria such as S. mutans (15).
Due to the development of nanotechnology (i.e., NGO and NCUR) in the field of antimicrobial activity, we decided to combine curcumin-reduced nano-graphene oxide (rGO-NCUR) with a composite resin used in orthodontics. Thus, achieving a composite with antimicrobial properties is considered as the practical goal of the present study.
Materials and Methods
Synthesis of NGO
In the present study, the following modified Hummers’ method was used to synthesize NGO. First, 1 g of graphite powder (Qingdao Tianhe Graphite Company, Ltd, China) with an average diameter of 4 mm and a purity of 99.95% was mixed with 23 mL of sulfuric acid (H2SO4, 98%). After adding 0.5 g of potassium persulfate (K2S2O8) and 0.5 g of phosphorus pentoxide (P2O5), they were mixed (600 rpm) for 6 hours at 20-25°C to dissolve the graphite powder. To remove excess acid, the resulting solution was centrifuged with 100 mL of deionized water at 10 000 rpm for 5 minutes. This process continued until the pH of the solution reached neutral. The resulting precipitate was dried by exposing to room temperature for 12 hours. Then, 23 mL of sulfuric acid was added to the resulting powder, the container containing the acid (reaction container) was placed in a cold-water bath, and finally 3 g of potassium permanganate was added to the solution. The temperature was kept below 20°C due to the exothermic temperature. The mixture was then homogenized by adding and stirring 46 mL of deionized water for 2 hours at 35°C. To convert excess permanganate to manganese ions, 2 mL of hydrogen peroxide (H2O2, 30%) was added to the solution after 15 minutes, and the reaction was stopped accordingly. After precipitating the material for 24 hours, the supernatant was discarded, and the precipitate was washed several times by hydrochloric acid (32%) and deionized water, and finally, centrifuged at 10°C at 10000 rpm for 10 minutes each time. To form monolithic GO plates, the solution was placed in a 300 W ultrasonic system at room temperature for 90 minutes. The remaining graphite grains and unopened graphene plates were centrifuged at 10 000 rpm at 15°C for 15 minutes, and the supernatant contained GO which is brown, uniform and clear. To obtain NGO powder, the solution was dried in the freezer system (16). The production of NGO was confirmed using scanning electron microscopy (SEM) (17).
Nano Curcumin Synthesis (NCUR)
In this study, NCUR was synthesized by physicochemical methods. CUR stock solution (5 mg/mL) was prepared by dissolving curcumin powder in 20 mL of dichloromethane. One milliliter of the stock solution was added to 50 mL of boiling water in ultrasound bath (a drop of 0.1 mL per minute). The solution was sonicated for 30 minutes. Then, the mixture was stirred at 800 rpm for 20 minutes to obtain an orange precipitate. The supernatant was discarded and thus the synthesized NCUR was obtained for further studies, and the production of NCUR was confirmed by applying SEM (17).
Reduction of NGO by NCUR
To reduce NGO, 5 mg of NCUR was dissolved in 10 mL of ethanol. While the NGO solution was placed on the stirrer, the NCUR solution was gradually added. To increase the pH of the solution to 10, ammonia was slowly added to the NGO-NCUR solution. The high pH of the solution promoted homogeneity and improved the stability of NGO-NCUR. The suspension was heated at 85°C for 120 minutes. Finally, the NGO-NCUR suspension (black) was dialyzed and centrifuged at 3000 rpm for 20 minutes to remove any residues (especially ammonia) and to control the pH (pH= 7). To obtain rGO-NCUR powder, the solution was dried in the freezer system (15).
Preparation of Transbond XT Composite Containing 5% w/w rGO -NCUR
Transbond XT composite (3M - Dentaltix) was mixed with rGO-NCUR powder at a concentration of 5% (w/w) using a court spatula on a glass slab in a semi-dark environment. The mixing continued until the modified composite appeared to be uniform and homogeneous.
Determination of Shear Bond Strength (SBS)
Initially, 20 bovine incisors without structural defects and visible cracks were collected from the bovine. After rinsing and separating the tissues around the teeth, they were kept in 0.5% chloramine for 1 week, and then they were transferred to distilled water for further experiments. Subsequently, the teeth were randomly divided into 2 groups (n=10): group 1 (Transbond XT composite containing 5% rGO-CUR) and group 2 (Transbond XT composite without rGO-CUR) (the control group).
The teeth were then etched using 35% phosphoric acid gel for 30 seconds, rinsed with water for 30 seconds, and dried using air without moisture and oil. A thin layer of adhesive was applied evenly to the enamel of all teeth and exposed to a light cure device for 20 seconds (Figure 1). In this study, standard stainless-steel brackets (OMB; 3S Dental group, Tehran, Iran) were used. The same amount of modified Transbond XT composite and original Transbond XT composite was added to the base of the brackets in the test and control groups, respectively. The brackets were then attached to the enamel by the light curing device (Figure 1).

Figure 1.
Preparation of Teeth for Bracket Bonding and Mounting it in Acrylic Blocks for Shear Bond Strength Testing.
.
Preparation of Teeth for Bracket Bonding and Mounting it in Acrylic Blocks for Shear Bond Strength Testing.
All teeth were kept in 37°C distilled water for 24 hours before the thermocycling. The teeth were thermocycled for 3000 cycles for 24 hours at 5 and 55°C, and then the teeth were mounted in acrylic blocks. Finally, the SBS was evaluated using the universal testing machine according to ISO/TS 11405: 2015 (Figure 2). The results of the test group (Transbond XT composite containing 5% rGO-CUR group) were compared with the control group (Transbond XT composite without rGO-CUR) using statistical analysis (18).

Figure 2.
Determination of Shear Bond Strength in the Sample Using Universal Testing Machine.
.
Determination of Shear Bond Strength in the Sample Using Universal Testing Machine.
Inhibition of Biofilm Formation
Prepared discs from a composite containing 5% rGO-NCUR were used to inhibit biofilm formation. Initially, the mold was placed on a glass slab, and a sufficient amount of composite was placed inside each hollow of the mold with a diameter of 5 mm. Then, gentle pressure was applied to the samples in each hollow using a glass slide so that the surface of the samples can get smooth and without porosity. The thickness of the samples was equal. Each sample was then cured by a light-curing device for 20 seconds (Figure 3a). After curing, the samples were separated from the mold by double-sided finger pressure and polished. All samples were made in the same environment at a temperature of 22-25°C by one person. Finally, the samples were sterilized by gamma radiation with a minimum dose of 25 kg in the gamma radiation center of the Iranian Institute of Nuclear Science and Technology.
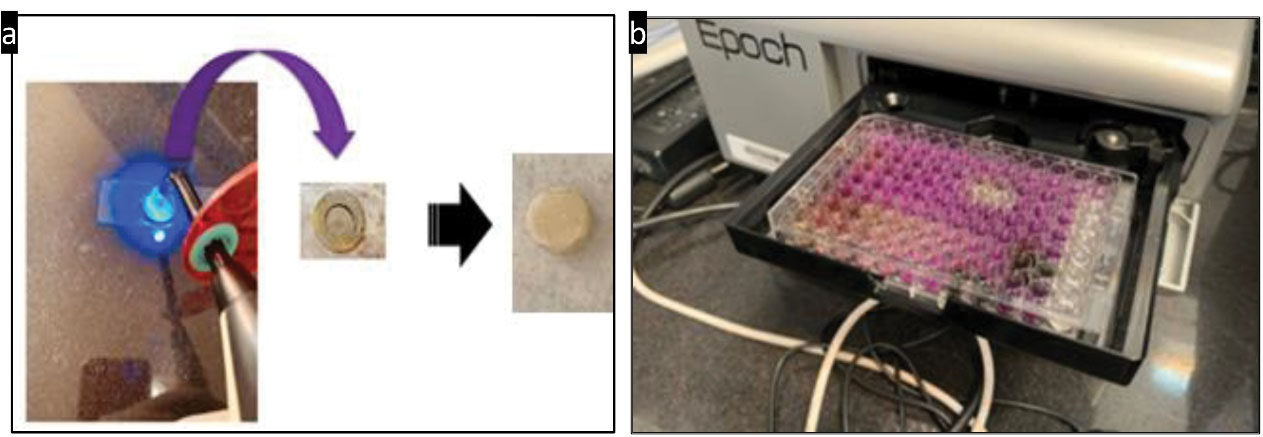
Figure 3.
(a) Preparation of Composite Discs Containing 5% rGO-NCUR and (b) Optical Density Reading of Microbial Biofilms With a Spectrophotometer at 570 nm. Note. rGO-NCUR: Curcumin-reduced nano-graphene oxide.
.
(a) Preparation of Composite Discs Containing 5% rGO-NCUR and (b) Optical Density Reading of Microbial Biofilms With a Spectrophotometer at 570 nm. Note. rGO-NCUR: Curcumin-reduced nano-graphene oxide.
Microbial biofilms were then formed by culturing S. mutans on the studied composite discs using a 24-well plate. For this purpose, after placing the composite discs in each well, a microbial suspension with a concentration of 1.5 × 108 CFU/mL was added to each well, and plate was incubated at 37°C in a candle jar to form mature biofilms. The composite discs were then washed by sterile saline to clear the discs from planktonic bacteria and loosely bound bacteria.
The biofilm mass attached to the surface of the composite discs was measured using a crystal violet assay. For this purpose, each composite disc was placed in a 24-well plate, and 1000 microliters of absolute methanol were added to each well for fixation of biofilm structures on the surface of each composite disc. Accordingly, 15 minutes were allotted for fixation. After this period, the methanol was removed and the composite discs were dried at room temperature. One milliliter of 1% crystal violet dye was added to each well of a 24-well plate containing composite discs, then the dye was removed 5 minutes after incubation at room temperature, and then the composite discs were washed by phosphate buffered saline (PBS). This step can remove the excess dye. Then, 1 mL of 33% acetic acid was added to each well to release the dye absorbed by the bacteria. The adsorption rate of the solution was read at 570 nm and compared to the control (19). In this assay, chlorhexidine 0.2% was used as a positive control.
Data Analysis
IBM SPSS software (version 22), one-way ANOVA test, and Tukey post hoc test with a significance level equal to or less than 0.05 were used to analyze the data.
Results
Confirmation of Synthesis of NGO, NCUR, and rGO -NCUR
As depicted in Figure 4a-c, successful synthesis of NGO, NCUR, and rGO-NCUR was confirmed by SEM. The average particle size of rGO-NCUR was in the range of 52 ± 6.03 nm.
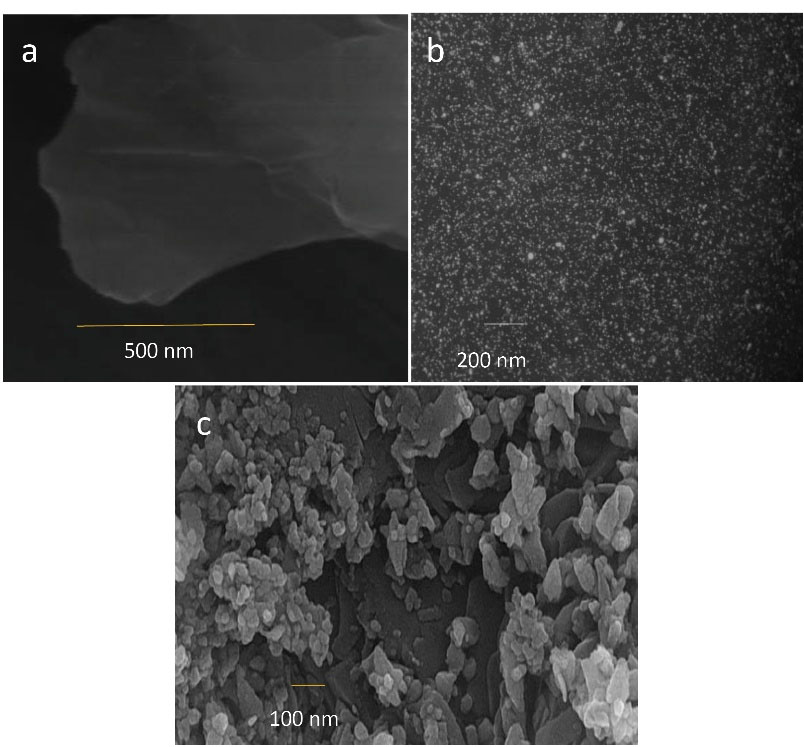
Figure 4.
Scanning Electron Microscopy From (a) NGO, (b) NCUR, and (c) rGO-NCUR
Note. NGO: Nanoparticle of graphene oxide; rGO-NCUR: Curcumin-reduced nano-graphene oxide.
.
Scanning Electron Microscopy From (a) NGO, (b) NCUR, and (c) rGO-NCUR
Note. NGO: Nanoparticle of graphene oxide; rGO-NCUR: Curcumin-reduced nano-graphene oxide.
SBS With Composite Contains rGO -NCUR
The amount of SBS in the composite sample with 5% w/w rGO-NCUR was 12.30 ± 0.65 MPa, which was not significantly different from the control group (P > 0.05).
Anti-biofilm Effect of Composite Containing rGO -NCUR
Effect of composite containing CUR (50 μmol), NGO (5% w/w), rGO-NCUR (5% w/w), and chlorhexidine (0.2% v/w) (as positive control) compared with the control (Transbond XT composite discs) on S. mutans biofilms are shown in Figure 5. Treatment of S. mutans with chlorhexidine 0.2% and composite containing 5%w /w rGO-NCUR led to a statistically significant decrease of 87.36% and 62.10%, respectively (both P < 0.05) in comparison with the control group. This could be attributed to the ability to form S. mutans biofilms. However, the treatment of S. mutans biofilms with CUR at a dose of 50 μmol and NGO 5% w/w, compared to the control group, indicated a statistically significant decrease of 16.14% and 11.92% (P > 0.05), respectively, suggesting the ability to form S. mutans biofilms.
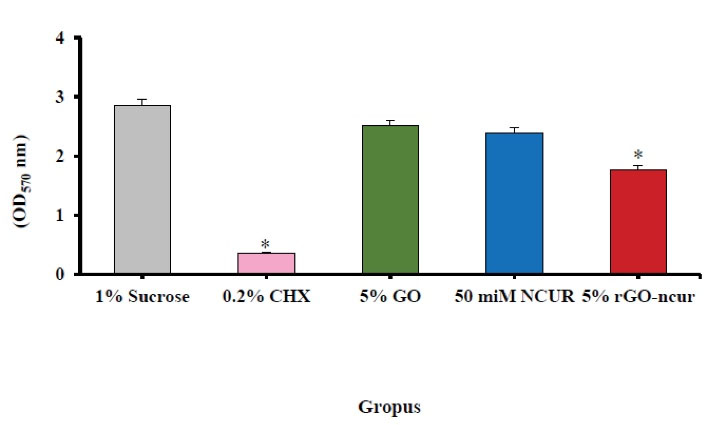
Figure 5.
Antimicrobial Effects of Composite Containing CUR (50 μmol), NGO (5% w/w), rGO-NCUR (5% w/w), and Chlorhexidine (0.2% v/w) on Biofilm Inhibition by Colorimetric Method. Note. NGO: Nanoparticle of graphene oxide; rGO-NCUR: Curcumin-reduced nano-graphene oxide.
.
Antimicrobial Effects of Composite Containing CUR (50 μmol), NGO (5% w/w), rGO-NCUR (5% w/w), and Chlorhexidine (0.2% v/w) on Biofilm Inhibition by Colorimetric Method. Note. NGO: Nanoparticle of graphene oxide; rGO-NCUR: Curcumin-reduced nano-graphene oxide.
Discussion
The results of this study demonstrated that SBS did not change significantly, compared to the control group, and did not decrease from the clinically accepted range (6-8 MPa) by adding 5% w/w rGO-NCUR to the Transbond XT composite used in orthodontics (20). It has been found that minimum bond strength of 6-8 MPa is sufficient for orthodontic forces and is considered sufficient for resistance to chewing (21). Therefore, the null hypothesis in this study, which stated that there is no statistically significant difference in SBS between the modified orthodontic composite and the original composite (without additives), was rejected. Clinically, the SBS values that are evaluated in real clinical cases are 40% lower than the laboratory-calculated SBS, resulting in SBS results from modified orthodontic composites containing 5% w/w rGO-NCUR in the present study should be within the acceptable range for in vivo evaluation. However, further clinical trial studies are needed to confirm the results of the present study and generalize it to the clinics.
According to a recent study by Pourhajibagher et al (18), Sodagar et al (22), and Akhavan et al (23), the obtained SBS from the bracket band by orthodontic composite (containing 10% w/w of additive to enamel) represented an unacceptable clinical score. They reported that SBS was significantly lower in the 10 % w/w of CUR/ZnONPs, NCUR, and AgNPs/hydroxyapatite groups compared with the control group (original composite). Contrary to the findings of the present study, Akhavan et al (23) reported a significant reduction in SBS from bracket bonds by a composite containing 5% w/w AgNPs/hydroxyapatite. The difference between the two studies can be attributed to the type of nanoparticles and the type of teeth. Akhavan et al (23) reported a significant increase in SBS in orthodontic composites containing 1% w/w AgNPs/hydroxyapatite compared with the control group, which was different from other studies (22,22,24). They showed that the effect of 1% w/w AgNPs/hydroxyapatite on the increase in SBS is due to the ability of hydroxyapatite to increase the mechanical strength of the composite layer. However, it should be noted that a noticeable increase in SBS is not always desirable, and if it is more than the clinically acceptable range, it may cause damage to the enamel when orthodontic brackets are deboning (23). Moreover, Oesterle et al (25) in an in vitro study revealed that orthodontic adhesive bond strength did not change within a month, followed by a decrease in bond strength from one month to 24 months. In another in vitro study, Choo et al (26) reported that the bond strength of orthodontic brackets using glass ionomer adhesives and modified orthodontic composites reduced over a one-year period of trial.
The results obtained in the present study suggested that Transbond XT composite contained 5% w/w of rGO-NCUR had antimicrobial properties against S. mutants biofilms compared to the control group. This finding is consistent with the study by Pourhajibagher et al (18) in which the addition of CUR/ZnONP increased the antimicrobial activity of composites used in orthodontics. Pourhajibagher et al (18) displayed that the anti-biofilm activity of orthodontic composites containing CUR/ZnONPs up to 90 days of rinsing by artificial saliva in a process called aging is evidence of the potential compatible use of CUR/ZnONPs as an additive to the composite orthodontics, controlling the biofilms of cariogenic bacteria.
Environmental effects such as the activity of other microorganisms, complex chewing process, pH and temperature fluctuations, and other factors were not considered in the present study. Therefore, they are regarded as the limitations of the present study. It should be noted that these physiological interventions in the mouth interfere with each other in SBS as well as the antimicrobial effect of rGO-CUR in vivo. Accordingly, further studies are needed to investigate the effects of those factors on rGO-CUR in vivo antimicrobial activities.
Conclusions
The data of the present study in terms of physical-mechanical properties (i.e., SBS) showed that the composite containing 5% w/w rGO-CUR is not significantly different from the original composites used in orthodontics. The present study indicated that the composite containing 5% w/w rGO-CUR has an anti-biofilm effect against S. mutans. Thus, it can be concluded that 5% w/w rGO-CUR without adverse effect on the physical-mechanical properties can be used as an additive in composites used in orthodontics with antimicrobial properties to control the formation of biofilms and caries around orthodontic brackets.
Acknowledgements
This research is a part of orthodontic residency thesis for the first author which has been scientifically and financially supported by Professor A. Bahador from Tehran University of Medical Sciences, Tehran, Iran.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this manuscript.
References
- Powers JM, Kim HB, Turner DS. Orthodontic adhesives and bond strength testing. Semin Orthod 1997; 3(3):147-56. doi: 10.1016/s1073-8746(97)80065-5 [Crossref] [ Google Scholar]
- Mizrahi E. Enamel demineralization following orthodontic treatment. Am J Orthod 1982; 82(1):62-7. doi: 10.1016/0002-9416(82)90548-6 [Crossref] [ Google Scholar]
- Kukleva MP, Shetkova DG, Beev VH. Comparative age study of the risk of demineralization during orthodontic treatment with brackets. Folia Med (Plovdiv) 2002; 44(1-2):56-9. [ Google Scholar]
- Matasa CG. Microbial attack of orthodontic adhesives. Am J Orthod Dentofacial Orthop 1995; 108(2):132-41. doi: 10.1016/s0889-5406(95)70075-7 [Crossref] [ Google Scholar]
- Wenderoth CJ, Weinstein M, Borislow AJ. Effectiveness of a fluoride-releasing sealant in reducing decalcification during orthodontic treatment. Am J Orthod Dentofacial Orthop 1999; 116(6):629-34. doi: 10.1016/s0889-5406(99)70197-6 [Crossref] [ Google Scholar]
- Podsiadlik BB. Orthodontic materials scientific and clinical aspects. N Y State Dent J 2001; 67(4):46. [ Google Scholar]
- Li Q, Hong L, Li H, Liu C. Graphene oxide-fullerene C60 (GO-C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light. Biosens Bioelectron 2017; 89(Pt 1):477-82. doi: 10.1016/j.bios.2016.03.072 [Crossref] [ Google Scholar]
- Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A. Improved synthesis of graphene oxide. ACS Nano 2010; 4(8):4806-14. doi: 10.1021/nn1006368 [Crossref] [ Google Scholar]
- Zhou T, Zhou X, Xing D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials 2014; 35(13):4185-94. doi: 10.1016/j.biomaterials.2014.01.044 [Crossref] [ Google Scholar]
- Neklyudov VV, Khafizov NR, Sedov IA, Dimiev AM. New insights into the solubility of graphene oxide in water and alcohols. Phys Chem Chem Phys 2017; 19(26):17000-8. doi: 10.1039/c7cp02303k [Crossref] [ Google Scholar]
- Mendes RG, Bachmatiuk A, Büchner B, Cuniberti G, Rümmeli MH. Carbon nanostructures as multi-functional drug delivery platforms. J Mater Chem B 2013; 1(4):401-28. doi: 10.1039/c2tb00085g [Crossref] [ Google Scholar]
- Sreedhar A, Sarkar I, Rajan P, Pai J, Malagi S, Kamath V. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a split mouth clinical and microbiological study. J Nat Sci Biol Med 2015; 6(Suppl 1):S102-9. doi: 10.4103/0976-9668.166100 [Crossref] [ Google Scholar]
- Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease?. Photochem Photobiol Sci 2004; 3(5):436-50. doi: 10.1039/b311900a [Crossref] [ Google Scholar]
- Liu Y, Qin R, Zaat SAJ, Breukink E, Heger M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J Clin Transl Res 2015; 1(3):140-67. [ Google Scholar]
- Ghorbanzadeh R, Assadian H, Chiniforush N, Parker S, Pourakbari B, Ehsani B. Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: an ex vivo biofilm model. Photodiagnosis Photodyn Ther 2020; 29:101643. doi: 10.1016/j.pdpdt.2019.101643 [Crossref] [ Google Scholar]
- Pak C, Lee DC. Crystalline transformation of colloidal nanoparticles on graphene oxide. ACS Appl Mater Interfaces 2012; 4(2):1021-9. doi: 10.1021/am201666q [Crossref] [ Google Scholar]
- Pourhajibagher M, Plotino G, Chiniforush N, Bahador A. Dual wavelength irradiation antimicrobial photodynamic therapy using indocyanine green and metformin doped with nano-curcumin as an efficient adjunctive endodontic treatment modality. Photodiagnosis Photodyn Ther 2020; 29:101628. doi: 10.1016/j.pdpdt.2019.101628 [Crossref] [ Google Scholar]
- Pourhajibagher M, Salehi Vaziri A, Takzaree N, Ghorbanzadeh R. Physico-mechanical and antimicrobial properties of an orthodontic adhesive containing cationic curcumin doped zinc oxide nanoparticles subjected to photodynamic therapy. Photodiagnosis Photodyn Ther 2019; 25:239-46. doi: 10.1016/j.pdpdt.2019.01.002 [Crossref] [ Google Scholar]
- Negri M, Silva S, Henriques M, Azeredo J, Svidzinski T, Oliveira R. Candida tropicalis biofilms: artificial urine, urinary catheters and flow model. Med Mycol 2011; 49(7):739-47. doi: 10.3109/13693786.2011.560619 [Crossref] [ Google Scholar]
- Barceló Santana HF, Hernández Medina R, Acosta Torres SL, Sánchez Herrera LM, Fernández Pedrero AJ, Ortíz González R. Evaluation of bond strength of metal brackets by a resin to ceramic surfaces. J Clin Dent 2006; 17(1):5-9. [ Google Scholar]
- Reynolds IR, von Fraunhofer JA. Direct bonding of orthodontic attachments to teeth: the relation of adhesive bond strength to gauze mesh size. Br J Orthod 1976; 3(2):91-5. doi: 10.1179/bjo.3.2.91 [Crossref] [ Google Scholar]
- Sodagar A, Bahador A, Pourhajibagher M, Ahmadi B, Baghaeian P. Effect of addition of curcumin nanoparticles on antimicrobial property and shear bond strength of orthodontic composite to bovine enamel. J Dent (Tehran) 2016; 13(5):373-82. [ Google Scholar]
- Akhavan A, Sodagar A, Mojtahedzadeh F, Sodagar K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol Scand 2013; 71(5):1038-42. doi: 10.3109/00016357.2012.741699 [Crossref] [ Google Scholar]
- Felemban NH, Ebrahim MI. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: an in vitro study. BMC Oral Health 2017; 17(1):43. doi: 10.1186/s12903-017-0332-2 [Crossref] [ Google Scholar]
- Oesterle LJ, Shellhart WC. Effect of aging on the shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop 2008; 133(5):716-20. doi: 10.1016/j.ajodo.2006.04.042 [Crossref] [ Google Scholar]
- Choo SC, Ireland AJ, Sherriff M. An in vitro investigation into the use of resin-modified glass poly(alkenoate) cements as orthodontic bonding agents. Eur J Orthod 2001; 23(3):243-52. doi: 10.1093/ejo/23.3.243 [Crossref] [ Google Scholar]