Avicenna Journal of Clinical Microbiology and Infection. 9(1):21-25.
doi: 10.34172/ajcmi.2022.04
Original Article
The Role of Efflux Pumps in the Antibiotic Resistance of Campylobacter spp. Isolated From Domestic Animals and Poultry
Parviz Moradi 1, Majid Baserisalehi 1, *
Author information:
1Department of Microbiology, Kazeroun Branch, Islamic Azad University, Kazeroun, Iran
Abstract
Background: Recently, the rate of antibiotic resistance of Campylobacter has been reported to be increasing and the mechanism of this resistance has been reported to be related to the activity of efflux pumps. The purpose of this study was to isolate Campylobacter strains from domestic animals such as poultry and cows and evaluate the role of efflux pumps in antibiotic resistance property of them.
Methods: A total of 300 fecal samples were collected from poultry and cows and subjected to isolation of Campylobacter by preT-KB method. The isolates were identified and confirmed by phenotypic and genotypic methods and their antibiotic susceptibility was evaluated using the disk diffusion method. Efflux pump activity in the isolates was assessed by EtBr-agar cartwheel method and the presence of efflux pump cmeABC was evaluated in all isolates. Finally, the correlation between efflux pump activity and antibiotic resistance was evaluated in the isolates using inhibition of efflux pump activity of Phe-Arg β-naphthylamide.
Results: Of all samples, 10 (3.3%) Campylobacter strains were isolated. Seven (70%) and three (30%) strains were isolated from poultry and cows, respectively. Of all isolates, 9 belonged to Campylobacter jejuni and 1 belonged to Campylobacter coli. The isolates were resistant to three antibiotics, namely Ciprofloxacin, Ceftriaxone, and Cefotaxime. Efflux pump activity was observed in all isolates; however, cmeABC genes were not present in all of them. In addition, resistance to Erythromycin and Ciprofloxacin was associated with efflux pump activity.
Conclusions: All Campylobacter isolates in the current study showed antibiotic resistance and the activity of efflux pumps could induce antibiotic resistance and decrease the antibacterial activity of many drug families in Campylobacter. In addition, the activity of efflux pumps can be considered a mechanism of antibiotic resistance and elimination of this activity might increase the effectiveness of antibiotics.
Keywords: Campylobacter, Antibiotic resistance, Efflux pumps
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Moradi P, Baserisalehi M. The role of efflux pumps in the antibiotic resistance of campylobacter spp. Isolated from domestic animals and poultry. Avicenna J Clin Microbiol Infect. 2022; 9(1):21-25. doi:10.34172/ajcmi.2022.04
Background
Campylobactereaceae include the genera Campylobacter, Achromobacter, andSulfurosprillium. 23SrRNA analysis opined that this family belongs to delta/epsilon of proteobacteria (1). Campylobacter species are gram-negative, motile, non-spore-forming, and spiral-shaped organisms. They are microaerophilic, nonsaccharolytic, nonproteolytic, and nonlipolytic so they do not ferment or oxidize carbohydrates. These bacteria live in the gastrointestinal tracts of birds and warm blooded animals. Campylobacter can grow on artificial media such as Kapadnis-Baseri (KB) medium under microaerophilic conditions. Recently, Campylobacter has been introduced as an important food poisoning agent and an emerging pathogen. Campylobacter genus is divided into two groups based on the production of catalase. Campylobacter jejuni, Campylobacter coli, and Campylobacter lari are catalase-positive human pathogens. However, the other species are considered catalase-negative or weak Campylobacter and are almost non-pathogenic (2). Campylobacter jejuniis the most important cause of diarrhea. Invasive factors of this bacterium are colonization, flagella, lipopolysaccharide (LPS), and production of toxins and antigens (3). The main transmission routes of Campylobacter include drinking of contaminated water and consumption of meat and dairy products (1). Nowadays, higher frequencies of antibiotic prescriptions culminated in the emergence of antibiotic-resistant Campylobacter. Moreover, antibiotic therapy in used in immunocompromised or elderly people to prevent infections. In this regard, HIV and diabetes mellitus patients are at risk of Campylobacter dissemination; therefore, the high rate of antibiotic prescription is needed for their treatment (4). In addition, plasmid-mediated antibiotic resistance in some Campylobacter isolates could increase the rate of Campylobacter infections among the human population (5). Gene modification and the production of drug-inactivating enzymes are the most important mechanisms of antibiotic resistance. On the other hand, the activity of efflux pumps could induce antibiotic resistance in many bacteria. Hydrolysis of ATP by these pumps reduces the antibiotic concentrations in the bacteria and subsequently increases the rate of antibiotic-resistant bacteria (6). Therefore, the present study was undertaken to investigate the role of efflux pumps (CmeABC) in inducing antibiotic resistance in Campylobacter isolates.
Materials and Methods
Sample Collection and Isolation of Campylobacter spp.
In this cross-sectional study, 300 fecal samples were collected from cows and poultry in different farms in Fars, Bushehr, and Khuzestan provinces in the south of Iran.The samples were collected using sterile sticks and polyethylene bags and transferred to the laboratory within one hour of sampling. The samples were subjected to isolation of Campylobacter using the preT-KB method (2). In this method, Campylobacter was cultivated on the Mueller-Hinton agar. To perform the experiment, 1 g of fecal samples was emulsified in sterile phosphate buffered saline (pH 7.0, 0.1 M) at 10% (w/v) concentration. The suspension was centrifuged at 8500 rpm for 10 minutes. Then, the tube was kept at room temperature for 10 minutes. Afterwards, a loopful of supernatant was cultivated on the Mueller-Hinton agar and the plates were incubated at 37°C for 48 hours. The isolates were phenotypically identified using Gram staining, glucose, oxidase, and catalase tests (2).
Confirmation of Campylobacter Isolates
Ten suspected Campylobacter strains were subjected to 16S rRNA gene sequencing. DNA extraction was performed using PCR kit (CinnaGen, Iran). The purity of the extracted DNA was evaluated by a biophotometer (Eppendorf, Germany) based on 260 and 280 nm wavelengths ratio. PCR mixture of each reaction contained master mix (CinnaGen, Iran) and forward and reverse primers of univrsal16S rRNA. Thermal program included 95°C for 4 minutes, followed by 32 cycles of 95°C for 5 minutes, 94°C for 35 seconds, 56°C for 40 seconds, and 72°C for 50 seconds with a final extension at 72°C for 5 minutes and storage at 4°C (Table 1) (7).
Table 1.
Primers Used in the Present Study
|
Primers
|
Sequence
|
Length
|
Reference
|
|
Camp.Fa |
5´-GGATGACACTTTTCGGAGC-3´ |
19 |
7 |
|
Camp.Ra |
5´-CATTGTAGCACGTGTGTC-3´ |
18 |
7 |
|
cmeA.F
b
|
5´-TGGGGTATTCATTGTTTTGGTAG-3´ |
23 |
9 |
|
cmeA.R
b
|
5´-ATACAAATGCCGCCTCAACC-3´ |
20 |
9 |
|
cmeB.F
b
|
5´-CCAAATACCGCAAAAGGTACAG -3´ |
22 |
9 |
|
cmeB.R
b
|
5´-CCTCTGTATTTAGCGCAGGAG -3´ |
21 |
9 |
|
cmeC.F
b
|
5´-GCCAATTTTGACGTGCCTCT -3´ |
20 |
9 |
|
cmeC.R
b
|
5´-GCGGTAGTCGTGCAAAAACA -3´ |
20 |
9 |
a16S rDNA primers; bEfflux pump primers
All PCR products were run on 1% (w/v) agarose gel along with 5 µL of 100 bp DNA ladder. The pure 16S rRNA PCR products were sent to Macrogen in South Korea (http://www.macrogen.com/) for DNA sequencing. Then, BLAST analysis was done (http://www.ncbi.nlm.nih.gov/BLAST/). It means similarity of the 16S rDNA sequence of all isolates was evaluated against corresponding nucleotide sequences retrieved from GenBank.
Antibiotic Susceptibility
Antimicrobial susceptibility of Campylobacter spp. isolates was assessed by the disc diffusion method. To perform the test, each isolate was inoculated in trypticase soy broth and incubated at 37°C for 48 hours under microaerobic conditions. Then, 0.1 mL of the suspension (0.5 McFarland standard tubes (1.5×108 cells mL-1)) was picked and streaked on the Mueller-Hinton agar. Afterwards, the antibiotic discs, including ampicillin (10 µg), ceftriaxone (30 µg), cefotaxime (30 µg), gentamicin (10 µg), erythromycin (15 µg), and ciprofloxacin (5 µg) (Patanteb, Iran), were placed on the plates and incubated at 37°C. After 48-72 hours, the inhibition zone of each disk was measured, and based on Clinical and Laboratory Standards Institute (CLSI) 2018 guidelines, the susceptibility of the isolates was analyzed by WHONET 5.6 and recorded (2).
Evaluation of Efflux Pump activity in Campylobacter Isolates
Detection of efflux pump activity was done by EtBr cartwheel method. To perform the test, Ethidium Bromide was serially diluted with sterile distilled water (1/2, 1/4, and 1/8). Then, 10 mL of each solution was added into 200 mL of melted sterile nutrient agar and plated (the process was done under laminar flow hood). Then, Campylobacter isolates were streaked on the solid agar medium and incubated at 37°C for 48-72 hours. The observation of visualized transillumination under UVTEC for each isolate was considered the inactivity of efflux pump and vice versa was considered the efflux pump activity (8).
Detection of Efflux Pump Genes
Genotypic detection of the efflux pump genes was performed using specific primers shown in Table 1 (9). The experiment was carried out as mentioned above except for primers and PCR temperatures (denaturation: 94 °C; annealing: cmeA: 56°C, cmeB:56°C, and cmeC: 58°C; extension : 72°C).
Correlation Between Antibiotic Resistance and efflux Pump Activity
Phe-Arg β-naphthylamide is a special compound for eliminating the efflux pump activity. Hence, this compound was used to achieve information concerning the correlation between antibiotic resistance and efflux pump activity. To perform the test, two flasks containing 200 mL of sterile melted Mueller Hinton agar mixed with 1 mL of Phe-Arg β-naphthylamide (concentration of 0.05 mg in 30 mL D.W). Then, the isolates were streaked on the medium and ampicillin (10 µg), ceftriaxone (30 µg), cefotaxime (30 µg), gentamicin (10 µg), erythromycin (15 µg), and ciprofloxacin (5 µg) discs were placed (distance of each disk from another was 24 mm) on the medium. Afterwards, the plates were incubated at 37°C. After 48-72 hours, the inhibition zone of each disk was measured, and based on CLSI, 2018 guidelines, the susceptibility of the isolates was evaluated and recorded (10).
Statistical Analysis
Paired student’s t test was used to determine the correlation between antibiotic resistance and efflux pump activity. P values of < 0.05 were considered significant.
Results
Isolation and Identification of Campylobacter spp.
Of all samples, 10 (3.3%) Campylobacter strains were isolated. Seven (70%) and 3 (30%) strains were isolated from poultry and cows, respectively. As seen in Table 2, 9 strains belonged to Campylobacter jejuni and 1 strain belonged to Campylobacter coli. Gel electrophoresis of 16S rDNA PCR products is shown in Figure 1. As seen in this figure, all Campylobacter 16SrDNA genes had a DNA fragment of 1232 bp.
Table 2.
Confirmation of Campylobacter Isolates
Campylobacter
Strains
|
Genotypic Confirmation
|
Accession Number
|
| C1 |
Campylobacter jejuni strain ZJB020 |
CP040613.1 |
| C2 |
Campylobacter jejuni strain AR-0419 |
CP044162.1 |
| P1 |
Campylobacter jejuni strain AR-0419 |
CP044162.1 |
| P2 |
Campylobacter jejuni strain AR-0413 |
CP044171.1 |
| P3 |
Campylobacter jejuni strain NCTC13257 |
LR134502.1 |
| P4 |
Campylobacter jejuni strain NCTC13261 |
LR134500.1 |
| P5 |
Campylobacter jejuni strain NCTC13266 |
LR134496.1 |
| P6 |
Campylobacter jejuni strain CFSAN032806 |
CP045789.1 |
| C3 |
Campylobacter coli RM4661 |
CP007181.1 |
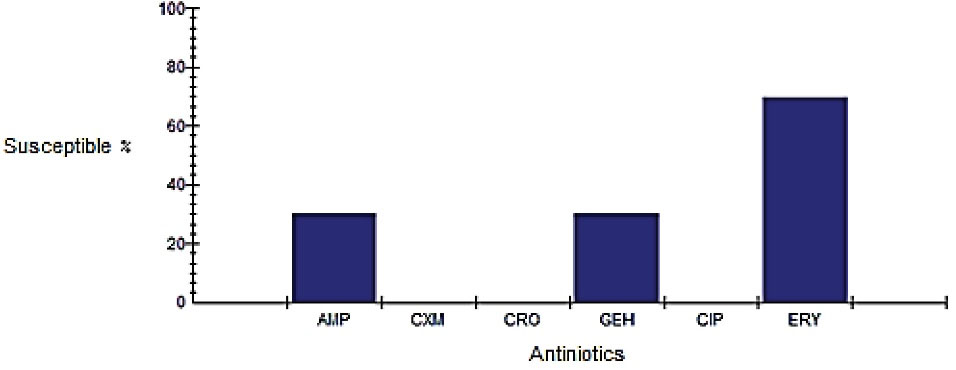
Figure 1.
Antibiotic Susceptibility of Campylobacter Isolates.AMP: Amoxicillin, CXM: Cefotaxime, Ceftriaxone, CIP: Ciprofloxacin, GEH: Gentamicin, ERY: Erythromycin.
.
Antibiotic Susceptibility of Campylobacter Isolates.AMP: Amoxicillin, CXM: Cefotaxime, Ceftriaxone, CIP: Ciprofloxacin, GEH: Gentamicin, ERY: Erythromycin.
Antibiotic Susceptibility
The results obtained indicated that all strains were resistant to ciprofloxacin, ceftriaxone, and cefixime. However, 70% and 20% of the isolates were resistant to erythromycin, ampicillin, and gentamicin, respectively (Figure 1).
Efflux Pump Activity in Campylobacter Isolates
The results obtained indicated that all the isolates showed efflux pump activity. In other words, transillumination was not observed in Campylobacter colonies. Furthermore, PCR products and gel electrophoresis showed the presence of cmeA, cmeB, and cmeC. As seen in these figures, DNA fragments of 661, 1153, and 838 bps were seen for cmeA, cmeB, and cmeC, respectively. As seen in Figure 2, cmeA gene was absent in three strains of Campylobacter jejuni (lines 1, 6, and 9). Figure 3 shows the detection of cmeB gene in all the isolates except for three strains of Campylobacter jejuni (columns 1, 5, and 10). Figure 3 shows the detection of cmeC gene in all the isolates except for two strains of Campylobacter jejuni (columns 6 and 9).
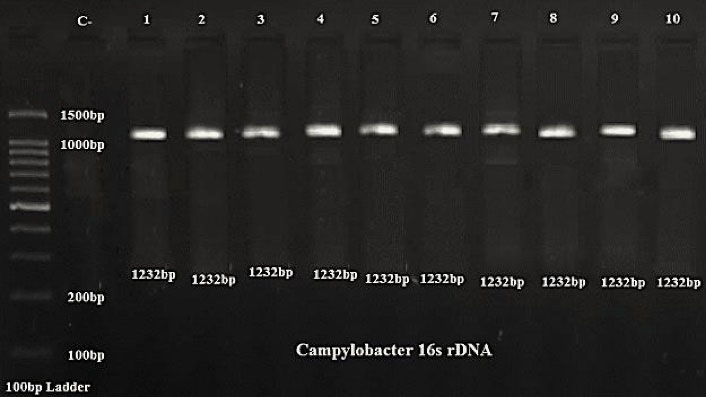
Figure 2.
Agarose Gel Electrophoresis of 16S rDNA PCR Products of Campylobacter.
.
Agarose Gel Electrophoresis of 16S rDNA PCR Products of Campylobacter.
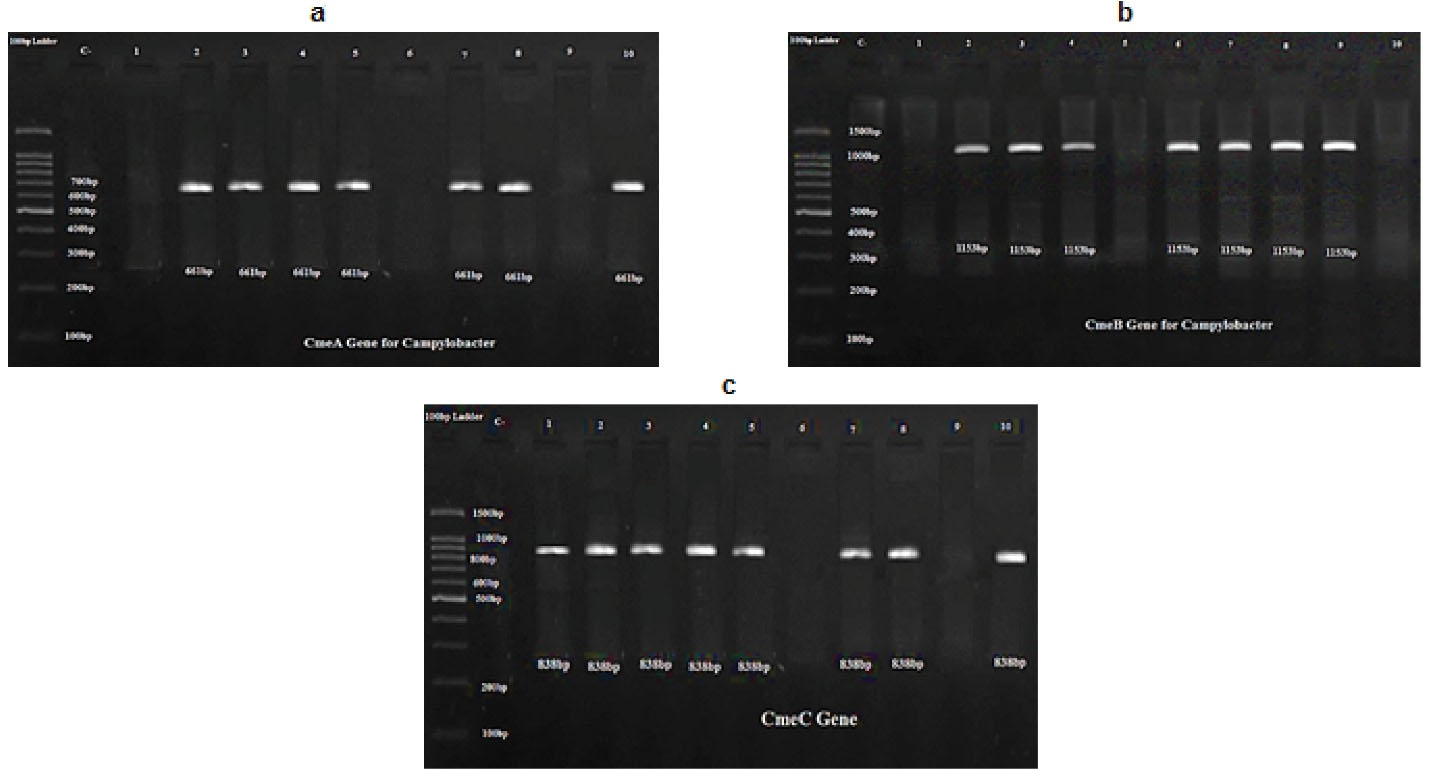
Figure 3.
PCR Product of Campylobacter Isolates showing Detection of (a) cmeA, (b)cmeB,and (c) cmeC Genes.
.
PCR Product of Campylobacter Isolates showing Detection of (a) cmeA, (b)cmeB,and (c) cmeC Genes.
Correlation Between Antibiotic Resistance and Efflux Pump Activity in Campylobacter Isolates
The results obtained indicated thatPhe-Arg β-naphthylamide could not increase the antimicrobial activity of 4 antibiotics including ampicillin, ceftriaxone, cefotaxime, and erythromycin. However, gentamicin and ciprofloxacin showed antimicrobial activity in the presence of Phe-Arg-β-naphthylamide. Statistical analysis of data in Table 3 shows significance values for ciprofloxacin and gentamicin were less than 0.05 and the value of confidence interval for both antibiotics were less than zero. Hence, a positive correlation was found between efflux pump activity and gentamicin and ciprofloxacin resistance in Campylobacter isolates. However, no correlation was found between efflux pump activity and resistance to other antibiotics in Campylobacter isolates.
Table 3.
Statistical Analysis of Efflux Pump Activity and Antibiotic Resistance in Campylobacter Isolates
|
Antibiotics
|
Levene's Test for Equality of Variances
|
|
|
T
test for Equality of Means
|
|
|
F
|
Sig.
|
T
|
df
|
Sig. (two-tailed)
|
Mean Difference
|
Standard Error Difference
|
95% CI of the difference
|
|
Lower
|
Upper
|
| Amoxicillin |
0.025 |
0.876 |
-0.331 |
18 |
0.744 |
-1.000 |
3.021 |
-7.346 |
5.346 |
|
|
|
-0.331 |
17.982 |
0.744 |
-1.000 |
3.021 |
-7.347 |
5.347 |
| Cefotaxime |
0.112 |
0.741 |
-1.857 |
18 |
0.080 |
-0.600 |
0.323 |
-1.279 |
0.079 |
|
|
|
-1.857 |
17.967 |
0.080 |
-0.600 |
0.323 |
-1.279 |
0.079 |
| Ceftriaxone |
0.112 |
0.741 |
-1.857 |
18 |
0.080 |
-0.600 |
0.323 |
-1.279 |
0.079 |
|
|
|
-1.857 |
17.967 |
0.080 |
-0.600 |
0.323 |
-1.279 |
0.079 |
| Ciprofloxacin |
6.369 |
0.021 |
-24.252 |
18 |
0.000 |
-17.600 |
0.726 |
-19.125 |
-16.075 |
|
|
|
-24.252 |
12.950 |
0.000 |
-17.600 |
0.726 |
-19.168 |
-16.032 |
| Gentamicin |
6.529 |
0.020 |
-22.204 |
18 |
0.000 |
-16.500 |
0.743 |
-18.061 |
-14.939 |
|
|
|
-22.204 |
14.015 |
0.000 |
-16.500 |
0.743 |
-18.094 |
-14.906 |
| Erythromycin |
0.067 |
0.798 |
-0.642 |
18 |
0.529 |
-1.100 |
1.714 |
-4.702 |
2.502 |
|
|
|
|
-0.642 |
17.932 |
0.529 |
-1.100 |
1.714 |
-4.703 |
2.503 |
Discussion
Campylobacter infection was recognized as a zoonosis, for which several antibiotics have been prescribed (2). In the present study, Campylobacter was isolated from cows and poultry with a prevalence of 3.3%. The rate of Campylobacter isolation in the present study was lower compared to other reports, which may be due to differences in the climate and diet of animals and poultry (11). In a different study, Baserisalehi et al reported that the prevalence of Campylobacter in poultry was relatively higher compared to camels because of their diet (1).
Recently, high consumption of antibiotics culminated in the emergence of antibiotic-resistant bacteria. In this regard, several mechanisms are responsible for developing the antibiotic-resistant bacteria (12). RNA efflux pump has an operon coded by three genes of cmeA, cmeB and cmeC which are essential for Campylobacter colonization in the intestinal tract (13). Yao et al in 2016 reported that efflux pumps increase antibiotic resistance in Campylobacter (14). Several studies showed thatefflux pumps can mediate resistance to norfloxacin, imipenem, ciprofloxacin, erythromycin, cefotaxim, and tetracycline (15,16). Our finding verified the existence of Campylobacter in the intestinal tract of domestic animals and poultry in our area. In addition, the isolates were resistant to some antibiotics such as ampicillin, cephalothin, ciprofloxacin, cefotaxime, erythromycin, and gentamicin with different percentages. In some countries such as Thailand and India, 80% and 77% of Campylobacter isolates, respectively, were resistant to fluoroquinolones (17). Even in China, 95.8%–99% of Campylobacter coli isolates were resistant to ciprofloxacin (18). Resistance to gentamicin and ciprofloxacin in the Campylobacter isolates was related to efflux pump activity. Hence, according to this data, resistance to aminoglycoside and quinolones in Campylobacter isolates was mediated by efflux pumps. Several reports supported our finding, for instance, Gibreel et al showed a relationship between resistance to Macrolides in Campylobacter spp. and efflux pump activity (18). In addition, Bolinger and Kathariou in 2017 reported a relationship between resistance to fluoroquinolones and Macrolides and the presence of cmeABC genes in Campylobacter jejuni. They stated that the MIC of macrolide-resistant Campylobacter was affected by mutations in the regulatory region of cmeABC (13). The results of the current study indicated the activity of efflux pumps in all Campylobacter isolates; however, cmeABC genes were not found in all of them.
Conclusions
Nowadays, several antibiotics are used for the treatment of Campylobacter disease. Hence, the rates of resistance to antibiotics among Campylobacter isolates are increasing. In this regard, our finding showed a high prevalence of antibiotic-resistant Campylobacter and efflux pumps activity was introduced as a major mechanism of antibiotic resistance. In addition, plasmid-mediated antibiotic resistance in some Campylobacter isolates could increase the rate of Campylobacter infections among the human population.
Acknowledgements
We appreciate the research deputy of Islamic Azad University, Kazeroun Branch, for providing laboratory facilities.
Authors’ Contribution
MBS designed the investigation and PM did all experimental procedures.
Conflict of Interests
The authors declared no conflict of interests.
References
- Baserisalehi M, Bahador N, Kapadnis BP. Isolation and characterization of Campylobacter spp from domestic animals and poultry in south of Iran. Pak J Biol Sci 2007; 10(9):1519-24. doi: 10.3923/pjbs.2007.1519.1524 [Crossref] [ Google Scholar]
- Baserisalehi M, Bahador N. A study on relationship of plasmid with antibiotic resistance in thermophilic Campylobacter spp isolates from environmental samples. Biotechnology 2008; 7(4):813-7. doi: 10.3923/biotech.2008.813.817 [Crossref] [ Google Scholar]
- Trust TJ, Logan SM. Outer membrane and surface structure of Campylobacter jejuni. In: Campylobacter Infection in Man and Animals. CRC Press; 1984. p. 133-42.
- Bravo F, Céspedes A, Morales P, Chanqueo L. [Campylobacter jejuni bacteremia in a patient with HIV infection in AIDS stage]. Rev Chilena Infectol 2019; 36(5):663-6. doi: 10.4067/s0716-10182019000500663 [Crossref] [ Google Scholar]
- Blair JM, Richmond GE, Piddock LJ. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol 2014; 9(10):1165-77. doi: 10.2217/fmb.14.66 [Crossref] [ Google Scholar]
- Grinnage-Pulley T, Zhang Q. Genetic basis and functional consequences of differential expression of the CmeABC efflux pump in Campylobacter jejuni isolates. PLoS One 2015; 10(7):e0131534. doi: 10.1371/journal.pone.0131534 [Crossref] [ Google Scholar]
- Chitra MA, Ponnusamy P, Ramesh A, Ronald BS. Isolation and identification of Campylobacter fetus subspp fetus from aborted bovine fetus. Haryana Vet 2017; 56(1):98-9. [ Google Scholar]
- Shaheli M, Baseri Salehi M, Bahador N. The influence of integrons on multidrug resistant Acinetobacter spp isolated from environment and clinical samples. Trop Biomed 2018; 35(2):354-64. [ Google Scholar]
- Lin J, Michel LO, Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother 2002; 46(7):2124-31. doi: 10.1128/aac.46.7.2124-2131.2002 [Crossref] [ Google Scholar]
- Taylor EV, Herman KM, Ailes EC, Fitzgerald C, Yoder JS, Mahon BE. Common source outbreaks of Campylobacter infection in the USA, 1997-2008. Epidemiol Infect 2013; 141(5):987-96. doi: 10.1017/s0950268812001744 [Crossref] [ Google Scholar]
- Tang Y, Fang L, Xu C, Zhang Q. Antibiotic resistance trends and mechanisms in the foodborne pathogen, Campylobacter. Anim Health Res Rev 2017; 18(2):87-98. doi: 10.1017/s1466252317000135 [Crossref] [ Google Scholar]
- Dai L, Shen Z, Yu EW, Zhang Q. Efflux pumps in Campylobacter: key players for antimicrobial resistance and environmental adaption. In: Li XZ, Elkins CA, Zgurskaya HI, eds. Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications. Cham: Springer; 2016. p. 471-87. 10.1007/978-3-319-39658-3_18.
- Bolinger H, Kathariou S. The current state of macrolide resistance in Campylobacter spp: trends and impacts of resistance mechanisms. Appl Environ Microbiol 2017; 83(12):e00416-17. doi: 10.1128/aem.00416-17 [Crossref] [ Google Scholar]
- Yao H, Liu D, Wang Y, Zhang Q, Shen Z. High prevalence and predominance of the aph(2″)-if gene conferring aminoglycoside resistance in Campylobacter. Antimicrob Agents Chemother 2017; 61(5):e00112-17. doi: 10.1128/aac.00112-17 [Crossref] [ Google Scholar]
- Hoge CW, Gambel JM, Srijan A, Pitarangsi C, Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis 1998; 26(2):341-5. doi: 10.1086/516303 [Crossref] [ Google Scholar]
- Jain D, Sinha S, Prasad KN, Pandey CM. Campylobacter species and drug resistance in a north Indian rural community. Trans R Soc Trop Med Hyg 2005; 99(3):207-14. doi: 10.1016/j.trstmh.2004.09.006 [Crossref] [ Google Scholar]
- Qin SS, Wu CM, Wang Y, Jeon B, Shen ZQ, Wang Y. Antimicrobial resistance in Campylobacter coli isolated from pigs in two provinces of China. Int J Food Microbiol 2011; 146(1):94-8. doi: 10.1016/j.ijfoodmicro.2011.01.035 [Crossref] [ Google Scholar]
- Gibreel A, Kos VN, Keelan M, Trieber CA, Levesque S, Michaud S. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob Agents Chemother 2005; 49(7):2753-9. doi: 10.1128/aac.49.7.2753-2759.2005 [Crossref] [ Google Scholar]