Avicenna Journal of Clinical Microbiology and Infection. 8(3):84-88.
doi: 10.34172/ajcmi.2021.15
Original Article
Evaluation of Slit-Skin Smear Against Punch Biopsy in Diagnosing Leprosy: A Cross-sectional Study in a Tertiary Care Centre of West Bengal
Lewith K Marak 1  , Sudipta Roy 2, Tanusree Mondal 3, Manisha Sarkar 4, *
, Sudipta Roy 2, Tanusree Mondal 3, Manisha Sarkar 4, *  , Tapashi Ghosh 1, Jayanta B Dey 1
, Tapashi Ghosh 1, Jayanta B Dey 1
Author information:
1Department of Microbiology, Bankura Sammilani Medical College, Bankura, West Bengal, India
2Department of Dermatology, Bankura Sammilani Medical College, Bankura, West Bengal, India
3Department of Community Medicine, Medical College, Kolkata, West Bengal, India
4Department of Community Medicine, Bankura Sammilani Medical College, Bankura, West Bengal, India
*Corresponding author: Manisha Sarkar, Jagatpur, Near Jagatpur High School, Post Office: Gouranga Nagar, District: North 24 Parganas, Kolkata, West Bengal, India, PIN 700159. Tel: 7974839033/ 9477464058, Email:
misdav2003@gmail.com
Abstract
Background: Leprosy is an infectious disease which faces diagnostic challenges. Slit-skin smear (SSS) is an age simple diagnostic technique, yet not commonly applied by health care providers. The study aimed to determine the effectiveness of SSS in terms of validity, diagnostic accuracy, and percentage agreement against punch biopsy in diagnosing leprosy among leprosy patients who were diagnosed with leprosy on clinical grounds only (i.e., number of skin lesions and/or peripheral nerve thickening).
Methods: An evaluation study of diagnostic tests with a cross-sectional design was conducted at a tertiary care center of Bankura. In general, 70 new untreated leprosy patients, diagnosed solely by clinical grounds (i.e., count of skin lesions and/or thickening of the nerve) and attending the dermatology outpatient department (From February 2019 to January 2020) were enrolled in this study. After excluding pure neuritic, relapse, and seriously ill patients by consecutive sampling, they were subjected to both SSS and punch biopsy using a standard process. SPSS for Windows (Version 16.0., Chicago, SPSS Inc.) was used to analyze data. Z test, chi-square test, and kappa test were conducted to test the statistical significance between the effectiveness of SSS and punch biopsy.
Results: The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio (DOR), and diagnostic accuracy of SSS were 81.81%, 95.83%, 90%, 92%, 20%, 0.19%, 102.87%, and 91.42%, respectively. Based on the results, SSS could detect acid-fast bacilli (AFB) in clinically diagnosed leprosy cases slightly less than punch biopsy, but it was statistically insignificant (Z=0.3689, P=0.71138, df=1). Finally, Cohen’s Kappa coefficient was 0.796, representing substantial agreement between SSS and punch biopsy (95% CI: 0.641-0.951).
Conclusions: Overall, SSS is more or less equally effective as compared to punch biopsy in confirming leprosy cases. Interest and training on SSS among resident doctors should be emphasized accordingly.
Keywords: Comparative evaluation, Effectiveness, Leprosy, Punch biopsy, Slit-skin smear, Sensitivity, Validity
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
New leprosy cases continue to occur in the world. In 2018, there were globally 208 619 newly registered leprosy cases (1). India contributes to nearly 60% of the global leprosy burden (2). As reflected from the child leprosy and disability cases in India, there is an increasing concern for continuous transmission and delayed case detection (2). Early detection needs an effective diagnostic tool.
Leprosy often faces diagnostic challenges, particularly at peripheral hospitals. Multiple changes in the definitions of paucibacillary (PB) and multibacillary (MB) leprosy in 1981, 1987, 1993, and 2017 (3-8) are considered as another issue in this regard. As per the World Health Organization (WHO), leprosy is diagnosed when at least one of the three cardinal signs is present, namely, definite sensory loss in a hypopigmented skin patch, thickening of peripheral nerve, and positive slit-skin smear (SSS) (7). Due to unavailability or in the expertise of health workers in SSS, most programs are using clinical criteria (i.e., the number of skin lesions and nerves involved) for classifying and providing leprosy treatment (9). However, this requires clinical expertise, and there can yet be doubtful cases that need a referral (10). The underdiagnosis of MB cases may lead to continuous transmission and increase in disability while overdiagnosis may lead to unnecessary treatment plus psychosocial consequences related to the diagnosis of leprosy.
Skin biopsy is the gold standard for diagnosis (11-13). However, biopsy has limitations, and even among the experts, some patients test negative even with definite signs of leprosy (14).
SSS is a safe, rapid, and feasible laboratory-based technique and has low sensitivity (10%-50%) but high specificity (100%) (15). There remains a hesitancy for learning and performing SSS even in the teaching institutes. This technique has its advantage. For instance, it is useful in the diagnosis of paucilesional MB cases and availability at peripheral institutions (16),but limited in the detection of bacilli less than 10 000 bacilli per milliliter (17). Although a DNA-based polymerase chain reaction has higher sensitivity, it is infeasible in resource-limited countries (18).
The recent focus of research has been shifted to the costlier molecular diagnosis which is infeasible on a routine basis in resource-poor settings. The current study can help in the confirmation of suspect cases and prevent under or over treatment of leprosy. It will also add to the existing evidence, the effectiveness of SSS in comparison to punch biopsy, and emphasize the need for better training and interest among the resident doctors for performing SSS.
The present study was thus undertaken aiming at determining the diagnostic effectiveness (i.e., validity, diagnostic accuracy, and percentage agreements) of SSS against punch biopsy in diagnosing leprosy among the new untreated clinically diagnosed leprosy patients based on the count of skin lesions and/or thickening of peripheral nerve.
Methods
An institution-based evaluation study of diagnostic tests with a cross-sectional design was conducted at the Dermatology Outpatient Department (OPD) and Microbiology Department of a Medical College and Hospital located at Bankura district of West Bengal from February 2019 to January 2020. New untreated clinically diagnosed leprosy patients based on skin lesions and nerve thickening, attending the Dermatology OPD of Bankura Sammilani Medical College and Hospital (BSMCH), Bankura were the study population. Those giving consent, having pure neuritic leprosy and relapse, and suffering from serious illness were excluded from the study. The sample size (SS) for the study was calculated based on the applied formula for the evaluation study of a provided diagnostic test. According to the existing literature, Sn for skin biopsy in detecting leprosy bacilli was 80.15% and the prevalence of leprosy in India was 30/100 000 population in districts where the disease was yet to bring under control (19,20). Considering the allowable error around the reported prevalence of the disease (6%) and putting all these values in the formula, the estimated SS was found to be approximately 63. Assuming a 10% non-participation rate, the revised SS was 70. Finally, 70 eligible patients were studied after considering the inclusion and exclusion criteria. As per records, 8-10 new cases attend the Dermatology OPD of BSMCH in a month on average. The data collection was continued for 12 months. Consecutive cases were included in the study based on eligibility criteria. If the selected case did not fulfill selection criteria and/or refused to participate in the study, the next very patients in the queue were attempted for participation. The process of case enrolment was continued until a total of 70 patients satisfying the inclusion and exclusion criteria could be interviewed and examined based on the study aim. Study variables included age, gender, religion, place of residence, and clinical examination findings of skin lesions and nerve thickening (Independent variables), as well as the result of SSS and punch biopsy test in patients and the diagnostic effectiveness in terms of validity, diagnostic accuracy, and percentage agreement (Dependent variables). The study was performed using the case record form and standard instruments required for conducting SSS (21), punch biopsy (11),and acid-fast bacilli (AFB) staining (21) by interviews, observation, clinical examination, and laboratory investigation techniques. Two samples were aseptically collected from each selected patient by performing SSS and punch biopsy to detect lepra bacilli as per a standard procedure. Mostly characteristic lesion was stained by the modified Ziehl–Neelsen stain using the standard protocol and looking for the presence of AFB (21). The active margins of lesions were chosen for punch biopsy. Hematoxylin and eosin (H&E) and Fite-Faraco stains were respectively used for histology and for demonstrating bacilli, according to the standard protocols (11). The smears and biopsy consistent with the diagnosis of leprosy were recorded, and the procedure, staining, and microscopic examinations for AFB were performed at the laboratory of the microbiology department of the institution. All patients were treated in accordance with the WHO guidelines. Data were compiled and coded in the Microsoft excel spreadsheet. Continuous variables were described by the mean and standard deviation while categorical variables were expressed in terms of proportion. Tables and figures were used for data display. Inferential statistics (e.g., sensitivity, specificity, positive and negative predictive values [PPV and NPV], diagnostic accuracy, and the like) were calculated considering the results of punch biopsy as the gold standard (11-13). Z test was employed to test the difference between two proportions while the difference between categorical variables was tested using the chi-square test. Kappa statistics was estimated to conclude about the agreement between two diagnostic tests. A P value less than 0.05 was considered significant for all statistical purposes, and the Statistical Package for the Social Sciences for Windows (version 16.0., Chicago, SPSS Inc.) was applied for analysis.
Results
The leprosy cases had a mean age of 36.79 ± 15.95 years ranging from 9 to 85 years. The socio-demographic characteristics of the leprosy cases are shown in Table 1.
Table 1.
Sociodemographic Characteristics of the Clinically Diagnosed Leprosy Patients (n = 70)
|
Socio-demographic Variables
|
Sub-variables
|
Number (%)
|
| Age |
<10 |
2 (2.8) |
| 11-20 |
7 (10.0) |
| 21-30 |
20 (28.6) |
| 31-40 |
20 (28.6) |
| 41-50 |
7 (10.0) |
| 51-60 |
7 (10.0) |
| >60 |
7 (10.0) |
| Gender |
Male |
46 (65.7) |
| Female |
24 (34.3) |
| Place of residence (district) |
Bankura |
66 (94.2) |
| Purulia |
2 (2.9) |
| Burdwan |
2 (2.9) |
Out of 70 clinically diagnosed leprosy patients, 20 cases were SSS positive. Among these SSS-positive cases, 3/4th and 1/4th were males and females, respectively. A total of 22 clinically diagnosed leprosy patients were reported to be punch biopsy positive, of whom 72.8% and 27.2% cases were male and female patients, respectively. Figure 1 shows the distribution of the smear-positive leprosy cases based on their positivity in SSS and punch biopsy individually and positivity in both diagnostic tests. Nearly two-thirds (65.7%) of the clinically diagnosed leprosy patients were negative by both the SSS and punch biopsy procedure.
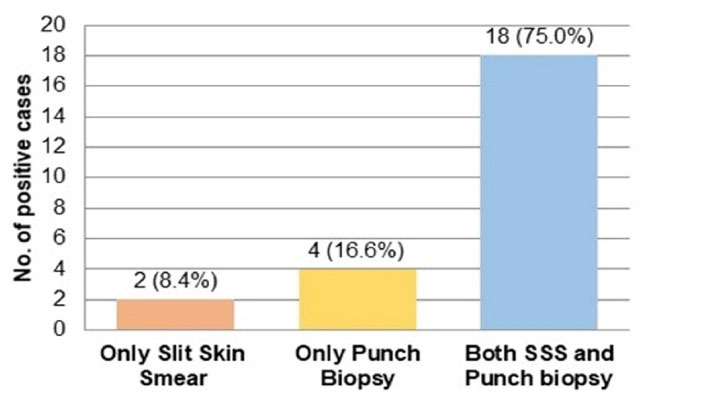
Figure 1.
Simple bar diagram showing the distribution of smear positive patients based on their positivity in SSS alone, punch biopsy alone and both SSS and punch biopsy (n=24)
Quality of is low. Provide us with original version of the figure. Leave
.
Simple bar diagram showing the distribution of smear positive patients based on their positivity in SSS alone, punch biopsy alone and both SSS and punch biopsy (n=24)
Quality of is low. Provide us with original version of the figure. Leave
There was an association between the test results of SSS and punch biopsy procedure among the new untreated clinically diagnosed leprosy patients (Table 2).
Table 2.
Comparison Between the Results of SSS (Index Test) and Punch Biopsy (Gold Standard Test) Among the Clinically Diagnosed Leprosy Patients (n = 70)
|
Procedure
|
|
Punch Biopsy
|
Total
Number (%)
|
Test Statistic
χ
2
, df,
P
-value
|
|
Positive Number (%)
|
Negative Number (%)
|
| SSS |
Positive |
18 (90) |
2 (10) |
20 (100.0) |
(χ2) = 44.572,
df = 1,
P < 0.00001 |
| Negative |
4 (8) |
46 (92) |
50 (100.0) |
| Total |
|
22 (31.4) |
48 (68.6) |
70 (100.0) |
Note. SSS: Slit skin smear.
Table 3 presents the sensitivity, specificity, PPV, NPV, positive likelihood ratio, and negative likelihood ratio of SSS as the diagnostic test against punch biopsy as the gold standard. The diagnostic odds ratio (DOR) measures the effectiveness of a diagnostic test. The DOR was 102.87. In other words, if the subject has leprosy (punch biopsy positive), the odds of positive SSS is 102.87 times that of being positive if the subject does not have leprosy (punch biopsy negative). The diagnostic accuracy of SSS represents the overall probability that a patient will be classified correctly. In the current study, the diagnostic accuracy of SSS was 91.42% against the gold standard, which is punch biopsy.
Table 3.
Diagnostic Evaluation of SSS in Comparison to Punch Biopsy in Clinically Diagnosed Leprosy Patients (n = 70)
|
Diagnostic Evaluation of SSS
|
Estimate
|
95% Confidence Interval
|
| Sensitivity |
0.818 |
0.615 to 0.927 |
| Specificity |
0.958 |
0.86 to 0.988 |
| PPV |
0.9 |
0.699 to 0.972 |
| NPV |
0.92 |
0.812 to 0.968 |
| LR+ |
19.476 |
4.985 to 77.349 |
| LR- |
0.19 |
0.078 to 0.461 |
Note. SSS: SSS: Slit skin smear; PPV: Positive predictive value; NPV: Negative predictive value; LR+: Positive likelihood ratio; LR−: Negative likelihood ratio.
The percentage positivity of punch biopsy and SSS are provided in Table 4. Although punch biopsy could detect AFB in clinically diagnosed leprosy cases slightly more compared to SSS, the difference was statistically insignificant. In other words, SSS is more or less equally effective in detecting leprosy cases as compared to punch biopsy.
The concordance between punch biopsy and SSS was evaluated by Cohen’s kappa coefficient. The number of observed agreements between punch biopsy and SSS and that of the expected agreements by chance between punch biopsy and SSS were 64 (91.43% of the observations) and 40.6 (57.96% of the observations), respectively. The Cohen’s kappa coefficient was computed as 0.796 with a standard error of kappa of 0.079 and a 95% confidence interval of 0.641-0.951. The Cohen’s kappa coefficient value of 0.796 represented substantial agreement between punch biopsy and SSS, and this was statistically significant (95% CI: 0.641-0.951).
Table 4.
Percentage Positivity of SSS and Punch Biopsy in Clinically Diagnosed Leprosy Patients (n = 70)
|
SSS
|
Punch Biopsy
|
Total Cases
No. (%)
|
Test Statistic
Z-value,
P
-value, df
|
Positive
No. (%)
|
Negative
No. (%)
|
Positive
No. (%)
|
Negative
No. (%)
|
| 20 (28.57%) |
50 (71.42%) |
22 (31.42%) |
48 (68.57%) |
70 (100.0%) |
Z = 0.3689, P = 0.71138, df = 1 |
Note. SSS: Slit skin smear.
Discussion
A one-year cross-sectional study was conducted among 70 new untreated leprosy cases diagnosed on clinical grounds (skin lesions and nerve thickening) who were subjected to both SSS and punch biopsy aiming at finding the diagnostic effectiveness of SSS against punch biopsy. The clinical diagnosis of leprosy relies upon the recognition of disease symptoms which is dependent on the expertise of the physician. There are problems with undertreatment of MB leprosy cases presenting with few lesions such as the continued transmission of the disease agent and an increase in disability which was otherwise preventable. On the other hand, some problems are linked with overtreatment, particularly in leprosy mimickers such as tinea, pityriasis alba, granuloma annulare, eczema, annular psoriasis, sarcoidosis, and mycosis fungoides (12). Overtreatment may have its psychosocial impact linked with the diagnosis of leprosy. It is noteworthy that the stigma attached to the disease and its consequences on mental health and well-being cannot be ignored with the globally increasing prevalence of mental illness. Moreover, the suspect leprosy cases may not show the cardinal signs of leprosy, or it may often be doubtful. SSS is no more mandatory for the initiation of MDT as per WHO as it is possible to classify leprosy without SSS results (22)although in the beginning the degree of SSS, positivity was needed to classify patients into MB and PB leprosy and to treat patients accordingly. This was due to the lack of interest and expertise among the staff and field workers for performing SSS. Considering that SSS is just an optional criterion as per WHO in order to diagnose leprosy based on cardinal signs, the interest to learn SSS has further decreased among the residents or health care workers.
Based on the results of the current study, the mean age of the cases was 36.79 ± 15.95 years. In addition, the ratio of male to female was 1.9: 1, indicating male preponderance, which is in line with the findings of Baddam et al (23). The majority of patients (28.57%) were of the age group of 21-40 years. Similar findings were found by Soni et al (24)and Patra et al (25). The maximum number of cases (94%) were reported from the Bankura district.
The findings further revealed that 24 patients (34.28%) were found to be smear-positive by either procedure while 46 cases (65.71%) were smear-negative by both procedures. Out of 24 smear-positive patients, 18 cases (75%) were positive by both procedures. In 20 patients, the detection of Mycobacterium leprae was possible by SSS (83% of smear-positive cases) while detection by punch biopsy was possible in 22 patients (91% of smear-positive cases). In this study, the microorganism was detected in 2 (8.33%) patients by SSS but not via punch biopsy. However, the Mycobacterium leprae microorganism was detected in 4 (16.66%) patients by punch biopsy rather than SSS. This finding is comparable with the obtained data by Patil et al (26),demonstrating that 11.36% and 7.95% of cases were positive by SSS alone and by punch biopsy alone, respectively.
In this study, there was a significant difference in detection by the two methods (P < 0.00001), which conforms to the obtained results by Ponnighaus et al (27)and Patil et al (26).
Considering punch biopsy as the gold standard, the sensitivity and specificity of SSS were 81.81% and 95.83% in the present study, respectively. The specificity of SSS reported by various studies is well correlated with that of the current study. The International Leprosy Association reported a sensitivity of 10%-50% and specificity of 100% (15). Patil et al also found the sensitivity and specificity of 26.47% and 95%, respectively (26).
PPV and NPV were 90% and 92%, respectively, which is in conformity with the results of Desikan et al (28) (PPV and NPV were 87.7% and 99.4%, respectively).
Based on the findings, the diagnostic accuracy of SSS was found to be 91.42%, indicating that SSS has a potential ability to detect lepra bacilli similar to punch biopsy. Thus, SSS can be used as an effective and safe diagnostic method for confirming leprosy in peripheral and remote centers where modern diagnostic tools are unavailable.
Implications of the Study
Although SSS is a conventional method, it is simple, reasonably reliable, valid, and generally highly effective for diagnosing leprosy in peripheral and referral centers where modern diagnostic tools are not accessible or available. Therefore, awareness should be created among the health care professionals through education regarding the simplicity and effectiveness of the procedure so that if needed, to easily perform it in remote locations where modern diagnostic modalities are not available. SSS should be made user-friendly with increased training and evaluation among the resident doctors. This method was found to be equally effective as punch biopsy. Therefore, SSS, which is a simpler procedure and equally effective as punch biopsy, may be an acceptable alternative for confirming leprosy.
Strengths
The study provided a comprehensive diagnostic evaluation of SSS as an effective and acceptable alternative to punch biopsy for leprosy confirmation. It may also help in developing an integrated diagnostic approach in resource-limited peripheral settings, particularly whenever there is a clinical dilemma in the diagnosis of leprosy.
Limitations
The quality control of the reagents and the instruments was as per the institutional norms, and no separate quality control methods were considered by the researcher.
Conclusions
Overall, SSS is a simple procedure that can be performed as an outpatient procedure and is equally effective in detecting Mycobacterium Leprae in comparison to punch biopsy. The findings revealed that there was substantial agreement (A kappa coefficient value of 0.796) between punch biopsy and SSS in diagnosing leprosy. SSS as a simple and valid procedure can effectively compete with costly procedures for the primary diagnosis of leprosy. Thus, there is a need to emphasize the capacity building of medical technologists, doctors, and other paramedical staff, and the like on how to perform the SSS and to make it user-friendly by training programs. Finally, SSS may be an acceptable alternative to punch biopsy for confirming leprosy, especially in remote locations.
Ethical Approval
Data were collected after obtaining ethical clearance from the Institutional Ethics Committee (Memo No. BSMC/Aca/71 dated 09-01-2019). Informed consent was taken from all eligible cases, and participants were ensured of anonymity and confidentiality.
Conflict of Interests
None.
Funding
None.
Acknowledgements
The author is grateful to all the faculties, postgraduate trainees, medical technologists, and other staff of the Microbiology Department of BSMCH, Bankura.
References
- WHO. Leprosy [Internet]. [updated 2019 Sep 10; cited 2021 Mar 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/leprosy.
- Central Leprosy Division, New Delhi and Central Leprosy Teaching & Research Institute, Chengalpattu, TN, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. National Leprosy Eradication Programme (NLEP) Training manual for Medical Officers 2019. New Delhi: 2019. p. 69.
- World Health Organization. A Guide to leprosy control [Internet]. Geneva: World Health Organization; Sold by WHO Publications Centre USA; 1980 [cited 2021 May 14]. Available from: https://primoa.library.unsw.edu.au/permalink/f/1gq3lal/UNSW_ALMA21119242100001731.
- Georgiev GD, McDougall AC. Skin smears and the bacterial index (B) in multiple drug therapy leprosy control programs: an unsatisfactory and potentially hazardous state of affairs. Int J Lepr Other Mycobact Dis 1988; 56(1):101-4. [ Google Scholar]
- WHO Expert Committee on Leprosy. Sixth report. World Health Organization Technical report series 768 [Internet]. Geneva: World Health Organization; 1988 [cited 2021 May 14]. 51 p. Report no.:6. Available from: https://apps.who.int/iris/handle/10665/37409.
- Mahajan VK. Slit-skin smear in leprosy: lest we forget it!. Indian J Lepr 2013; 85(4):177-83. [ Google Scholar]
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy [Internet]. New Delhi: World Health Organization, Regional Office for South-East Asia; 2017 [cited 2021 May 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/274127/9789290226383-eng.pdf.
- World Health Organization. Global Leprosy Strategy 2016–2020. Accelerating towards a leprosy-free world. Monitoring and Evaluation Guide [Internet]. New Delhi: World Health Organization, Regional Office for South-East Asia; 2017 [cited 2021 May 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/254907/9789290225492-eng.pdf?sequence = 1&isAllowed = y.
- World Health Organization. Leprosy elimination. Classification of Leprosy [Internet]. World Health Organization. [cited 2021 May 14]. Available from: https://www.who.int/lep/classification/en/.
- World Health Organization. Leprosy elimination. Diagnosis of Leprosy [Internet]. World Health Organization. [cited 2021 May 14]. Available from: https://www.who.int/lep/diagnosis/en/.
- Singh A, Weng X, Nath I. Skin biopsy in leprosy. In: Khopkar U, ed. Skin Biopsy-Perspectives. Croatia: IntechOpen; 2011. p. 73-86. 10.5772/22227
- Naveed T, Shaikh ZI, Anwar MI. Diagnostic accuracy of slit skin smears in leprosy. Pak Armed Forces Med J 2015; 65(5):649-52. [ Google Scholar]
- Gulati A, Kaushik R, Kaushal V. Cytological diagnosis of lepromatous leprosy: a report of two cases with review of literature. J Cytol 2012; 29(3):203-4. doi: 10.4103/0970-9371.101178 [Crossref] [ Google Scholar]
- The diagnosis and classification of leprosy. Int J Lepr 2002;70(1 Suppl):S23-31. Available from: http://ila.ilsl.br/pdfs/v70n1s1a05.pdf.
- Oskam L. Diagnosis and classification of leprosy. Lepr Rev 2002; 73:S17-26. [ Google Scholar]
- Gautam M, Jaiswal A. Forgetting the cardinal sign is a cardinal sin: slit-skin smear. Indian J Paediatr Dermatol 2019; 20(4):341-4. doi: 10.4103/ijpd.IJPD_74_19 [Crossref] [ Google Scholar]
- Shepard CC, McRae DH. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis 1968; 36(1):78-82. [ Google Scholar]
- Siwakoti S, Rai K, Bhattarai NR, Agarwal S, Khanal B. Evaluation of polymerase chain reaction (PCR) with slit skin smear examination (SSS) to confirm clinical diagnosis of leprosy in eastern Nepal. PLoS Negl Trop Dis 2016; 10(12):e0005220. doi: 10.1371/journal.pntd.0005220 [Crossref] [ Google Scholar]
- de Campos Soriani Azevedo M, Ramuno NM, Fachin LR, Tassa M, Rosa PS, de Faria Fernandes Belone A. qPCR detection of Mycobacterium leprae in biopsies and slit skin smear of different leprosy clinical forms. Braz J Infect Dis 2017; 21(1):71-8. doi: 10.1016/j.bjid.2016.09.017 [Crossref] [ Google Scholar]
- Rao PN, Suneetha S. Current situation of leprosy in India and its future implications. Indian Dermatol Online J 2018; 9(2):83-9. doi: 10.4103/idoj.IDOJ_282_17 [Crossref] [ Google Scholar]
- Central Leprosy Division and Central Leprosy teaching and training institute. Ministry of Health and Family Welfare. Government of India. National Leprosy eradication. Training manual for medical officers 2019 [Internet]. India: 2019 Feb 22 [cited 2021 May 14]. 69 p. Available from: https://cltri.gov.in/MO%20Training%20Manual.pdf.
- WHO Expert Committee on Leprosy. Seventh report. World Health Organization Technical report series 874 [Internet]. Geneva: World Health Organization; 1998 [cited 2021 May 14]. 43 p. Report no.:7. Available from: https://apps.who.int/iris/handle/10665/42060.
- Baddam GS, Sana VP, Maddali M, Ramachandra S. Validity of FNAC for the diagnosis of leprosy. Indian J Lepr 2018; 90(2):137-46. [ Google Scholar]
- Soni S, Shah N, Bhalodia J. Clinicopathological correlation in leprosy. Int J Med Sci Public Health 2019; 8(6):459-64. [ Google Scholar]
- Patra AC, Sarkar S, Burman SD, Vittal KP. Leprosy among the tribal population: a profile from a rural and tribal based tertiary care leprosy center of eastern India. IP Indian J Clin Exp Dermatol 2019; 5(1):75-9. [ Google Scholar]
- Patil AB, Singh AL, Madke B, Ghatge A, Jawade S, Singh S. Comparison of efficacy of slit skin smear and fite faraco stain on histopathology specimens in cases of leprosy. J Clin Diagn Res 2020; 14(3):1-4. doi: 10.7860/jcdr/2020/43019.13543 [Crossref] [ Google Scholar]
- Ponnighaus JM, Lienhardt C, Lucas S, Fine PE, Sterne JA. Comparison of bacillary indexes in slit-skin smears, skin and nerve biopsies; a study from Malawi. Int J Lepr Other Mycobact Dis 1997; 65(2):211-6. [ Google Scholar]
- Desikan KV, Rao KV, Bharambe MS, Rao PV. Appraisal of skin smear reports of field laboratories. Lepr Rev 2006; 77(4):311-6. [ Google Scholar]