Avicenna Journal of Clinical Microbiology and Infection. 8(4):117-122.
doi: 10.34172/ajcmi.2021.22
Original Article
Prevalence and Antibiotic Resistance Pattern of Pathogenic Bacteria Isolated From Urinary Tract Infections in Qal’at Saleh Hospital, Iraq
Mohammed Allami 1, 2  , Eman J. Mohammed 3
, Eman J. Mohammed 3  , Faten Alazzawi 4, Masoumeh Bahreini 2, *
, Faten Alazzawi 4, Masoumeh Bahreini 2, * 
Author information:
1Department of Dentistry, Al-Manara College for Medical Sciences, Misan, Iraq
2Department of Biology, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran
3Department of Biology, Faculty of Science, Mustansiriyah University, Baghdad, Iraq
4Microbiology Laboratory, Qal’at Saleh Hospital, Misan, Iraq
*
Corresponding author: Masoumeh Bahreini, Department of Biology, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran, Tel: +98 9153152856, Fax: +98 5138796416, Email:
mbahreini@um.ac.ir
Abstract
Background: Antibiotic resistance emerged in the pathogens causing urinary tract infections (UTIs) and became widespread. Moreover, increasing drug resistance has highlighted the need to evaluate the antibiotic resistance pattern to improve experimental treatment. The purpose of this study was to evaluate the bacteria causing UTIs and their susceptibility patterns based on the geographical area.
Methods: The present study was conducted on outpatients referred to Qal’at Saleh Hospital in Iraq from January 2018 to January 2019. The pathogenic bacteria were detected using API 20E kit. The antimicrobial susceptibility testing was conducted using the disk diffusion method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI).
Results: Of 216 isolates, 87.9% contained gram-negative bacteria and 12.03% contained gram-positive bacteria. In this study, Escherichia coli was identified as the main cause of UTIs. Of all the isolates, 73.61% were resistant to three or more classes of antibiotics. The antibiotic susceptibility and resistance patterns of all isolates showed that amikacin and ciprofloxacin had the highest activity against gram-negative bacteria and vancomycin, amikacin, and levofloxacin had the highest activity against gram-positive bacteria.
Conclusions: Due to the widespread resistance to drugs used in the treatment of UTIs, it is difficult to select the appropriate drugs for treating UTIs. UTI affects different age groups; therefore, sufficient knowledge should be transferred to the community to prevent these infections. If urine culture is unavailable, or it is impossible to wait for antibiotic susceptibility testing, Amikacin and Vancomycin might be the best candidates for UTI treatment.
Keywords: Urinary tract infections, Drug resistance, Anti-bacterial agents, Multidrug resistance
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Urinary tract infections (UTIs) are among the most common infections, affecting 150 million people worldwide each year (1). Most UTIs are caused by the transmission of bacteria from the fecal flora and through the urethra (2). Some human physiological and anatomical factors, incomplete bladder emptying, and vesicoureteral reflux, especially in the elderly and pregnant women, play major roles in increasing the prevalence of UTI. UTIs are mainly caused by bacteria, and sometimes by viruses, fungi, and parasites (3,4). Among bacteria, gram-negative bacteria are the most common causes of UTIs, including Escherichia coli, Klebsiella spp., Proteus mirabilis, Pseudomonas aeruginosa, Acinetobacter spp., and Serratia spp. E. coli is the most prevalent agent that has been isolated from the urine samples of 70% to 90% of infected people (5). Only 10% of reported UTI cases are caused by gram-positive bacteria, including Staphylococcus aureus, Streptococcus agalactiae,and Enterococcus faecalis (6).
The most appropriate treatment for bacterial infections is the selection of an antibiotic with high efficiency. Due to the acquisition of antibiotic resistance genes by bacteria over time and the changing antimicrobial resistance pattern of bacteria, choosing the right antibiotic for treatment has become a challenge, mostly based on information obtained from their antimicrobial resistance pattern in the area (7-9). Researchers believe that the causes of UTIs and drug susceptibility patterns can vary depending on geographical, social, and biological conditions (10-12). In many infectious diseases, including UTI, the physician needs to begin treatment before identifying the cause of the infection and its antibiotic susceptibility, which requires sufficient knowledge of the possible cause of the infection and its antibiotic susceptibility pattern in order to prescribe the appropriate medication (13).
The emergence of antibiotic resistance in UTIs is a serious public health problem, especially in developing countries, where there is a high level of ignorance, poverty, and poor hygiene practices (14). Over the past few decades, reports of antibiotic resistance in UTI-causing bacteria have increased dramatically worldwide. Rapid detection and culture facilities are not available in many areas in developing countries, which may lead to misdiagnosis or self-medication, resulting in increased antibiotic resistance among urinary tract pathogens (13). Therefore, the selection of appropriate antibiotics for the treatment of UTI should be based on antibiotic susceptibility profiles of different bacteria causing the infection.
In developing countries, including Iraq, UTIs occur every year, and the administration of inappropriate antibiotics, due to lack of information about pathogens and their resistance profiles, increases the duration of treatment and drug resistance in other bacteria. The aim of this study was to evaluate the bacterial species isolated from outpatients with UTI and to determine their antibiotic susceptibility pattern at Qal’at Saleh Hospital in Maysan Governorate, Iraq.
Materials and Methods
Uropathogenic Isolates
This study was performed from January 2018 to January 2019 at Qal’at Saleh Hospital in Maysan Governorate, southern Iraq. A total of 830 patients with clinical symptoms of UTI were referred to the bacteriology laboratory, including 460 (55.4%) females and 370 (44.5%) males.
Bacterial Culture
The inclusion criteria in this study included not taking specific antibiotics, absence of any infectious disease, and not being hospitalized for two weeks before referring to the laboratory. The samples were collected from the mid-stream urine, cultured on blood agar and MacConkey agar media (Salucea, New Zealand), and incubated at 37°C for 24 hours. The samples with a colony count of ≥105 CFU/mL were considered positive UTI samples (15). The isolates were identified based on their morphology and using Api20 kit (BioMérieux, France) and divided into two groups of gram-negative and gram-positive bacteria.
Antibiotic Susceptibility Test
The antimicrobial susceptibility testing was performed using the Kirby-Bauer disk diffusion method on Müller-Hinton agar medium (Merck Co., Germany) according to the guidelines of Clinical and Laboratory Standards Institute (CLSI, 2017) (16). Twenty-four commercial antibiotic discs (Bioanalyse Co., Turkey) were used including amikacin (AK, 30 μg), vancomycin (VA, 10 μg), nalidixic acid (NA, 30 μg), augmentin (AMC, 30 μg), gentamicin (GEN, 10 μg), norfloxacin (NOR, 10 μg), ticarcillin (TI, 75 μg), piperacillin (PI, 100 μg), doxycycline (Do, 30 μg), ceftazidime (CAZ, 30 μg), cefixime (CFM, 5 μg), nitrofurantoin (NIT, 100 μg), imipenem (IMP,10 μg), aztreonam (AZT,30 μg), ciprofloxacin (CIP, 5 μg), trimethoprim (TMP, 5 μg), ceftriaxone (CTR, 30 μg), ampicillin (AMP, 25 μg), cefoxitin (CX, 30 μg), linezolid (L, 10 μg), clindamycin (CD, 2 μg), co-trimethoprim (COT, 30 μg), erythromycin (E, 10 μg), and levofloxacin (Lev,5 μg).
Statistical Analysis
Data were analyzed using IBM SPSS version 22.0 (IBM SPSS Inc., USA). Fisher’s exact test was used for statistical analysis at a significance level of P < 0.05.
Results
Etiological Characteristics of UTIs
In this study, 830 patients with clinical symptoms of UTI were referred to the laboratory; out of 216 positive urine culture samples, 63.89% were female (n=138) and 36.11% were male (n=78). According to the chi-square test, there was a significant relationship between female gender and UTI (P < 0.05). The patient’s ages ranged from 8 months to 87 years. Among all isolates, 190 isolates (87.97%) were gram-negative bacteria and 26 isolates (12.03%) were gram-positive bacteria. No sample was found to contain both gram-negative and gram-positive bacteria. in this study, E. coli, P. mirabilis, E. aerogenesis, S. aureus, and E. faecalis were isolated from UTI samples. Among gram-negative uropathogens, E. coli and P. mirabilis were the most frequently isolated bacteria, with prevalence rates of 47.2% and 29.62%, respectively. The most common gram-positive uropathogen was S. aureus, with a prevalence of 9.7% (Figure 1). The overall prevalence of bacteria was higher among women than among men and E. faecalis was not observed among male patients (Table 1). The highest prevalence was observed in the age group of 0-5 years (32.4%), while the lowest number of isolates was seen in the age group of 50-84 years (3.7%), all of which were E. coli. The number of UTI cases decreased with increasing age (Figure 2).
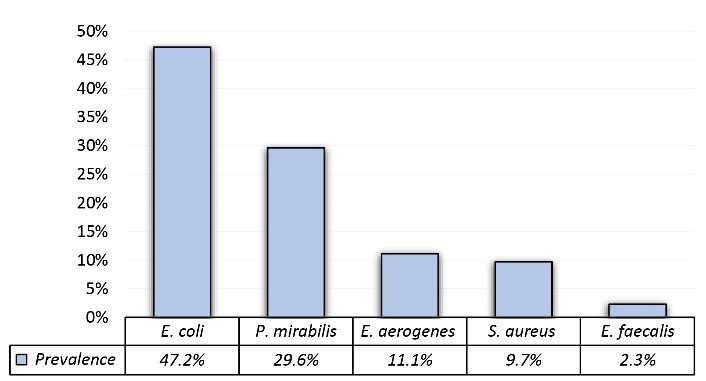
Figure 1.
Prevalence of Bacteria Isolated from Urine Samples of Outpatients.
.
Prevalence of Bacteria Isolated from Urine Samples of Outpatients.
Table 1.
Comparison of the Distribution of Isolates in Both Males and Females
|
Gender
|
E. coli
|
P. mirabilis
|
E. aerogenes
|
S. aureus
|
E. faecalis
|
Total
|
| Female |
65 |
35 |
19 |
14 |
5 |
138 |
| Man |
37 |
29 |
5 |
7 |
0 |
78 |
| Total |
102 |
64 |
24 |
21 |
5 |
216 |
|
P value |
0.03 |
0.02 |
0.001 |
0.02 |
0.06 |
|
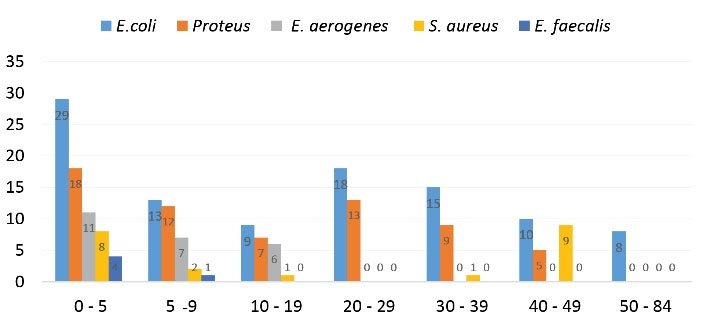
Figure 2.
Frequency of Pathogens among Different Age Groups.
.
Frequency of Pathogens among Different Age Groups.
Drug Resistance Pattern
The antibiotics used in this study were selected according to the guidelines of the Iraqi Ministry of Health for culture of urine samples. The antibiotic resistance and sensitivity of gram-negative and gram-positive bacteria are detailed in Tables 2 and 3. AK and CIP showed the highest activity against E. coli and P. mirabilis. While AMC and TI showed the lowest activity against E. coli, and TMP and NIT showed the lowest activity against P. mirabilis. E. aerogenes showed the highest sensitivity to AK and CAZ and lowest sensitivity to AMC. In general, the antibiogram profiles of gram-negative bacteria showed that AK and CIP were the most effective drugs. E. faecalis showed resistance to AMC, COT, and CTR, while the most effective antibiotics included VA and AK. S. aureus showed the highest resistance to AMP and the lowest resistance to VA and LEV. Only one isolate of P. aeruginosa was identified, which was highly resistant to most of the antibiotics used; however, IMP (78%) and AK (63%) were the most effective antibiotics against the bacterium. The resistance rates of gram-negative and gram-positive UPEC isolates to different antibiotics are shown in Figures 3 and 4.
Table 2.
Antibiotic Susceptibility Pattern of Gram-Negative Bacteria Isolated from Urine Samples of Outpatients
|
Ab
|
E. coli =
102
|
P. mirabilis
= 64
|
E. aerogenes
= 24
|
Total
|
P
Value
|
|
R (%)
|
I (%)
|
S (%)
|
R (%)
|
I (%)
|
S (%)
|
R (%)
|
I (%)
|
S (%)
|
R (%)
|
I (%)
|
S (%)
|
| AK |
2.94 |
14.7 |
82.35 |
9.37 |
21.87 |
68.75 |
8.33 |
4.16 |
87.5 |
6.88 |
13.57 |
79.53 |
0.014 |
| CIP |
36.27 |
8.82 |
54.9 |
37.5 |
3.12 |
59.37 |
25 |
4.16 |
66.66 |
32.92 |
5.36 |
60.31 |
0.001 |
| GEN |
29.41 |
20.58 |
50 |
50 |
10.93 |
39.06 |
29.16 |
4.16 |
45.83 |
36.19 |
11.89 |
44.96 |
0.001 |
| NIT |
27.45 |
26.47 |
46.07 |
81.25 |
6.25 |
12.5 |
58.33 |
29.16 |
12.5 |
55.67 |
20.62 |
23.69 |
0.018 |
| NOR |
40.19 |
13.72 |
46.07 |
40.62 |
7.81 |
51.56 |
29.16 |
4.16 |
66.66 |
36.65 |
8.56 |
54.76 |
0.085 |
| CAZ |
48.03 |
14.7 |
37.2 |
53.12 |
0 |
46.87 |
16.66 |
12.5 |
70.83 |
39.27 |
9.06 |
51.63 |
0.03 |
| CTR |
55.88 |
7.84 |
36.27 |
53.12 |
0 |
46.87 |
50 |
0 |
50 |
53 |
2.61 |
44.38 |
0.043 |
| AT |
62.74 |
2.94 |
34.31 |
73.43 |
6.25 |
20.31 |
62.5 |
4.16 |
33.33 |
66.22 |
4.45 |
29.31 |
0.001 |
| CFM |
53.92 |
13.72 |
32.35 |
53.12 |
1.56 |
45.31 |
50 |
4.16 |
45.83 |
52.34 |
6.48 |
41.16 |
0.054 |
| NA |
74.5 |
10.78 |
14.7 |
68.75 |
9.37 |
21.87 |
58.33 |
25 |
16.66 |
67.19 |
15.05 |
17.74 |
0.004 |
| TMP |
78.43 |
6.86 |
14.7 |
85.93 |
10.93 |
3.12 |
87.5 |
4.16 |
8.33 |
83.95 |
7.31 |
8.71 |
0.241 |
| PI |
80.39 |
6.86 |
12.74 |
70.31 |
0 |
29.68 |
83.33 |
8.33 |
8.33 |
78.01 |
5.06 |
16.91 |
0.118 |
| TI |
81.37 |
7.84 |
10.78 |
68.75 |
1.56 |
29.68 |
83.33 |
8.33 |
8.33 |
77.81 |
5.91 |
16.26 |
0.006 |
| AMC |
81.37 |
11.76 |
6.86 |
70.31 |
7.81 |
21.87 |
83.33 |
12.5 |
4.16 |
78.33 |
32.07 |
10.96 |
0.341 |
Ab: antibiotic, R: resistant, I: intermediate, S: susceptibility, Total: The amount of susceptibility, intermediate activity, and resistance to each antibiotic in total Gram-negative isolates.
Table 3.
Antibiotic Susceptibility Pattern of Gram-Positive Bacteria Isolated from Urine Samples of Outpatients
|
E. faecalis =
5
|
S. aureus
= 21
|
|
Ab
|
S (%)
|
I (%)
|
R (%)
|
Ab
|
S (%)
|
I (%)
|
R (%)
|
| VA |
100 |
0 |
0 |
VA |
100 |
0 |
0 |
| AK |
76.19 |
14.28 |
9.52 |
LEV |
100 |
0 |
0 |
| L |
52.38 |
4.76 |
42.85 |
CIP |
80 |
20 |
0 |
| CIP |
47.61 |
4.76 |
47.61 |
NOR |
80 |
20 |
0 |
| CX |
42.85 |
4.76 |
52.38 |
DO |
40 |
20 |
40 |
| NOR |
33.33 |
9.52 |
57.14 |
L |
40 |
0 |
60 |
| GEN |
28.57 |
4.76 |
66.66 |
E |
20 |
20 |
60 |
| CD |
28.57 |
4.76 |
66.66 |
NIT |
20 |
20 |
60 |
| DO |
23.8 |
19.04 |
57.14 |
AMP |
20 |
0 |
80 |
| NIT |
9.52 |
9.52 |
80.95 |
|
|
|
|
| AMP |
4.76 |
4.76 |
90.47 |
|
|
|
|
| AMC |
4.76 |
0 |
95.23 |
|
|
|
|
| COT |
4.76 |
0 |
95.23 |
|
|
|
|
| CTR |
0 |
4.76 |
95.23 |
|
|
|
|
Ab: antibiotic; R: resistant; I: intermediate; S: susceptibility.
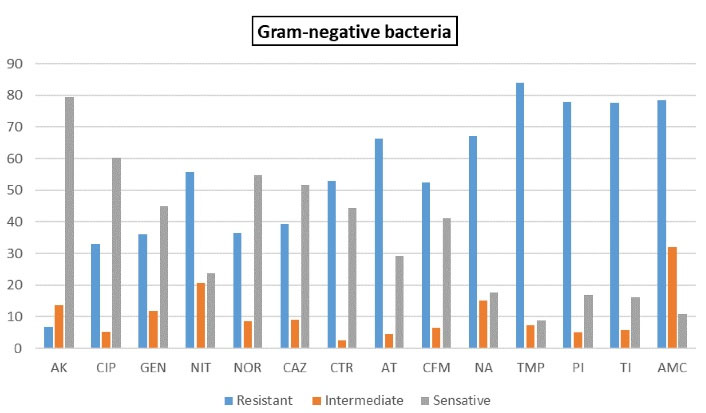
Figure 3.
Drug Resistance in Gram-Negative Bacteria.
.
Drug Resistance in Gram-Negative Bacteria.
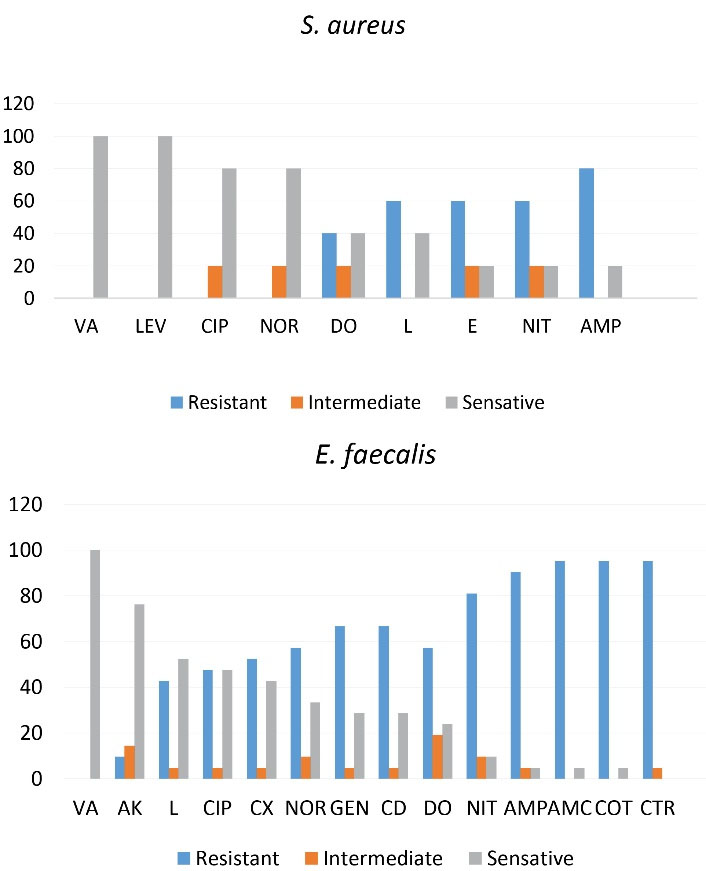
Figure 4.
Drug Resistance in E. faecalis and S. aureus.
.
Drug Resistance in E. faecalis and S. aureus.
Multidrug Resistance
Of all the isolates, 73.61% were resistant to three or more classes of antibiotics. The prevalence of multidrug resistance (MDR) of E. coli, P. mirabilis, and E. aerogenes was reported to be 83.3%, 90.62%, and 87.5%,respectively. All S. aureus isolates were MDR and resistant to all classes of antibiotics. In contrast, the lowest MDR rate belonged to E. faecalis (40%). The resistance to the highest number of antibiotic classes was observed in S. aureus (9 classes) and P. mirabilis (8 classes), respectively. The MDR rate of each strain is shown in Figure 5.
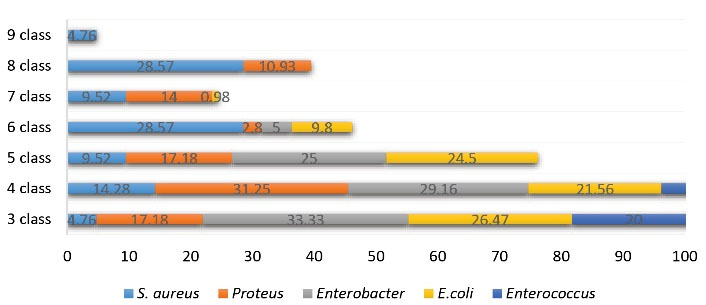
Figure 5.
Multidrug Resistance of Bacteria.
.
Multidrug Resistance of Bacteria.
Discussion
The antibiotic resistance of uropathogenic strains is a significant concern for treating UTIs and it is increasing day by day. Improper administration and incomplete course of antibiotics to treat this infection increased the drug resistance of pathogens (17,18). Therefore, there is a need for continuous monitoring of UTI-causing agents and their resistance/sensitivity pattern in a region.
The present study showed that the prevalence of UTI is higher in women than in men (138 versus 78), which is consistent with studies conducted in Germany (19), France (20), Turkey (21), Iran (22), and India (7). UTIs have been reported in all age groups, and some studies have reported that the prevalence of the disease increases with age (1,23). However, in this study, the highest prevalence of the disease (32.4%) was observed in the age range of 0-5 years, which is in line with a study conducted by Luty et al in Iraq (18). The high prevalence of infection in childhood may be due to the poor culture and lack of mother’s knowledge about child health.
The results showed that gram-negative bacteria are the most common causes of UTI; the highest prevalence of gram-negative and gram-positive bacteria was 87.9% and 12.03%, respectively. Ullah et al in Pakistan (73%) and Kimando et al in Kenya (89.5%) reported a higher prevalence of gram-negative bacteria than gram-positive bacteria, which is consistent with our study (24,25). Gram-negative bacteria have several unique structures or virulence factors that help them attach to cells in the urinary tract and not be excreted in the urine, which allows them to multiply and invade tissue (26). The present results showed that E. coli is the most prevalent gram-negative bacterium (47.2%) among UTI patients, which is consistent with studies conducted in Iraq (18,27), Iran (22), and India (28). Enterococcus has been reported as the second leading cause of UTIs (29), but it had the lowest prevalence (2.3%) in the present study, which is consistent with studies conducted in Iraq (18,30), India (7), Iran (29), and Uganda (31). Yolbaş et al in Turkey (15) and Vakili et al in Iran (32) showed that 2.7% of UTI cases were caused by Klebsiella and 6% were caused by Streptococcus, which is inconsistent with the results of the present study. Differences in the prevalence and diversity of uropathogens can be due to differences in cultural traditions, environmental factors, personal hygiene, and healthcare facilities (18).
Antibiotic resistance is a major clinical problem in the treatment of infections, especially UTIs. Drug resistance has increased over time, and the rate of resistance varies from country to country. In general, the isolates from Latin American countries show the lowest susceptibility to all antimicrobial drugs, followed by isolates from Asia-Pacific and European strains. Strains from Canada showed the best global susceptibility testing results (7).
In this study, E. coli isolates were susceptible to Ak (82.35%), and the highest resistance was observed against AMC (81.37%), which is consistent with the results of studies conducted by Demirci et al and Shakhatreh et al, respectively (33,34). P. mirabilis showed the highest susceptibility to AK (68.75%) and CIP (59.37%) and the lowest susceptibility to TMP (3.12%) and NIT (12.5%). In a study by Mama et al (2014), P. mirabilis was 100% resistant to NIT (100%) and TMP/SMX (100%), but it was sensitive to AK (40%) (35). E. aerogenes showed the highest susceptibility to AK (87.5%) and CAZ (70.83%). Prakash et al (2013) reported that the susceptibility to AK and CAZ in E. aerogenes were 81.82% and 90.91%, respectively (36). In the present study, the prevalence of S. aureus was 9.7% and it showed the lowest sensitivity to AMP, but all isolates were 100% sensitive to VA and LEV, which is in line with the results of some previous studies (31,37). All isolates of E. faecalis were susceptible to VA (100%) and AMP (90.47%). Yolbaş et al reported the highest susceptibility (93.3%) to VA and the highest resistance to AMP (15). Pseudomonas aeruginosawas isolated from only one patient, which is inconsistent with a study conducted by Luty et al (18). It was highly resistant to most of the antibiotics used; however, IMP (78%) and AK (63%) were most active against P. aeruginosa.
Of all the isolates, 73.61% were resistant to three or more classes of antibiotics, which is consistent with studies conducted by Bajpai et al and Eshetie et al (38,39). MDR rates are lower in some countries than in Iraq (23); this difference may be due to the use of different MDR detection methods, the number and types of antibiotics used, changes in the pattern of bacterial strains tested, and differences in the social demographic characteristics and lifestyle of the study population (8,40). The high prevalence of MDR UTI isolates in our study may be related to the increased misuse of antibiotics, leading to the development of organisms carrying resistance genes.
Conclusions
In this study, gram-negative bacteria were the most prevalent among the samples of patients referred to the hospital. Five species of UTI-causing bacteria were isolated from patients, with E. coli being the most common. The susceptibility of all bacteria tested in this study to CIP and AK in gram-negative bacteria and to VA, LEV, and AK in gram-positive bacteria was significantly higher than other antibiotics. Due to the increasing use of antibiotics and the spread of antibiotic resistance, it is necessary to control the emergence of antibiotic resistance. One of the most important factors influencing this phenomenon is the improper and incorrect use of antibiotics, and efforts should be made to use antibiotics properly and correctly.
Acknowledgments
The authors would like to thank the staff of the Bacteriology Laboratory who helped us in all the practical steps of this research.
Conflict of Interests
None.
References
- Keyhan H, Sedighi S, Mashayekhi B, Fathi M, Mokhtari M. Community acquired urinary tract infections’ etiological organisms and antibiotics susceptibility patterns. Nephrourol Mon 2017; 9(5):e62146. doi: 10.5812/numonthly.62146 [Crossref] [ Google Scholar]
- Inabo HI, Obanibi HB. Antimicrobial susceptibility of some urinary tract clinical isolates to commonly used antibiotics. Afr J Biotechnol 2006; 5(5):487-9. [ Google Scholar]
- Komala M, Kumar KP. Urinary tract infection: causes, symptoms, diagnosis and it’s management. Indian J Res Pharm Biotechnol 2013; 1(2):226-33. [ Google Scholar]
- Paduch DA. Viral lower urinary tract infections. Curr Urol Rep 2007; 8(4):324-35. doi: 10.1007/s11934-007-0080-y [Crossref] [ Google Scholar]
- Behrman RE, Kliegman RM, Jenson HB. Nelson Textbook of Pediatrics. Vol 1. Philadelphia, PA: Saunders Co; 2004.
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008; 27(4):302-8. doi: 10.1097/INF.0b013e31815e4122 [Crossref] [ Google Scholar]
- Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in JNMC hospital Aligarh, India. Ann Clin Microbiol Antimicrob 2007; 6:4. doi: 10.1186/1476-0711-6-4 [Crossref] [ Google Scholar]
- Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother 2001; 45(5):1402-6. doi: 10.1128/aac.45.5.1402-1406.2001 [Crossref] [ Google Scholar]
- Grude N, Tveten Y, Jenkins A, Kristiansen BE. Uncomplicated urinary tract infections Bacterial findings and efficacy of empirical antibacterial treatment. Scand J Prim Health Care 2005; 23(2):115-9. doi: 10.1080/02813430510015287 [Crossref] [ Google Scholar]
- Molazade A, Shahi A, Gholami MS, Najafipour S, Mobasheri F, Jafari S. The antibiotic resistance pattern of gram-negative bacilli isolated from urine cultures of adult outpatients admitted to Vali Asr Hospital of Fasa Clinical Laboratory in 2012-13. J Jahrom Univ Med Sci 2014; 12(3):22-15. doi: 10.29252/jmj.12.3.22.[Persian] [Crossref] [ Google Scholar]
- Shaifali I, Gupta U, Mahmood SE, Ahmed J. Antibiotic susceptibility patterns of urinary pathogens in female outpatients. N Am J Med Sci 2012; 4(4):163-9. doi: 10.4103/1947-2714.94940 [Crossref] [ Google Scholar]
- Gupta S, Kapur S, Padmavathi D. Comparative prevalence of antimicrobial resistance in community-acquired urinary tract infection cases from representative States of northern and southern India. J Clin Diagn Res 2014; 8(9):DC09-12. doi: 10.7860/jcdr/2014/9349.4889 [Crossref] [ Google Scholar]
- Lee DS, Lee SJ, Choe HS. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int 2018; 2018:7656752. doi: 10.1155/2018/7656752 [Crossref] [ Google Scholar]
- Hooton TM, Gupta K. Acute Simple Cystitis in Women. In: Waltham, Mass: UpToDate; 2018.
- Yolbaş I, Tekin R, Kelekci S, Tekin A, Okur MH, Ece A. Community-acquired urinary tract infections in children: pathogens, antibiotic susceptibility and seasonal changes. Eur Rev Med Pharmacol Sci 2013; 17(7):971-6. [ Google Scholar]
- Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 2009; 49(11):1749-55. doi: 10.1086/647952 [Crossref] [ Google Scholar]
- Alizade H. Escherichia coli in Iran: an overview of antibiotic resistance: a review article. Iran J Public Health 2018; 47(1):1-12. [ Google Scholar]
- Luty RS, Fadil AG, Najm JM, Abduljabbar HH, Kashmar SA. Uropathogens antibiotic susceptibility as an indicator for the empirical therapy used for urinary tract infections: a retrospective observational study. Iran J Microbiol 2020; 12(5):395-403. doi: 10.18502/ijm.v12i5.4599 [Crossref] [ Google Scholar]
- Toval F, Köhler CD, Vogel U, Wagenlehner F, Mellmann A, Fruth A. Characterization of Escherichia coli isolates from hospital inpatients or outpatients with urinary tract infection. J Clin Microbiol 2014; 52(2):407-18. doi: 10.1128/jcm.02069-13 [Crossref] [ Google Scholar]
- Lavigne JP, Bruyère F, Bernard L, Combescure C, Ronco E, Lanotte P. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: a French prospective multicentre study. J Med Microbiol 2016; 65(6):530-7. doi: 10.1099/jmm.0.000247 [Crossref] [ Google Scholar]
- Düzgün A, Okumuş F, Saral A, Çiçek A, Cinemre S. Determination of antibiotic resistance genes and virulence factors in Escherichia coli isolated from Turkish patients with urinary tract infection. Rev Soc Bras Med Trop 2019; 52:e20180499. doi: 10.1590/0037-8682-0499-2018 [Crossref] [ Google Scholar]
- Ghanbari F, Khademi F, Saberianpour S, Shahin M, Ghanbari N, Naderi K. An epidemiological study on the prevalence and antibiotic resistance patterns of bacteria isolated from urinary tract infections in central Iran. Avicenna J Clin Microbiol Infect 2017; 4(3):e42214. doi: 10.5812/ajcmi.42214 [Crossref] [ Google Scholar]
- Saha S, Rahman S, Hassan FMN, Sarkar S, Islam K, Saha P. Antimicrobial resistance in uropathogen isolates from patients with urinary tract infections. Biomed Res Ther 2015; 2(5):11. doi: 10.7603/s40730-015-0011-3 [Crossref] [ Google Scholar]
- Ullah A, Shah SR, Almugadam BS, Sadiqui S. Prevalence of symptomatic urinary tract infections and antimicrobial susceptibility patterns of isolated uropathogens in Kohat region of Pakistan. MOJ Biol Med 2018; 3(4):85-9. [ Google Scholar]
- Kimando JM, Okemo PO, Njagi EN. Resistance to antibiotics in urinopathogenic bacteria isolated in patients attending Kenyatta University Health Clinic, Nairobi. East Afr Med J 2010; 87(3):115-9. doi: 10.4314/eamj.v87i3.62197 [Crossref] [ Google Scholar]
- Zak D. Managing uncomplicated recurrent urinary tract infections in reproductive aged women: a primary care approach. J Am Assoc Nurse Pract 2014; 26(12):658-63. doi: 10.1002/2327-6924.12110 [Crossref] [ Google Scholar]
- Al-Jebouri MM, Mdish SA. Antibiotic resistance pattern of bacteria isolated from patients of urinary tract infections in Iraq. Open J Urol 2013; 3(2):124-31. doi: 10.4236/oju.2013.32024 [Crossref] [ Google Scholar]
- Chaurasia D, Shrivastava RK, Shrivastava S, Dubey D, Songra M. Bacterial pathogens and their antimicrobial susceptibility pattern isolated from urinary tract infection in a tertiary care centre. Int J Pharm Bio Sci 2015; 1:20-4. [ Google Scholar]
- Karimian M, Kermani R, Khaleghi M, Kelishadi R, Ataei B, Mostafavi N. Antibiotic susceptibility patterns of isolates from children with urinary tract infection in Isfahan, Iran: impact on empirical treatment. J Glob Antimicrob Resist 2017; 9:3-7. doi: 10.1016/j.jgar.2016.12.014 [Crossref] [ Google Scholar]
- Naqid IA, Hussein NR, Balatay A, Saeed KA, Ahmed HA. Antibiotic susceptibility patterns of uropathogens isolated from female patients with urinary tract infection in Duhok province, Iraq. Jundishapur J Health Sci 2020; 12(3):e105146. doi: 10.5812/jjhs.105146 [Crossref] [ Google Scholar]
- Odoki M, Almustapha Aliero A, Tibyangye J, Nyabayo Maniga J, Wampande E, Drago Kato C. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi district, Uganda. Int J Microbiol 2019; 2019:4246780. doi: 10.1155/2019/4246780 [Crossref] [ Google Scholar]
- Vakili M, Khazaei Z, Ayatollahi J, Khazaei S, Poorrahim H, Goodarzi E. The pattern of antibiotic resistance of pathogens isolated from urine cultures of patients referred to Yazd Central Laboratory in 2012-2013. Biomed Res Ther 2018; 5(5):2271-8. doi: 10.15419/bmrat.v5i5.440 [Crossref] [ Google Scholar]
- Demirci M, Ünlü Ö, İstanbullu Tosun A. Detection of O25b-ST131 clone, CTX-M-1 and CTX-M-15 genes via real-time PCR in Escherichia coli strains in patients with UTIs obtained from a university hospital in Istanbul. J Infect Public Health 2019; 12(5):640-4. doi: 10.1016/j.jiph.2019.02.017 [Crossref] [ Google Scholar]
- Shakhatreh MA, Swedan SF, Al-Odat MA, Khabour OF. Uropathogenic Escherichia coli (UPEC) in Jordan: prevalence of urovirulence genes and antibiotic resistance. J King Saud Univ Sci 2019; 31(4):648-52. doi: 10.1016/j.jksus.2018.03.009 [Crossref] [ Google Scholar]
- Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob 2014; 13:14. doi: 10.1186/1476-0711-13-14 [Crossref] [ Google Scholar]
- Prakash D, Saxena RS. Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of Meerut city, India. ISRN Microbiol 2013; 2013:749629. doi: 10.1155/2013/749629 [Crossref] [ Google Scholar]
- Beyene G, Tsegaye W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci 2011; 21(2):141-6. doi: 10.4314/ejhs.v21i2.69055 [Crossref] [ Google Scholar]
- Bajpai T, Bhatambare GS, Pandey M, Varma M. Prevalence of multi, extensively and pan drug resistant uropathogens among the women patients visiting a tertiary care hospital in central India. International Journal of Health System and Disaster Management 2014; 2(1):38-43. doi: 10.4103/2347-9019.135357 [Crossref] [ Google Scholar]
- Eshetie S, Unakal C, Gelaw A, Ayelign B, Endris M, Moges F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral hospital, Northwest Ethiopia. Antimicrob Resist Infect Control 2015; 4:12. doi: 10.1186/s13756-015-0054-7 [Crossref] [ Google Scholar]
- Wolfensberger A, Kuster SP, Marchesi M, Zbinden R, Hombach M. The effect of varying multidrug-resistence (MDR) definitions on rates of MDR gram-negative rods. Antimicrob Resist Infect Control 2019; 8:193. doi: 10.1186/s13756-019-0614-3 [Crossref] [ Google Scholar]