Avicenna Journal of Clinical Microbiology and Infection. 8(2):74-80.
doi: 10.34172/ajcmi.2021.13
Review Article
Scrub Typhus and its Risk Factors in Asian Countries: A Meta-analysis
Mohd Shahrol Abd Wahil 1  , Muhammad Faiz Mohd Ishak 1, Nor Akila Binti Mahmood 1, Zahir Izuan Azhar 2, Mohammad Saffree Jeffree 3, Syed Sharizman Syed Abdul Rahim 3, Hasanain Faizal Ghazi 4, *
, Muhammad Faiz Mohd Ishak 1, Nor Akila Binti Mahmood 1, Zahir Izuan Azhar 2, Mohammad Saffree Jeffree 3, Syed Sharizman Syed Abdul Rahim 3, Hasanain Faizal Ghazi 4, *  , Mohd Rohaizat Hassan 1, *
, Mohd Rohaizat Hassan 1, * 
Author information:
1Department of Community Health, University Kebangsaan Malaysia, Kuala Lumpur, 56000, Malaysia
2Department of Public Health Medicine, Faculty of Medicine, University Teknologi MARA, Selangor, Malaysia
3Department of Public Health Medicine, Faculty of Medicine and Health Sciences, Universiti Malaysia Sabah
4College of Nursing, Al-Bayan University, Baghdad, Iraq
*
Corresponding author: Mohd Rohaizat Hassan, Department of Community Health, University Kebangsaan Malaysia, Kuala Lumpur, 56000, Malaysia. Email:
rohaizat@ppukm.ukm.edu.my
Abstract
Background: Scrub typhus is an infectious disease with potentially fatal consequences. It is caused by Orientia tsutsugamushi and transmitted to humans via the bites of infected larval mites (chiggers). Scrub typhus is one of the most neglected and severe diseases despite its easy treatment. Delay in diagnosis and treatment is not uncommon, resulting in a 10% fatality rate in cases of inappropriate treatment. The main aim of this study was to determine the prevalence of scrub typhus in Southeast Asia and the risk factors contributing to the disease in order to aid the development of effective control and prevention strategies.
Methods: In this meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) were used as a guideline. The systematic literature search was performed for the relevant titles, abstracts, and keywords in the journal databases of PubMed, EBSCOhost, Ovid, and Google Scholar in November 2018. The keywords and terms were derived from population, intervention, comparison, outcome analysis, and a total of 720 studies were retrieved accordingly. Only original research, published articles, and articles written in the English language were selected for this purpose. Screening of abstracts had shortlisted 36 studies and data extraction was conducted accordingly. However, only 9 studies were accepted after a review of the full texts. The other 27 articles were excluded due to ecological studies and different outcome measures.
Results: Several factors in this meta-analysis were identified to have significant risk of having scrub typhus (P<0.05), including people who are involved in the agriculture sector (OR: 2.90, 95% CI: 2.33, 3.77), those having direct contact or being exposed to the vector habitat (OR: 2.17, 95% CI: 1.49, 3.16), and house yard conditions (OR: 3.02, 95% CI: 2.1, 4.33). Other factors were indoor house conditions (OR: 1.96, 95% CI: 1.45, 2.67), those having close contact with rodents or domestic animals (OR: 2.51, 95% CI: 1.67, 3.77), those working in paddy fields or vegetable farms (OR: 5.17, 95% CI: 3.15, 8.47), and bad occupational safety practices (OR: 1.71, 95% CI: 1.12, 2.62).
Conclusions: The identified risk factors from this meta-analysis highlight the importance of public health intervention strategies for the prevention of scrub typhus among high-risk populations.
Keywords: Scrub typhus, Orientia tsutsugamushi, Agriculture sector, Outdoor activities, Rodents
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Scrub typhus is considered as an infectious disease with potentially fatal consequences. It is caused by arthropod-borne Gram-negative obligately intracellular bacilli Orientia tsutsugamushi and transmitted to humans through the bites of infected larval mites, namely, chiggers (1). It is further known to be prevalent but not limited to the rural areas of Asia Pacific, and one billion people are at the risk of infection and one million cases are annually reported around the world (1). The traditional endemic area of this disease is widely known as the “tsutsugamushi triangle”, extending from the Russian Far East in the north to Pakistan in the west, Australia in the south, and Japan in the east.
Currently, globalization and advancement in transportation allow the spread of scrub typhus to non-endemic areas as illustrated by sporadic cases reported in the United Arab Emirates, Chile, and Africa (2). It has also been reported that the burden of the disease in rural Asia is especially tremendous with up to 20% hospital admission due to febrile conditions (1). Despite the considerable consequences, a statement by the World Health Organisation (WHO) in 1999 conveyed scrub typhus to be one of the most under-diagnosed and underreported febrile diseases that requires hospitalization in the area (3).
This statement is further supported by Paris et al (1), adding that scrub typhus is one of the most neglected and severe diseases despite its simple treatment. Delay in diagnosis and treatment is not uncommon, causing a 10% rate of fatality in cases of inappropriate treatment. This is perhaps due to clinicians’ lack of awareness of scrub typhus as it has long been a neglected disease worldwide. Therefore, it is important to design strategies to increase the awareness of scrub typhus among clinicians and the population. This is to prevent and control the spread of scrub typhus, reduce the burden of disease, and reduce the rate of fatality.
To design such strategies, the prevalence of scrub typhus and its risk factors should be investigated thoroughly. Knowing the prevalence of scrub typhus in Asia will underline the seriousness of the issue and identifying the risk factors will guide policymakers in their efforts to strategized and implement appropriate actions. This study aimed to determine the prevalence of scrub typhus in Asia and the contributing risk factors to the disease in order to help the development of effective control and prevention strategies.
Materials and Methods
Search Method
This is a meta-analysis which applies the approach of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) as a guideline (4). Systematic literature search was conducted for relevant titles, abstracts, and keywords in several databases such as PubMed, EBSCOhost, Ovid, and Google Scholar in November 2018 (Figure 1). Overall, 720 studies were retrieved accordingly. The derived keywords and terms from the population, intervention, comparison, outcome analysis were (“Scrub Typhus” OR “Orientia Tsutsugamushi”) AND (“Risk factor” OR “Associated factor” OR “Contributing factor” OR “Predisposing factor” OR “Predict*” OR “Determinant”) AND (“Prevalence” OR “Incidence” OR “Occurrence” OR “Frequency” OR “Epidemiology”) with restrictions on the publication date from 2013 until 2018 (5). Only original research, published articles, and articles written in the English language were selected based on the study aim. Based on abstract screening, 36 studies were selected, and data were extracted accordingly. However, only 9 studies were accepted after reviewing the full texts. The other 27 articles were excluded due to ecological studies and various outcome measures. The results of the review were separated according to the risk factors of scrub typhus.
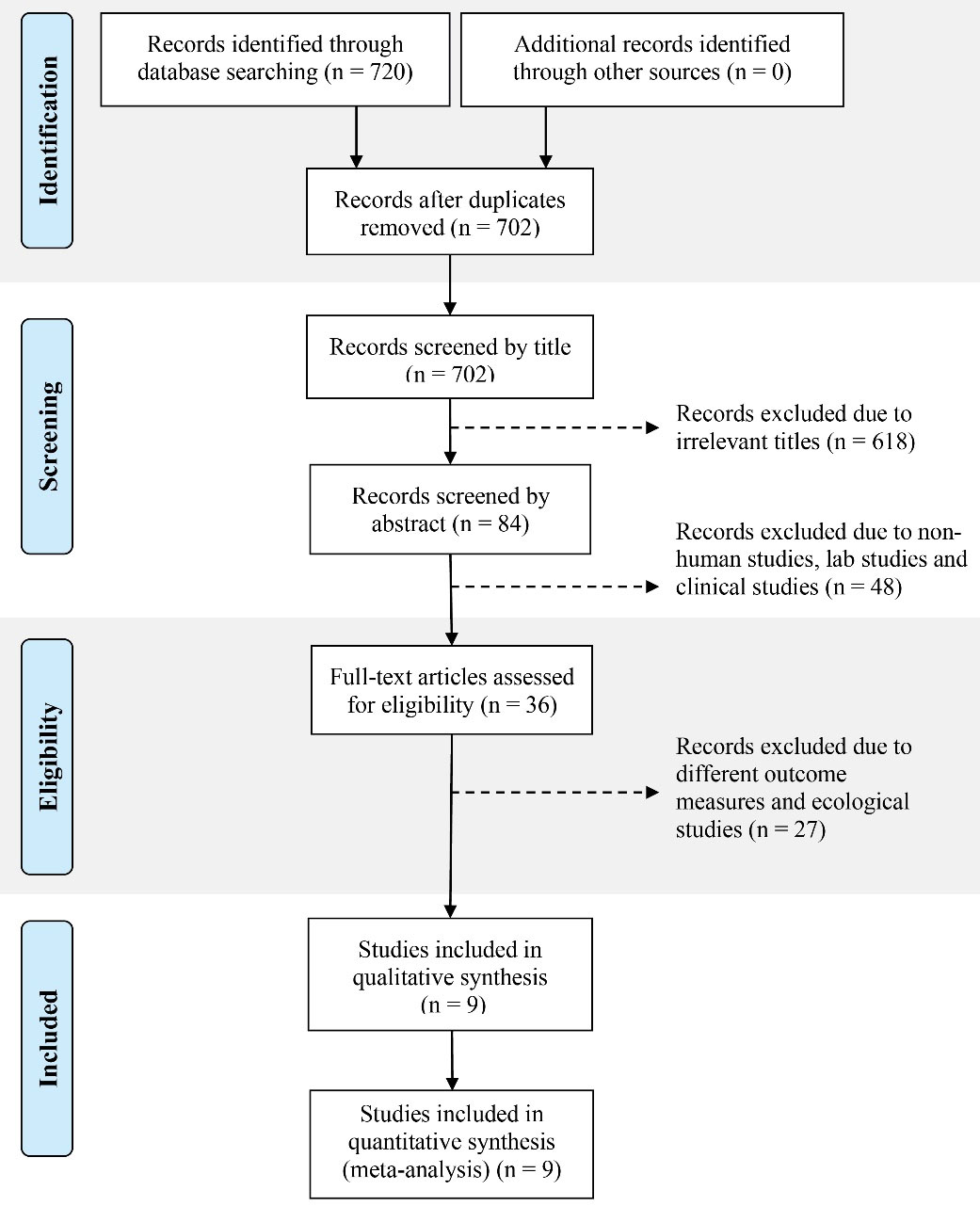
Figure 1.
Search Method Using PRISMA Flow Diagram. Note. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses.
.
Search Method Using PRISMA Flow Diagram. Note. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Data Synthesis and Quality Assessment
The data were extracted from the 9 selected studies and the information including study details, methods, and outcomes were recorded by two independent authors for each selected article. The third author was consulted at the disagreement point. The Newcastle-Ottawa Quality Assessment (Tables 1 and 2) was used to assess the quality of the evidence (6). Studies meeting at least five out of nine criteria were considered to be of high quality and were proceeded to meta-analysis.
Table 1.
Newcastle Ottawa Scale for Case-Control Studies
|
Author
|
Year
|
Adequate Definition of Cases
|
Representativeness of Cases
|
Selection of Control
|
Definition of Control
|
Comparability
|
Exposure
|
Ascertainment for Cases & Control
|
Non-response Rate
|
Final Score
|
| George |
2018 |
* |
* |
* |
* |
** |
- |
* |
- |
7 |
| Hu |
2015 |
* |
* |
* |
- |
** |
- |
- |
- |
5 |
| Kim |
2018 |
* |
* |
* |
* |
* |
- |
* |
- |
6 |
| Li |
2014 |
* |
* |
* |
* |
* |
- |
* |
- |
6 |
| Lyu |
2013 |
* |
* |
* |
* |
** |
* |
- |
- |
7 |
| Varghese |
2016 |
* |
* |
* |
* |
* |
* |
* |
- |
7 |
| Wei |
2014 |
* |
* |
* |
- |
** |
- |
* |
- |
6 |
Table 2.
Newcastle Ottawa Scale for Cross-sectional Studies
|
Author
|
Year
|
Representativeness of the Sample
|
Sample Size
|
Non-respondents
|
Ascertainment of the Exposure (Risk Factor)
|
Comparability
|
Assessment of Outcomes
|
Statistical Test
|
Final Score
|
| Tay |
2013 |
* |
* |
* |
* |
* |
* |
* |
7 |
| Trowbridge |
2017 |
* |
* |
- |
* |
* |
* |
* |
6 |
The Review Manager 5.2 (7) was applied to perform all the analyses. The effect measurements for dichotomous outcomes are estimated by the odds ratio (OR) as the summary statistic. According to previous research (8), the inconsistency in study results is assessed using statistical heterogeneity by reviewing forest plots that display the study-specific estimates of outcome measures, along with a 95% CI where a poor overlap of results indicates the presence of statistical heterogeneity (I2statistic). A result greater than 50% represents heterogeneity. The random-effect model was applied when the heterogeneity was detectable. Subgroup analysis was not performed due to the limited number of studies. A funnel plot was employed to check for the existence of publication bias (9)
Results
In total, 9 studies were included to determine the risk factors for scrub typhus in Asian countries. The included studies were 7 case-control and 2 cross-sectional studies from endemic countries (i.e., India, China, Malaysia, and Korea). The majority of the included studies (n=4) were conducted in China, followed by India (n=3), Korea (n=1), and Malaysia (n=1). The factors contributing to the risk of contracting the disease were discussed in all studies. It should be noted that only significant risk factors gathered from the included studies were analyzed in this study. Overall, the recruited respondents in all studies were adults and the sample population ranged from 29 to the largest sample size of 663 (Table 3).
Table 3.
Summary of the Included Studies and the Risk Factors of Scrub Typhus
|
Author
|
Year
|
Significant Risk Factors
|
Case
|
Control
|
OR
(95% CI)
|
Sig.
|
| George (11) |
2018 |
Farming/Gardening |
30 (n=75) |
10 (n=75) |
4.2 (1.5-11.5) |
* |
| Sitting without mat/on grass |
41 (n=75) |
29 (on=75) |
1.8 (0.9-3.2) |
* |
| No bathrooms in the house |
51 (n=75) |
41 (n=75) |
1.8 (0.9-3.40) |
* |
| Not using proper footwear |
19 (n=75) |
10 (n=75) |
2.1 (0.9-4.9) |
* |
| Hu (14) |
2015 |
Agriculture labour |
125 (n=134) |
182 (n=214) |
2.9 (1.5-5.8) |
* |
| Working at the rice field |
122 (n=132) |
171 (n=239) |
2.8 (1.5-5.0) |
* |
| Bundling up/moving waste straws |
115 (n=134) |
80 (n=241) |
9.8 (5.0-19.2) |
* |
| Living at the edge of village |
34 (n=107) |
112 (n=214) |
0.5 (0.4-0.8) |
* |
| Stacking indoor waste straws |
33 (n=107) |
29 (n=214) |
1.6 (1.1-2.4) |
* |
| Kim (18) |
2018 |
Awareness |
27 (n=57) |
78 (n=114) |
0.41 (0.21-0.79) |
* |
| Wearing long-sleeve clothing |
33 (n=57) |
87 (n=114) |
0.42 (0.21-0.84) |
* |
| Not sitting on the grass |
35 (n=57) |
87 (n=114) |
0.49 (0.24-0.98) |
* |
| Taking off work clothes after work |
34 (n=57) |
87 (n=114) |
0.45 (0.23-0.90) |
* |
| Showering after working |
35 (n=57) |
87 (n=114) |
0.49 (0.24-0.98) |
* |
| Separating work with daily clothes |
35 (n=57) |
88 (n=114) |
0.47 (0.23-0.93) |
* |
| Puddle of water around the house |
17 (n=57) |
16 (n=114) |
2.60 (1.19-5.65) |
* |
| Dry field farming |
44 (n=57) |
66 (n=114) |
2.46 (1.19-5.06) |
* |
| Involvement in livestock industry |
16 (n=57) |
18 (n=114) |
2.08 (0.96-4.47) |
* |
| Li (15) |
2014 |
Harvesting corn |
25 (n=45) |
26 (n=135) |
3.2 (1.5-6.9) |
* |
| Wearing long-sleeve shirt |
10 (n=45) |
53 (n=135) |
0.2 (0.2-0.6) |
* |
| Lyu (16) |
2013 |
Place of workat farmland |
38 (n=56) |
60 (n=168) |
3.8 (2.0-7.2) |
* |
| Vegetables fields |
19 (n=56) |
34 (n=168) |
3.7 (1.1-11.9) |
* |
| Vinyl house |
4 (n=56) |
2 (n=168) |
6.4 (1.1-35.9) |
* |
| Woodlands/hilly areas |
22 (n=56) |
45 (n=168) |
1.8 (0.9-3.3) |
* |
| Raising animals |
36 (n=56) |
73 (n=168) |
2.3 (1.3-4.4) |
* |
| Working in the fields - harvesting in autumn |
38 (n=56) |
53 (n=168) |
4.6 (2.4-8.8) |
* |
| Sowing |
22 (n=56) |
45 (n=168) |
1.8 (0.9-3.3) |
* |
| Outdoor picking wild fruit and collecting firewood |
25 (n=56) |
49 (n=168) |
2.0 (1.1-3.7) |
* |
| Leisure activities-lying on grass without a mat |
17 (n=56) |
29 (n=168) |
2.1 (1.0-4.2) |
* |
| House yard without cement floor |
17 (n=56) |
30 (n=168) |
4.2 (1.0-17.0) |
* |
| Houses near grassland, vegetables field, or ditch |
29 (n=56) |
27 (n=168) |
6.7 (2.3-19.5) |
* |
| Education < 6 years |
26 (n=56) |
70 (n=168) |
16.0 (1.8-139.2) |
* |
| Tay (19) |
2013 |
Age > 18 years old |
28 (n=114) |
22 (n=166) |
1.15 (1.01-1.3) |
* |
| Occupation-working in agriculture |
14 (n=49) |
35 (n=49) |
1.18 (0.98-1.42) |
* |
| Household income < RM 500 |
40 (n=183) |
143 (n=183) |
1.30 (1.14-1.64) |
* |
| Keeping animals/pets |
47 (n=236) |
189 (n=236) |
1.17 (1.05-1.06) |
* |
| Trowbridge (12) |
2017 |
Age > 60 years old |
86 (n=197) |
14 (n=67) |
3.06 (1.22-7.68) |
* |
| Female |
147 (n=451) |
82 (n=270) |
1.60 (1.05-2.45) |
* |
| Defecation/urinate outdoors |
94 (n=293) |
131 (n=409) |
1.48 (1.01-2.18) |
* |
| Clustered house |
218 (n=663) |
10 (n=53) |
2.12 (1.05-4.27) |
* |
| Varghese (13) |
2016 |
Agriculture labourer |
39 (n=128) |
26 (n=128) |
1.79 (1.01-3.16) |
* |
| Residential with single room |
98 (n=128) |
86 (n=128) |
1.75 (1.02-3.01) |
* |
| Not wearing a shirt at home |
64 (n=128) |
50 (n=128) |
4.23 (1.12-16.3) |
* |
| Scrubs/bushes around the house |
106 (n=128) |
70 (n=128) |
1.95 (1.08-3.53) |
* |
| Wei (17) |
2014 |
Outdoor activities |
22 (n=29) |
64 (n=116) |
2.6 (1.0-6.4) |
* |
| Sitting on the lawn |
5 (n=29) |
3 (n=116) |
8.0 (1.8-35.8) |
* |
| Close contact with rats |
9 (n=29) |
15 (n=116) |
3.3 (1.2-9.6) |
* |
| Sitting near rat holes |
7 (n=29) |
9 (n=116) |
3.8 (1.3-11.2) |
* |
| Wearing long-sleeve clothing |
8 (n=29) |
62 (n=116) |
0.3 (0.1-0.8) |
* |
Note. *P-value<0.05; OR: Odds ratio; CI: Confidence interval. Sig.: Level of significance.
Risk Factors Associated With Scrub Typhus
Based on the findings of this meta-analysis, the overall odds of having scrub typhus for those who are involved in agriculture was about 3 times as compared to those who are not involved in the industry (OR: 2.90, 95% CI: 2.23, 3.77), and the finding was statistically significant in this regard (P < 0.05). The overall odds of having scrub typhus for those possibly exposing to chigger habitat was nearly 2 times in comparison to those who experienced no exposure (OR: 2.17, 95% CI: 1.49, 3.16), and the finding was statistically significant (P < 0.05). However, the overall odds of having scrub typhus based on house location was not statistically significant (P>0.05). On the other hand, the overall odds of having scrub typhus for those having a house yard or the surrounding environment favours vector presences was 3 times compared to clean and good sanitation (OR: 3.02, 95% CI: 2.1, 4.33), and the related finding demonstrated a statistical significance (P < 0.05). According to the obtained data, the overall odds of having scrub typhus for those living in poor house conditions was approximately 2 times as compared to those living in proper house conditions (OR: 1.96, 95% CI: 1.45, 2.67), and the result is statistically significant (P < 0.05).
The overall odds of having scrub typhus among those having close contact with animals that favour vector presences was 2.5 times in comparison with those who did not have such close contact (OR: 2.51, 95% CI: 1.67, 3.77), and the finding was statistically significant (P < 0.05). Based on statistically significant findings, the overall odds of having scrub typhus among those working in paddy fields or vegetable farms was 5 times compared to those who did not work in these areas (OR: 5.17, 95% CI: 3.15, 8.47, P < 0.05). Additionally, the overall odds of having scrub typhus among those performing outdoor or outdoor work-related activities was nearly 3 times as compared to those who did not perform these activities (OR: 2.78, 95% CI: 0.81, 9.51). However, the finding was not statistically significant (P>0.05). The results also revealed that the overall odds of having scrub typhus among those with bad occupational safety practices was 1.7 times in comparison to those who did not have bad practices (OR: 1.71, 95% CIL: 1.12, 2.62), and the finding was statistically significant (P < 0.05). Those who applied good occupational safety practices had a 60% risk reduction of having scrub typhus as compared to those who did not apply them (OR: 0.40, 95% CI: 0.26,0.63), and the result was represented a statistical significance (P < 0.05). A summary of the meta-analysis results is listed in Table 4, and the Forest plots are provided as the supplementary items of this article.
Table 4.
Summary of Meta-analysis for the Risk Factors of Scrub Typhus
|
Risk Factors
|
Case
|
Control
|
OR (95% CI)
|
P
Value
|
| Involvement in the agriculture sector |
544 |
1065 |
2.90 (2.23,3.77) |
<0.00001 |
| Direct contact with the chigger habitat |
217 |
473 |
2.17 (1.49,3.16) |
<0.0001 |
| House locations |
190 |
409 |
1.47 (0.11,19.91) |
0.77 |
| House yard conditions |
270 |
526 |
3.02 (2.11,4.33) |
<0.0001 |
| House conditions |
900 |
4.87 |
1.96 (1.45,2.67) |
<0001 |
| Close contact with domestic animals |
378 |
442 |
2.51 (1.67,3.77) |
<0.0001 |
| Farming areas |
188 |
407 |
5.17 (3.15,8.47) |
<0.00001 |
| Outdoor or outdoor work-related activities |
512 |
934 |
2.78 (0.81,9.51) |
0.10 |
| Bad occupational safety practices |
203 |
203 |
1.71 (1.12,2.62 |
0.01 |
| Good occupational safety practices |
131 |
365 |
0.40 (0.26,0.63) |
<0.0001 |
Note. OR: Odds ratio; CI: Confidence interval.
Discussion
Scrub typhus is a vector-borne zoonotic disease. The tsutsugamushi triangle is home to more than half of the world’s population, indicating the annual occurrence of 2 billion at-risk and 1 million cases of scrub typhus (1). This triangle includes South Asia, Southeast Asia, East Asia, the Pacific Island, and Northern Australia although some similar reports also come from Africa, Middle East, and South America (10). Based on the findings of this review, 3 and 4 articles were related to countries such as India (11-13) and China (14-17), respectively. The two other ones belonged to Korea (18) and Malaysia (19), respectively, which mainly evaluated the risk factors. The included studies were 7 case-control and 2 cross-sectional studies focusing on determining risk factors for scrub typhus. To our knowledge, this is the first systematic review that has addressed the risk factors of scrub typhus, highlighting the risk of contracting this disease for a group of people in a certain working or living environment, as well as sanitation and hygiene practices.
This meta-analysis produced significant identified risk factors for contracting scrub typhus. The results suggested that people involved in agriculture are 3 times more likely to get infected with the disease, which becomes an occupational risk, especially for the farmers. Those who are involved in the agriculture sector, particularly those working in paddy fields, vegetable fields, high altitude woodlands, and doing work activities such as harvesting were found to have a higher risk of getting the disease. This can be explained in terms of the risk of having direct contact with the chigger habitat which is abundant in agriculture localities with relatively high humidity (60%-85%), low temperature (20-30 ̊C), and dense vegetative canopy (20).
The vector for this disease is found in great numbers in forest clearings, riverbanks, and grassy regions, explaining why another possible factor is the location of the house that is situated in those areas (21). In addition, farms and other agriculture places with bush, straw piles, rodents, and domestic animals have significantly increased the risk of scrub typhus transmission. Information on all these risk factors should be disseminated by related agencies in public health and agriculture to create awareness among communities who are residing in the area or those who are economically dependent on the agriculture industry such as palm oil plantations, rubber plantations, and paddy fields.
The house condition and its surrounding areas, which favour the infestation of rodents and expose humans to other domestic animals acting as the vector, are shown to significantly increase the risk of their residents getting infected with the disease. Therefore, habitat modifications (either indoor or outdoor including the house yard and its surroundings) such as providing good sanitation, clearing vegetations around the fields, making houses less favourable for rodent infestation, and preventing vector breeding would markedly reduce the risk of contracting scrub typhus (22). However, long-term maintenance of this habitat modification and vector control measures is difficult. Successful strategies for maintaining rodent control activities and habitat modification will largely depend on the awareness of those in the high-risk groups and can be achieved through public health education (2).
Outdoor activities for recreation or work have been shown to contribute to the risk of infection with an OR of 2.78 (95% CI: 0.81, 9.51). This higher odd was because people, who spent time outdoor, would be more likely to encounter mite’s island compared to those who spend most of the time indoors as the chiggers thrived in areas with grass and weeds. Agricultural workers, especially those working and harvesting in the fields, were most likely to be infected with the mite’s island. Conversely, the threat of scrub typhus was not limited to agricultural fields in rural areas as grassland parks could also be infested with mite’s islands as suggested by Wei et al (17), who conducted their study among the attendants of a park in the city. Therefore, any outdoor activities that increased contact with mite’s island pose a risk of scrub typhus infection.
Increased skin exposure while doing outdoor activities is suggested to be one of the most defining factors that contributes to scrub typhus infection. This risk was further accentuated by the habits of not wearing suitable garments, lying on grasses and fields without a mat, and not changing undergarments after returning from outdoor activities as suggested by George et al (11) and Varghese et al (13). These two studies agreed that certain behavioural practices contributed to the increased likelihood of scrub typhus infection, as demonstrated by the OR of 1.71 (95% CI: 1.12, 2.62). A person who does not wear protective garments and lies on the grass without the protection of a mat increases exposure to mite’s island, probably leading to a higher likelihood for chigger bites. Moreover, chiggers prefer to live in a moist environment, thus, a person who does not change the undergarments after returning from outdoor activities is even at more risk.
Some studies recommended good practice of wearing long sleeves and proper shoes or boots provided a protective effect against scrub typhus infection (15,17,18). Covering skin while working outdoor reduced the potential of mite bites even when encountering mite’s island, and this was evidenced by an OR of 0.40 (95% CI: 0.26, 0.63) in this review. In general, three included studies considered various sociodemographic conditions as risk factors to scrub typhus infection. However, these studies measured different sociodemographic factors with a wide range of outcome measures, this direct comparison of studies was unavailable. Gender was one common sociodemographic factor that was measured by the three studies and demonstrated conflicting results in this regard. Lyu et al (16) found that males had 2.7 odds (95% CI: 1.0, 7.1) of having scrub typhus whereas Trowbridge et al (12) discovered that females were more likely to be infected with this disease.
Although insignificant, Tay et al also concluded that females have a slightly higher odds of infection compared to males. The conflicting results on gender were probably due to different cultures in the countries where the studies were conducted. Lyu et al (16) argued that males were at higher risk of infection as they were more involved in outside works compared to females whereas Tay et al (19) suggested that more females were involved in agricultural works, especially in rural areas. Trowbridge et al (12) further reported that their results were due to the under-recruitment of male subjects, where only 270 (37.4%) of the participants were males. Age was a factor in two of the studies. Tay et al (19) concluded that those above the age of 18 years old had higher exposure to scrub typhus in comparison with younger participants. This could be explained by the fact that those above 18 years of age accounted for the majority of the workforce, thus increasing their exposure to mite’s island. Likewise, Trowbridge et al (12) found higher odds of scrub typhus in participants above 60 years old and speculated that accumulated life exposure plays a role in the obtained results since these participants were most likely to be at their prime working lives when scrub typhus started to re-emerge 20-30 years ago. Therefore, adults with previous work experiences were more likely to contract the disease compared to younger generations.
Lyu et al (16) and Tay et al (19) collected data on the education level as one of their sociodemographic parameters but only Lyu et al (16) reported education less than 6 years to be a contributing factor to the infection. On the other hand, Tay et al (19) found that an income of less than RM500 to be a factor. As education was known to be an influential factor on work opportunities and income generation, the lower level of education could lead to less favourable economic conditions, thus forcing participants to live in conditions that increased their risk of infection. People with low education were also more likely to work outdoors, further increasing their risk of getting scrub typhus. To take preventive measures, public education on case recognition and personal protection is the priority. The WHO recommended that advocacy, awareness, and educational activities should be targeted at school children, teachers, and women in endemic areas (23). Field workers and outdoor travellers may have higher risks of becoming infected, thus educational programmes should include them as well. For field workers and those people who cannot avoid the risk factors, taking protective precautions (e.g., wearing a long-sleeve shirt when going outdoors and proper footwear) will help to prevent the disease (2).
Our review mostly involved studies from Asian countries while not including native languages. Hence, there is a possibility of missing other articles published in non-English medium journals. The results could suggest further studies on investigating the effectiveness of any public health intervention strategies for the prevention of scrub typhus among the high-risk population.
Conclusions
Overall, this review identified several risk and protective factors for scrub typhus related to housing and occupational environment. Future interventions for prevention and control programs of scrub typhus should be targeting the specific high-risk occupational group and those having problems with poor hygiene and environment.
Ethical Approval
Not applicable.
Conflict of Interests
None.
References
- Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 2013; 89(2):301-7. doi: 10.4269/ajtmh.13-0064 [Crossref] [ Google Scholar]
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 2017; 11(11):e0006062. doi: 10.1371/journal.pntd.0006062 [Crossref] [ Google Scholar]
- WHO 1999. WHO Recommended Surveillance Standards WHO/CDS/CSR/ISR/99.2. 2nd Ed. http://www.who.int/csr/resources/publications/surveillance/whocdscsrisr992.pdf?ua=1. Accessed December 23, 2018.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [Crossref] [ Google Scholar]
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007; 7:16. doi: 10.1186/1472-6947-7-16 [Crossref] [ Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25(9):603-5. doi: 10.1007/s10654-010-9491-z [Crossref] [ Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Cochrane Collaboration; 2012.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11):1539-58. doi: 10.1002/sim.1186 [Crossref] [ Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56(2):455-63. doi: 10.1111/j.0006-341x.2000.00455.x [Crossref] [ Google Scholar]
- Horton KC, Jiang J, Maina A, Dueger E, Zayed A, Ahmed AA. Evidence of Rickettsia and Orientia infections among abattoir workers in Djibouti. Am J Trop Med Hyg 2016; 95(2):462-5. doi: 10.4269/ajtmh.15-0775 [Crossref] [ Google Scholar]
- George T, Rajan SJ, Peter JV, Hansdak SG, Prakash JAJ, Iyyadurai R. Risk factors for acquiring scrub typhus among the adults. J Glob Infect Dis 2018; 10(3):147-51. doi: 10.4103/jgid.jgid_63_17 [Crossref] [ Google Scholar]
- Trowbridge P, P D, Premkumar PS, Varghese GM. Prevalence and risk factors for scrub typhus in South India. Trop Med Int Health 2017; 22(5):576-82. doi: 10.1111/tmi.12853 [Crossref] [ Google Scholar]
- Varghese GM, Raj D, Francis MR, Sarkar R, Trowbridge P, Muliyil J. Epidemiology & risk factors of scrub typhus in south India. Indian J Med Res 2016; 144(1):76-81. doi: 10.4103/0971-5916.193292 [Crossref] [ Google Scholar]
- Hu J, Tan Z, Ren D, Zhang X, He Y, Bao C. Clinical characteristics and risk factors of an outbreak with scrub typhus in previously unrecognized areas, Jiangsu province, China 2013. PLoS One 2015; 10(5):e0125999. doi: 10.1371/journal.pone.0125999 [Crossref] [ Google Scholar]
- Li Y. Emergence of Scrub Typhus in Northern Jiangsu, China and Risk Factors Associated with its Outbreak. Research; 2014. 10.13070/rs.en.1.974
- Lyu Y, Tian L, Zhang L, Dou X, Wang X, Li W. A case-control study of risk factors associated with scrub typhus infection in Beijing, China. PLoS One 2013; 8(5):e63668. doi: 10.1371/journal.pone.0063668 [Crossref] [ Google Scholar]
- Wei Y, Luo L, Jing Q, Li X, Huang Y, Xiao X. A city park as a potential epidemic site of scrub typhus: a case-control study of an outbreak in Guangzhou, China. Parasit Vectors 2014; 7:513. doi: 10.1186/s13071-014-0513-7 [Crossref] [ Google Scholar]
- Kim DS, Acharya D, Lee K, Yoo SJ, Park JH, Lim HS. Awareness and work-related factors associated with scrub typhus: a case-control study from South Korea. Int J Environ Res Public Health 2018; 15(6):1143. doi: 10.3390/ijerph15061143 [Crossref] [ Google Scholar]
- Tay ST, Mohamed Zan HA, Lim YA, Ngui R. Antibody prevalence and factors associated with exposure to Orientia tsutsugamushi in different aboriginal subgroups in West Malaysia. PLoS Negl Trop Dis 2013; 7(8):e2341. doi: 10.1371/journal.pntd.0002341 [Crossref] [ Google Scholar]
- Tsai PJ, Yeh HC. Scrub typhus islands in the Taiwan area and the association between scrub typhus disease and forest land use and farmer population density: geographically weighted regression. BMC Infect Dis 2013; 13:191. doi: 10.1186/1471-2334-13-191 [Crossref] [ Google Scholar]
- Prakash JAJ. Scrub typhus: risks, diagnostic issues, and management challenges. Res Rep Trop Med 2017; 8:73-83. doi: 10.2147/rrtm.s105602 [Crossref] [ Google Scholar]
- Kuo CC, Huang JL, Shu PY, Lee PL, Kelt DA, Wang HC. Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl 2012; 22(6):1803-16. doi: 10.1890/12-0031.1 [Crossref] [ Google Scholar]
- World Health Organization. Frequently Asked Questions: Scrub Typhus. WHO; 2016.