Avicenna Journal of Clinical Microbiology and Infection. 8(1):11-16.
doi: 10.34172/ajcmi.2021.03
Original Article
Synthesis and Antimicrobial Evaluation of New Series of 1,3,4-Oxadiazole Containing Cinnamic Acid Derivatives
Yasin SarveAhrabi 1, *, Nakisa Zarrabi Ahrabi 1, *, Ali Souldozi 2
Author information:
1Department of Biology, Central Tehran Branch, Islamic Azad University, Tehran, Iran
2Department of Biology, Urmia Branch, Islamic Azad University, Urmia, Iran
*
Corresponding author: Nakisa Zarrabi Ahrabi, Assistant Professor, Department of Biology, Central Tehran Branch, Islamic Azad University, Tehran, Iran. Tel: +989906962429, Email:
na_zarrabi@yahoo.com. Yasin SarveAhrabi, PhD in Microbiology, Department of Biology, Central Tehran Branch, Islamic Azad University, Tehran, Iran. Tel: +989141483263, Email:
yasin.ahrabi2016@gmail.com
Abstract
Background: New drugs must be designed and synthesized for combating resistant pathogens. In this study, antibacterial and antifungal activities of 4 new derivatives of 1,3,4-oxadiazole were assessed against 8 bacterial and 2 fungal pathogens.
Methods: To this end, the cinnamic acid derivatives were dissolved in acetonitrile solvent and N-iso-ciano-imino-triphenyl-phosphorane was added to the above-mentioned solution, followed by applying Petroleum ether and Ethyl acetate as solvent and base. Then, antimicrobial susceptibility tests were used to determine inhibition zone diameter, minimum inhibitory concentration, the minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) values.
Results: The chemical structure of all compounds was characterized with infrared spectra, 1H-NMR, and 13C-NMR. A variety of inhibitory effects were observed by the synthesized compounds. Methoxyphenyl derivative (3c) affected bacterial strains, especially Streptococcus mutans. Other compounds also had antibacterial properties. Additionally, compound 3c showed the greatest effect on fungal samples, especially Aspergillus flavus.
Conclusions: In general, our new derivatives of 1,3,4-oxadiazole are able to destroy Gram-positive bacteria. In addition, developing new derivatives of 1,3,4-oxadiazole in future research can improve therapeutic properties. It seems that with the addition of other functional groups and increasing the destructive power of compounds, inhibitory effects on fungal samples can also be observed.
Keywords: Oxadiazoles, Antibacterial activity, Antifungal activity, Methoxyphenyl
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
The increase of drug resistance in bacteria and fungi has increased the need for new drug compounds (1). In addition, improper use of drugs and mutations in pathogens have increased drug resistance (2). As a result, the discovery, design, and synthesis of new antimicrobial compounds have become inevitable requirements in the field of treatment (3). 1,3,4-oxadiazole derivatives are biologically active organic compounds. 1,3,4-Oxadiazole is a N2 (nitrogen) and O2 (oxygen) containing heterocycle and one of the 4 isomers of oxadiazoles. 1,3,4-oxadiazole itself is not generally used in chemistry, but many of its derivatives are significant (4). For instance, raltegravir is an anti-HIV drug which contains a 1,3,4-oxadiazole ring (5). Other pharmaceutical drugs containing the 1,3,4-oxadiazole ring include fenadiazole (6), zibotentan (7), and doxazosin (8). Numerous available functional groups in 1,3,4-oxadiazole have given them diverse therapeutic properties such as antibacterial (9), antifungal (10), anti-cancer (11), antioxidant (12), anti-malarial (13), and anti-inflammatory (14). The varied biological properties of 1,3,4-oxadiazole prompted us to synthesize several derivatives of this family via the reaction of different carboxylic acid derivatives and 2-pyridinecarboxaldehyde, and their antimicrobial properties were assessed against a wide range of bacterial and fungal pathogens.
Methods
Chemicals
All the required materials were prepared from Merck Company (Germany) and used without further purification. The infrared spectrum was measured by a Shimadzu IR-460 spectrometer. Nuclear magnetic resonance spectrum was obtained by a Bruker DRX-300 AVANCE spectrometer (1H NMR at 300 Hz, 13C NMR at 75 Hz) in CDCl3. Chromatography columns were prepared using silica gel powder (Merck, Germany).
One-Step Process for the Synthesis of 1,3,4-Oxadiazole Derivatives (3a-d)
According to Figures 1 and 2, N-iso-ciano-imino-triphenyl-phosphorane [2] through its isocyanide carbon, separates acidic hydrogen from cinnamic acid derivatives [1] to form ion pairs [5]. Then, the protonated carbon of N-iso-ciano-imino-triphenyl-phosphorane is attacked by the anion of cinnamic acid derivatives (Figure 3)and an intermediate is created [6]. The intermediate [6] reacts with the intramolecular Wittig to form product [3] together with triphenylphosphine oxide [4]. It is noteworthy that these structures were synthesized for the first time.
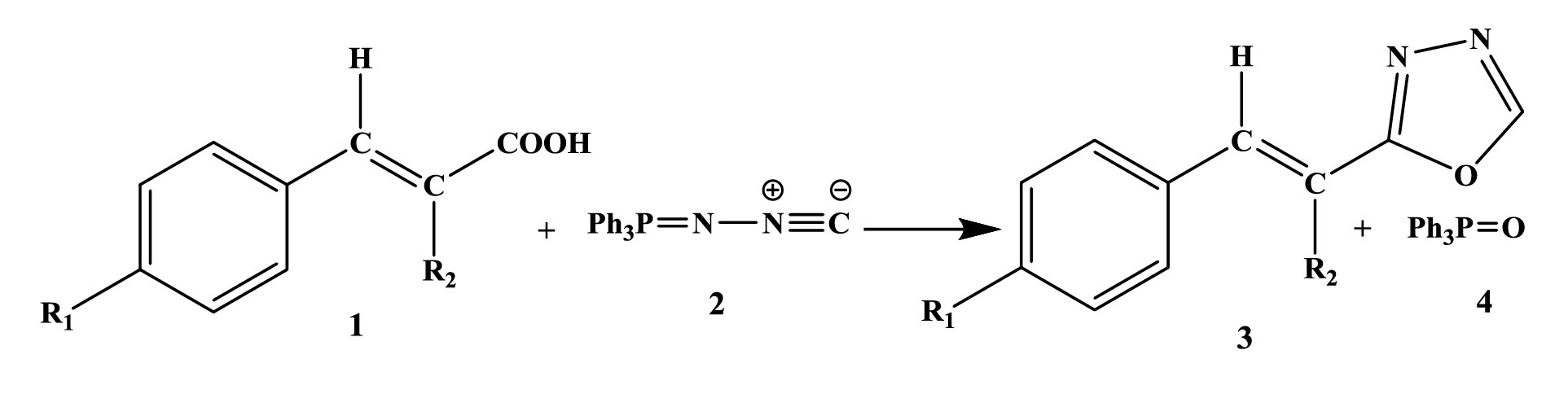
Figure 1.
Overall Reaction.
.
Overall Reaction.
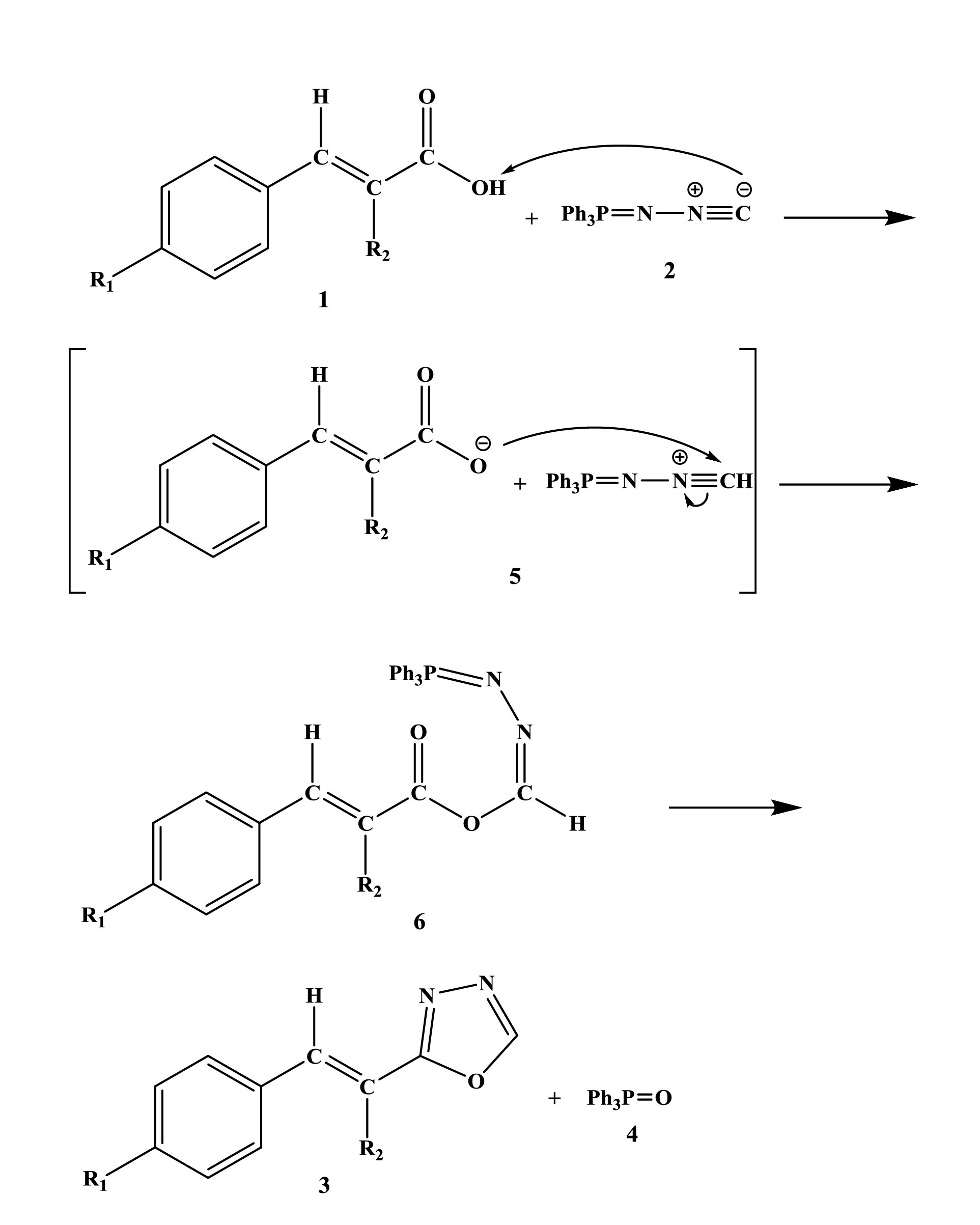
Figure 2.
Reaction Theory
.
Reaction Theory
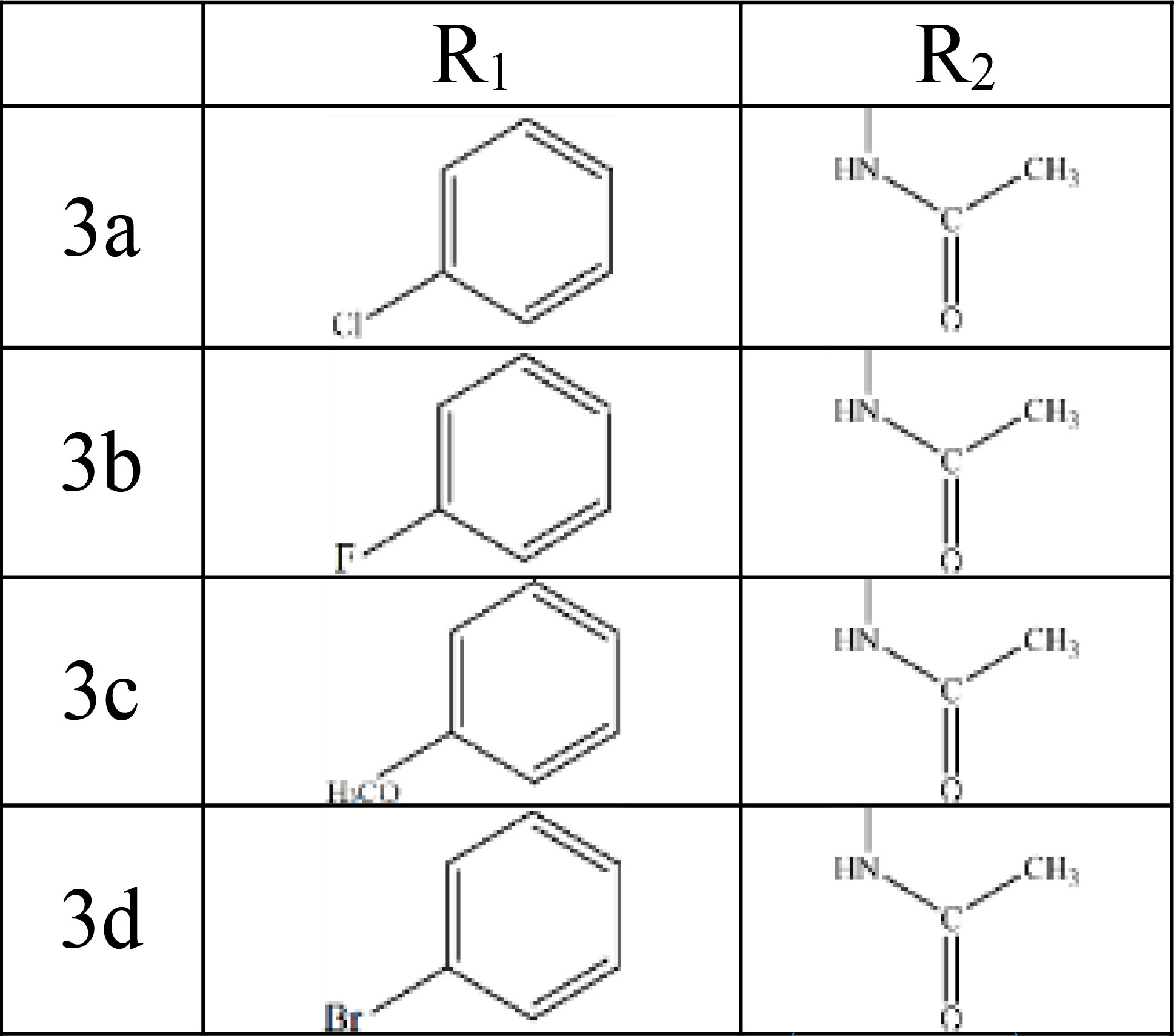
Figure 3.
Functional Groups.
.
Functional Groups.
Antimicrobial Assay
Microorganisms
Culture media (Mueller-Hinton agar, Mueller-Hinton broth, Sabouraud dextrose agar) were obtained from Merck Company (Germany). Gram-negative and gram-positive bacterial and fungal strains were prepared from the Iranian Industrial Microorganisms Collection Center (Lyophilized). Microbiological tests were performed using a Memmert-INC153T2T3 incubator. Gram-negative strains included Escherichia coli (PTCC 1276), Proteus mirabilis (PTCC1793), Salmonella typhi (PTCC1609), and Pseudomonas aeruginosa (PTCC1310). Gram-positive strains included Staphylococcus epidermidis (PTCC1435), Staphylococcus aureus subsp. aureus (PTCC1917), Streptococcus mutans (PTCC1683), and Streptococcus pseudopneumoniae (PTCC1662). Fungal strains included Candida albicans (PTCC5027) and Aspergillus flavus (PTCC5018). Inhibition zone and Broth microdilution (minimum bactericidal concentration [MBC], minimum inhibitory concentration [MIC] and minimum fungicidal concentration [MFC]) were applied to evaluate antibacterial and antifungal susceptibility tests (11). The results were reported as the mean of three independent experiments.
Inhibition Zone
After preparing the suspension of bacteria and fungi (separately) in the tube with distilled water and adjusting the turbidity of the suspensions to match 0.5 McFarland standard (1.5 × 106 CFU/mL), some of each suspension was removed with sterile cotton swabs and was cultivated as grass in Mueller-Hinton agar medium for bacterial culture and Sabouraud dextrose agar for fungal culture. Then, wells were made in the plate by Pasteur pipette. Then, 10 μL of solutions prepared from oxadiazole derivatives (at concentrations of 0.5 mg/mL), ciprofloxacin (positive control for bacteria), and clotrimazole (positive control for fungi) was injected into the wells. Positive control samples were used to compare their growth areas with new compounds. Finally, the plates were closed and they were incubated for 24 hours at 37°C for bacterial samples and 48 hours at 35°C for fungal samples. After the time mentioned, the plates were examined for the presence of growth inhibition zone (15).
Minimum Inhibitory Concentration Experiment
MIC of the compounds was determined using the two-fold serial dilution method (bacterial-fungal). Concentrations of 1000, 500, 250, 125, 62.50, 31.25, and 15.62 µg/mL were prepared in sterile molten Mueller-Hinton agar from the stock solution. The microplates were inoculated with 1.5×106 CFU/mL of bacterial suspension at 37°C for 24 hours. The MIC was defined as the lowest concentration of the compounds that prevented visible growth of microorganisms after 24 hours of incubation. The antibacterial activity of the compounds was compared with ciprofloxacin as standard antibacterial agents. Next, the fungal suspension was made and adjusted to turbidity equivalent to a 0.5 McFarland standard (1.5×106 CFU/mL). The inoculated agar was poured into the assay plate and allowed to cool down on a leveled surface. Once the medium solidified, wells were created on the agar, and 70 µL of the antifungal agents was poured into each well. After adding the synthesized compounds, the plate was incubated at 35oC for 48 hours (16). Antifungal susceptibility test was performed by the agar dilution method. Stock solutions of 1,3,4-oxadiazole derivatives were prepared at a concentration of 0.5 mg/mL in dimethyl sulfoxide (0.001 g of each derivative in 2 mL of DMSO). Next, 1.6 mL of molten Sabouraud dextrose agar was poured into sterile microplates and allowed to cool to 50°C. Then, 0.4 mL of dilutions prepared from the stock solutions of the clotrimazole was added in descending order of concentration. In addition, 10 μL of the standardized fungal inoculum was added to all microplates except for the sterility control. The microplates were incubated at 35°C for 7 days and visualized for growth. The lowest concentration that inhibited the growth of fungi was defined as the MIC. All experiments were performed in duplicate and results were reported as mean ± standard deviation.
MBC and MFC Experiments
To determine the MBC and the MFC of the synthesized compounds, a loopful was taken from the MIC tubes and streaked on Mueller-Hinton agar for bacterial culture and Sabouraud dextrose agar for fungal culture. Growth was observed after incubation at 37ºC for 24 hours. The lowest concentration at which no growth was observed was determined as the MIC and MFC (17).
Results and Discussion
Chemicals
Structures, Infrared, C-NMR, and H-NMR of all compounds were obtained (Figure 4).
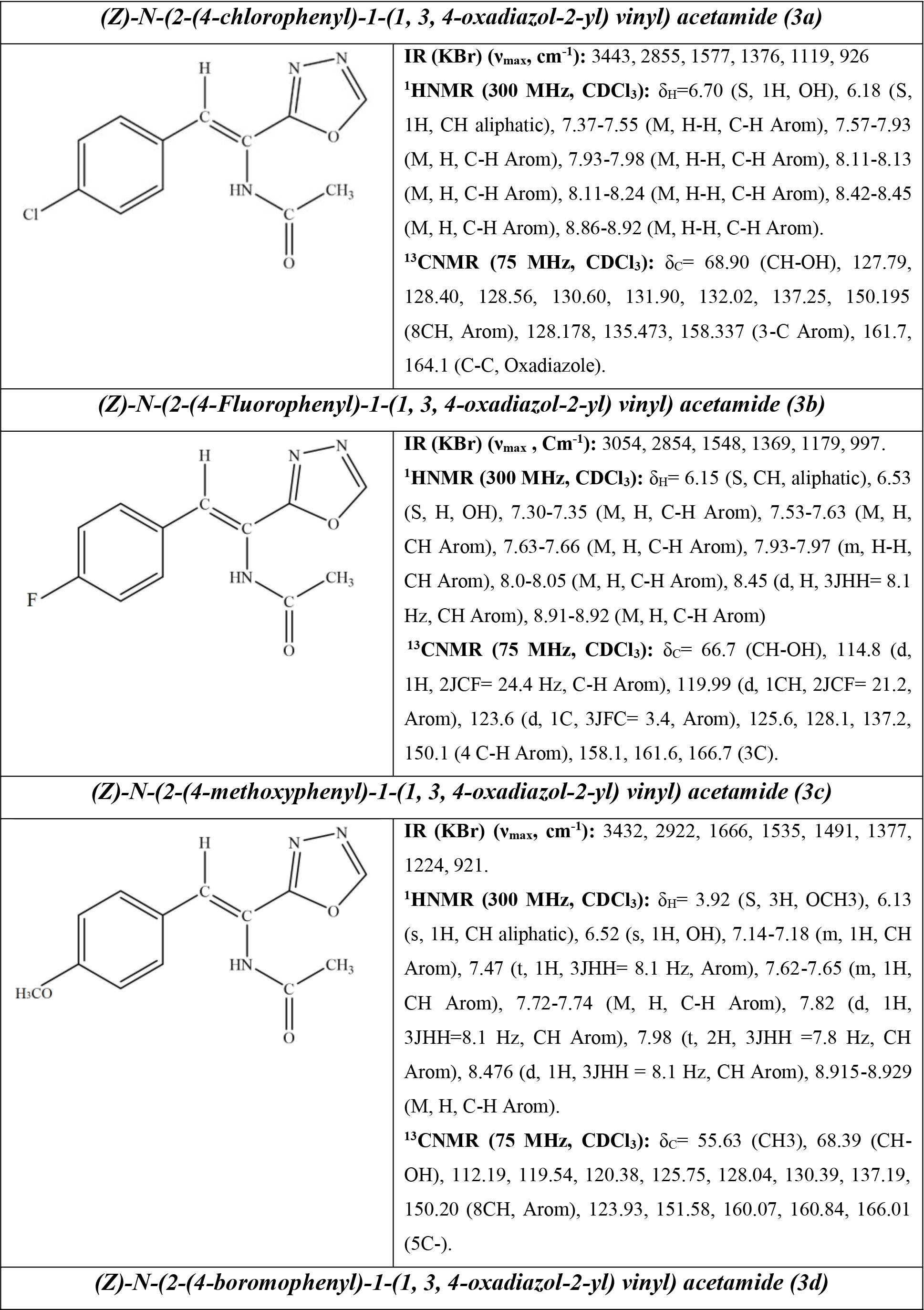
Figure 4.
The Final Structure of Compounds and their Spectral Information.
.
The Final Structure of Compounds and their Spectral Information.
Determination of the In Vitro Antibacterial and Antifungal Properties
Antibacterial and antifungal properties of new derivatives of 1,3,4-oxadiazole (3a-3d) were evaluated in terms of their structures (Figure 4). The inhibition zone diameters of the synthesized compounds against the tested bacteria and fungi are presented in Tables 1 and 2.
Table 1.
Antibacterial Properties of 1,3,4-Oxadiazole Compounds (3a-d)
Bacterial
Strains
|
|
1,3,4-Oxadiazole Compounds
|
Antibiotic
Ciprofloxacin
|
|
3a
|
3b
|
3c
|
3d
|
| 1276 |
IZ |
15.33 ± 0.50 |
17.33 ± 0.50 |
13.33 ± 0.50 |
15.33 ± 0.50 |
35.33 ± 0.50 |
| MIC |
≥1000 |
500 |
- |
500 |
62.50 |
| MBC |
≥1000 |
≥1000 |
- |
≥1000 |
125 |
| 1793 |
IZ |
14.33 ± 0.50 |
20.33 ± 0.50 |
12.33 ± 0.50 |
15.33 ± 0.50 |
22.33 ± 0.50 |
| MIC |
- |
500 |
- |
500 |
500 |
| MBC |
- |
≥1000 |
- |
≥1000 |
≥1000 |
| 1609 |
IZ |
14.33 ± 0.50 |
15.33 ± 0.50 |
15.33 ± 0.50 |
12.33 ± 0.50 |
21.33 ± 0.50 |
| MIC |
≥1000 |
500 |
500 |
- |
500 |
| MBC |
≥1000 |
≥1000 |
≥1000 |
- |
≥1000 |
| 1310 |
IZ |
11.33 ± 0.50 |
11.33 ± 0.50 |
11.33 ± 0.50 |
10.33 ± 0.50 |
30.33 ± 0.50 |
| MIC |
- |
- |
- |
- |
125 |
| MBC |
- |
- |
- |
- |
250 |
| 1435 |
IZ |
24.66 ± 0.50 |
23.66 ± 0.50 |
27.66 ± 0.50 |
28.66 ± 0.50 |
41.66 ± 0.50 |
| MIC |
500 |
500 |
≤250 |
250 |
31.25 |
| MBC |
≥1000 |
≥1000 |
500 |
500 |
62.50 |
| 1917 |
IZ |
22.66 ± 0.50 |
24.66 ± 0.50 |
25.66 ± 0.50 |
23.66 ± 0.50 |
29.66 ± 0.50 |
| MIC |
500 |
500 |
250 |
500 |
125 |
| MBC |
≥1000 |
≥1000 |
500 |
≥1000 |
250 |
| 1683 |
IZ |
31.33 ± 0.50 |
20.33 ± 0.50 |
39.33 ± 0.50 |
21.66 ± 0.50 |
35.66 ± 0.50 |
| MIC |
125 |
500 |
62.50 |
500 |
62.50 |
| MBC |
250 |
≥1000 |
125 |
≥1000 |
125 |
| 1662 |
IZ |
20.33 ± 0.50 |
25.66 ± 0.50 |
26.33 ± 0.50 |
22.66 ± 0.50 |
41.66 ± 0.50 |
| MIC |
500 |
500 |
≤250 |
500 |
31.25 |
| MBC |
≥1000 |
≥1000 |
500 |
≥1000 |
62.50 |
Note. IZ (mm): inhibition zone; MIC (μg/mL-1): Minimum inhibitory concentration; MBC (μg/mL-1): minimum bactericidal concentration.
Table 2.
Antifungal Properties of 1,3,4-Oxadiazole Derivatives (3a-d)
|
Fungal Strains
|
|
1,3,4-Oxadiazole Derivatives
|
Antifungal
Clotrimazole
|
|
3a
|
3b
|
3c
|
3d
|
| 5027 |
IZ |
11.33 ± 0.50 |
11.33 ± 0.50 |
12.33 ± 0.50 |
10.33 ± 0.50 |
29.33 ± 0.50 |
| MIC |
- |
- |
500 |
- |
250 |
| MFC |
- |
- |
≥1000 |
- |
500 |
| 5018 |
IZ |
21.33 ± 0.50 |
25.33 ± 0.50 |
33.33 ± 0.50 |
19.33 ± 0.50 |
40.33 ± 0.50 |
| MIC |
500 |
500 |
250 |
- |
31.25 |
| MFC |
≥1000 |
≥1000 |
500 |
- |
62.50 |
Note. IZ (mm): inhibition zone; MIC (μg/mL-1): minimum inhibitory concentration; MFC (μg/mL-1); minimum fungicidal concentration
As shown in Table 1, the greatest effect of compound 3a was observed on S. mutans with IZ=31.33 ± 0.50 mm, MIC=125 mg/mL, and MBC=250 mg/mL. This result can be due to the presence of chlorophenyl group in the main compound (18). The greatest effect of compound 3b was observed on S. aureus with IZ=24.66 ± 0.50 mm, MIC=500 mg/mL, and MBC=1000 mg/mL. This result can be due to the presence of fluorophenyl group in the main compound (19). The greatest effect of compound 3c was observed on S. mutans with IZ=39.33 ± 0.50 mm, MIC=62.50 mg/mL, and MBC=125 mg/mL. This result can be due to the presence of methoxyphenyl group in the main compound (9). The greatest effect of compound 3d was observed on S. epidermidis with IZ=28.66 ± 0.50 mm, MIC=250 mg/mL, and MBC=500 mg/mL. This result can be due to the presence of bromophenyl group in the main compound (20).
As shown in Table 2, the greatest effect is related to compound 3c on A. flavus with IZ=33.33 ± 0.50 mm, MIC=250 mg/mL, and MFC=500 mg/mL. This result can be due to the presence of methoxyphenyl group in the main compound.
As shown in Tables 1 and 2, the significant results obtained in this study displayed that most of the synthesized compounds performed very well against the gram-positive samples; however, compound 3C showed acceptable inhibitory effects with high IZ, MIC, and MBC among all.
Conclusions
In general, 1,3,4-oxadiazoles are multi-functionalized compounds with a variety of biological properties; therefore, synthesis of their new derivatives is of great importance. This study evaluated the antibacterial and antifungal effects of all 4 synthesized 1,3,4-oxadiazole derivatives on pathogenic bacteria and fungi. These compounds, chiefly methoxyphenyl, showed relatively acceptable antibacterial effects on Gram-positive strains such as S. mutans and S. epidermidis, as well as Gram-negative strains such as P. mirabilis. In addition, good to excellent antifungal activities were observed for compound 3c. Our results show that 1,3,4-oxadiazole derivatives would be helpful structures for the possible development of new drugs; however, this result needs to be confirmed by other extensive clinical trials that will be part of our future plans. Furthermore, the easy workup procedure, high yield, and short reaction times make the method a useful addition for preparing modern pharmaceutical synthetics.
These derivatives were synthesized for the first time andthe relevant tests were done to ensure the biological properties of the compounds. Thisstudy was done to provide new structures and confirm the existence of antibacterial and antifungal activity of the compounds. In future research, several concentrations will be used for other resistant bacteria and cell lines as well as MTT assay and so on.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgements
This study was conducted as a part of the author’s PhD thesis (Code: 10330507951009) at Islamic Azad University, Central Tehran Branch.
Ethical Approval
The disclaimer implies that ethical principles were considered in relation to the proposed work and no ethical issues were found to be applied to this study.
Authors’ Contribution
YSA: supervision, writing original draft, reviewing, and editing. NZA: data analysis. AS: investigation, methodology.
Funding/Support
Islamic Azad University of Central Tehran Branch provided financial support for this study.
References
- You Z, Ran X, Dai Y, Ran Y. Clioquinol, an alternative antimicrobial agent against common pathogenic microbe. J Mycol Med 2018; 28(3):492-501. doi: 10.1016/j.mycmed.2018.03.007 [Crossref] [ Google Scholar]
- Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 2018; 11:1645-58. doi: 10.2147/idr.s173867 [Crossref] [ Google Scholar]
- Sweeney MT, Lubbers BV, Schwarz S, Watts JL. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J Antimicrob Chemother 2018; 73(6):1460-3. doi: 10.1093/jac/dky043 [Crossref] [ Google Scholar]
- Ningegowda R, Chandrashekharappa S, Singh V, Mohanlall V, Venugopala KN. Design, synthesis and characterization of novel 2-(2, 3-dichlorophenyl)-5-aryl-1,3,4-oxadiazole derivatives for their anti-tubercular activity against Mycobacterium tuberculosis. Chem Data Collect 2020; 28:100431. doi: 10.1016/j.cdc.2020.100431 [Crossref] [ Google Scholar]
- Parizadeh N, Alipour E, Soleymani S, Zabihollahi R, Aghasadeghi MR, Hajimahdi Z. Synthesis of Novel 3-(5-(Alkyl/arylthio)-1,3,4-Oxadiazol-2-yl)-8-Phenylquinolin-4(1H)-One Derivatives as Anti-HIV Agents. Phosphorus Sulfur Silicon Relat Elem 2018; 193(4):225-31. doi: 10.1080/10426507.2017.1394302 [Crossref] [ Google Scholar]
- Parameshwar A, Selvam V, Ghashang M, Guhanathan S. Synthesis and antibacterial activity of 2-(2-(cyclopropylmethoxy) phenyl)-5-methyl-1,3,4-oxadiazole. Orient J Chem 2017; 33(3):1502-12. [ Google Scholar]
- Madhavi S, Sreenivasulu R, Raju RR. Synthesis and biological evaluation of oxadiazole incorporated ellipticine derivatives as anticancer agents. Monatsh Chem 2017; 148(5):933-8. doi: 10.1007/s00706-016-1790-y [Crossref] [ Google Scholar]
- Ahmed MN, Yasin KA, Hameed S, Ayub K, Haq IU, Tahir MN. Synthesis, structural studies and biological activities of three new 2-(pentadecylthio)-5-aryl-1,3,4-oxadiazoles. J Mol Struct 2017; 1129:50-9. doi: 10.1016/j.molstruc.2016.09.057 [Crossref] [ Google Scholar]
- Sarve Ahrabi Y, Tahaye Abadi S. Single-step synthesis of 1,3,4-oxadiazoles containing fluorophenyl ring and evaluation of their antibacterial effect on gram-negative pathogens in vitro. Paramedical Sciences and Military Health 2020; 14(4):1-9. [ Google Scholar]
- Sarve Ahrabi Y, Hemmati Eslamlou P. Synthesis and evaluation of the antifungal effects of [5-aryl-[1,3,4] oxadiazole-2-yl] phenyl-methanol. Paramedical Sciences and Military Health 2019; 14(1):1-9. [ Google Scholar]
- Glomb T, Szymankiewicz K, Świątek P. Anti-cancer activity of derivatives of 1,3,4-oxadiazole. Molecules 2018; 23(12):3361. doi: 10.3390/molecules23123361 [Crossref] [ Google Scholar]
- Sauer AC, Leal JG, Stefanello ST, Leite MTB, Souza MB, Soares FAA. Synthesis and antioxidant properties of organosulfur and organoselenium compounds derived from 5-substituted-1,3,4-oxadiazole/thiadiazole-2-thiols. Tetrahedron Lett 2017; 58(1):87-91. doi: 10.1016/j.tetlet.2016.11.106 [Crossref] [ Google Scholar]
- Al-Wahaibi LH, Santhosh Kumar N, El-Emam AA, Venkataramanan NS, Ghabbour HA, Al-Tamimi AMS. Investigation of potential anti-malarial lead candidate 2-(4-fluorobenzylthio)-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole: Insights from crystal structure, DFT, QTAIM and hybrid QM/MM binding energy analysis. J Mol Struct 2019; 1175:230-40. doi: 10.1016/j.molstruc.2018.07.102 [Crossref] [ Google Scholar]
- Mahapatra DK, Shivhare RS, Ugale VG. Anti-inflammatory potentials of some novel Murrayanine containing 1,3,4-oxadiazole derivatives. Asian J Pharm Technol 2018; 8(1):47-51. doi: 10.1016/j.molstruc.2018.07.102 [Crossref] [ Google Scholar]
- Zarrabi N, Souldozi A, Sarve Ahrabi Y. In vitro evaluation of antibacterial and antifungal properties of some new 1,3,4-oxadiazole derivatives containing phenyl group. Infect Epidemiol Microbiol 2020; 6(3):177-92. [ Google Scholar]
- Magaldi S, Mata-Essayag S, Hartung de Capriles C, Perez C, Colella MT, Olaizola C. Well diffusion for antifungal susceptibility testing. Int J Infect Dis 2004; 8(1):39-45. doi: 10.1016/j.ijid.2003.03.002 [Crossref] [ Google Scholar]
- Ikegbunam M, Ukamaka M, Emmanuel O. Evaluation of the antifungal activity of aqueous and alcoholic extracts of six spices. Am J Plant Sci 2016; 7(1):118-25. [ Google Scholar]
- Hassanzadeh F, Sadeghi-Aliabadi H, Jafari E, Sharifzadeh A, Dana N. Synthesis and cytotoxic evaluation of some quinazolinone-5-(4-chlorophenyl) 1,3,4-oxadiazole conjugates. Res Pharm Sci 2019; 14(5):408-13. doi: 10.4103/1735-5362.268201 [Crossref] [ Google Scholar]
- Al-Wahaibi LH, Alsfouk A, El-Emam AA, Blacque O. Crystal structures and Hirshfeld surface analysis of 2-(adamantan-1-yl)-5-(4-fluoro-phen-yl)-1,3,4-oxa-diazole and 2-(adamantan-1-yl)-5-(4-chloro-phen-yl)-1,3,4-oxa-diazole. Acta Crystallogr E Crystallogr Commun 2019; 75(Pt 5):611-5. doi: 10.1107/s2056989019004651 [Crossref] [ Google Scholar]
- Shajari N, Yahyaei H. Ab initio and DFT Calculation of Conformational Properties and Thermodynamic Properties of Sterically Congested 5-(4-Bromophenyl)-N-(trichloroacetyl)-1,3,4-oxadiazole-2-carboxamide. Kish Island: 18th Iranian Physical Chemistry Conference; 2016.