Avicenna Journal of Clinical Microbiology and Infection. 7(4):99-103.
doi: 10.34172/ajcmi.2020.22
Original Article
Synthesis and Characterization of Silver Nanoparticles with Ultrasound-Assisted Reverse Micelles Method and Their Antibacterial Effects on Methicillin-Resistant Staphylococcus aureus Isolates
Sima Sedrizadeh-Bami 1, Ashraf Kariminik 1, *  , Mehdi Ranjbar 2
, Mehdi Ranjbar 2 
Author information:
1Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran.
2Pharmaceutics Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
*
Corresponding author: Ashraf Kariminik, Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran. Email:
a.kariminik@iauk.ac.ir
Abstract
Background: Serious infections are associated with methicillin-resistant Staphylococcus aureus (MRSA) bacteria and this can lead to many deaths in the world. The aim of the present study was to evaluate the antibacterial effect of silver nanoparticles (AgNPs) against MRSA isolates from clinical samples.
Methods: Ag nanoparticles were synthesized by ultrasound-assisted reverse micelles method. The as-prepared Ag nanoparticles were characterized by X-Ray diffraction (XRD) and scanning electron microscopy (SEM). The antibacterial effect of AgNPs was investigated using agar well diffusion assay and minimum inhibitory concentration (MIC) was determined.
Results: The XRD studies showed that pure Ag nanoparticles have been produced after calcination. Synthesized AgNPs showed favorable effects on the bacteria used. MIC and minimum bactericidal concentration (MBC) values were determined to be 0.015 and 0.07 mg/mL, respectively. All MRSA isolates were susceptible to AgNPs. In contrast, they showed high resistance to multiple classes of antibiotics.
Conclusions: AgNPs had high inhibitory activity against MRSA; therefore, they can be proposed as an alternative or adjuvant to antibiotics for the treatment of MRSA infections. Further investigations are required to assess the safety and efficacy of AgNPs in the body.
Keywords: Silver nanoparticles, Methicillin-resistant Staphylococcus aureus, Antibacterial effects, Ag nanostructure
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
At the beginning of the 20th century, the outbreak of infectious diseases was one of the leading causes of death worldwide (1). Following the discovery of antimicrobial agents in the present century, morbidity and mortality from infectious diseases reduced. Nowadays, the emergence of antibiotic-resistant strains is a new challenge in the treatment of infectious diseases. The attention of researchers has been drawn to the search for new drugs and antimicrobial agents (2). One of the recent efforts to address the challenge was the use of antimicrobial nanomaterials. Nanoparticles have advantages over antibiotics in terms of reducing toxicity, cost-effectiveness, and broad-spectrum efficacy against bacteria in comparison with conventional antibiotics (3). Antibacterial effects of silver have long been considered. Silver compounds such as metallic silver, silver nitrate, and silver sulfadiazine are used for the treatment of various types of wound infections and disinfection of water (4). Compared to other nanomaterials, silver nanoparticles (AgNPs) have the highest antimicrobial activity against bacteria, viruses, and fungi (5). Production of nanoparticles can be achieved by different methods. Chemical approaches are the most commonly used methods for the production of nanoparticles. Biological methods, enzymes, and plant extracts are also used in the production of nanoparticles (6). Several systemic infections are associated with methicillin-resistant Staphylococcus aureus (MRSA). The aim of this study was to synthesize AgNPs by ultrasonic method and evaluate the in vitro antibacterial effects against MRSA isolated from clinical specimens.
Methods
Synthesis and Characterization of Silver Nanoparticles
In a typical approach, AgNPs are prepared by ultrasound-assisted reverse micelles method (7). For this purpose, 0.05 g of AgNO3 was dissolved in 20 mL of propylene glycol and then, 13 mmol of an anionic surfactant (SDS) was subsequently added to the solution and stirred with 400 rpm at 50◦C. The solvent was evaporated to dryness in reflux system under reduced pressure at room temperature. During drying, the magnet was rotated at 100 rpm speed until a smooth dry film layer was obtained. It was kept in a vacuum desiccator for 48 hours. The film containing non-ionic surfactant-based vesicle was hydrated using double distilled water. After thermal treatment, the system was allowed to cool to room temperature naturally. Then, the obtained precipitate was collected by filtration and washed with absolute ethanol and distilled water several times. X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro X-ray diffractometer using Ni-filtered Cu Ka radiation at the scan range of 10<2θ<80. Scanning electron microscopy (SEM) images were obtained using LEO-1455VP equipped with an energy dispersive X-ray spectroscopy.
Antibacterial Activity of the Silver Nanoparticles
A total of 50 S. aureus isolates were obtained from clinical specimens at the microbiology laboratory of Kerman hospital. Sampling was carried out using sterilized swabs and the samples were transferred to the normal saline solution. Handling, transporting, and storing of collected samples were done at 4°C. All samples were inoculated on blood agar and incubated at 37°C for 48 hours. The obtained colonies were recognized as S. aureus by morphology, gram staining, catalase, coagulase, and DNase tests (8). The identified S. aureus isolates were evaluatedfortheir susceptibility to methicillinusingdisk diffusion test based on the guidelines of Clinical and Laboratory Standard Institute (CLSI) (9). Oxacillin agar-screening test was used to isolate MRSA. All isolates were cultured on Mueller-Hinton agar (Merck, Germany). A 1-μg oxacillin disk (Padtan Teb, Iran) was placed and incubated at 37°C and the results were recorded after 24 hours of incubation. Isolates showing inhibition zone size ≤13 mm were considered to be resistant (10). After overnight incubation at 37˚C, the antibiotic susceptibility was determined by measuring the zone of inhibition in mm and the isolates were identified as MRSA based on their resistance to methicillin. Antibacterialactivities of the synthesized AgNPs against MRSA isolates were measured using the well diffusion method. The bacterial concentration was adjusted to McFarland standard (1.5×108CFU/mL in sterile normal saline). The Mueller-Hinton agar (Merck, Germany) medium with 4 mm depth was poured into petri dishes to give a solid plate and inoculated with 100 μL of the prepared suspension of MRSA isolates by sterile cotton swabs. Wells with 6 mm diameter were punctured in the media by sterile cork borers and filled with 20 μL of the silver nano-particles. The first concentration used was 80 mg/m. The plates were incubated at 37˚C for 24 hours. The antibacterial activity was determined by measuring the inhibition zones around each of the wells in mm. Minimum inhibitory concentration (MIC) was defined as the lowest concentration of silver nano-particles that prevented bacterial growth. Different concentrations (80, 40, 20, 10, 5, 2.5, 1.25, 0.625, 0.3, and 0.1 mg/mL) of silver nano-particles solution in DMSO were used. Methanol (1:1 v/v) solvent was prepared and bioassayed on Mueller Hinton agar medium (Merck, Germany) by well diffusion method as mentioned above. Methanol (1:1 v/v) solvent was considered as the negative control. Briefly, the plates were incubated at 37°C for 24 hours. Next, the inhibition zone (IZ) diameter of AgNPs was measured in mm (10,11).
Results
The crystalline size and diameter (Dc) of Cu NPs can be determined from the diffraction patterns obtained from the full width of the half maximum (FWHM) using the Debye-Scherrer equation:
Dxrd = 0.9λ (57.3)/Wsize cos Ө (1)
Where Wsize = (Wb2- Ws2)1/2 (2)
Wsize = FWHM (3)
For sample Wsize = FWHM,
For standard use λ = 1.542 A°.
Of 50 S. aureus isolates, 10 were identified as MRSA by beta hemolysis on blood agar, catalase, coagulase, DNase positive tests, and resistance to methicillin. All 10 MRSA isolates were resistant to 7 used antibiotics. The information about the susceptibility and resistance rate of the MRSA isolates against the 10 different antibiotics used in this study is shown in Figure 1. This finding showed that all of MRSA isolates were resistant to different antibiotics such as penicillin, cotrimoxazole, vancomycin, clindamycin, erythromycin,andofloxacin. The synthesized AgNPs showed a dose-dependent growth inhibitory effect on the MRSA isolates (Figure 2)withthe mean MIC and minimum bactericidal concentration (MBC) of 0.015 and 0.07 mg/mL, respectively, and a mean IZ of 10 mm. The effects of different concentrations of AgNPs against10 MRSA isolates are shown in Table 1.
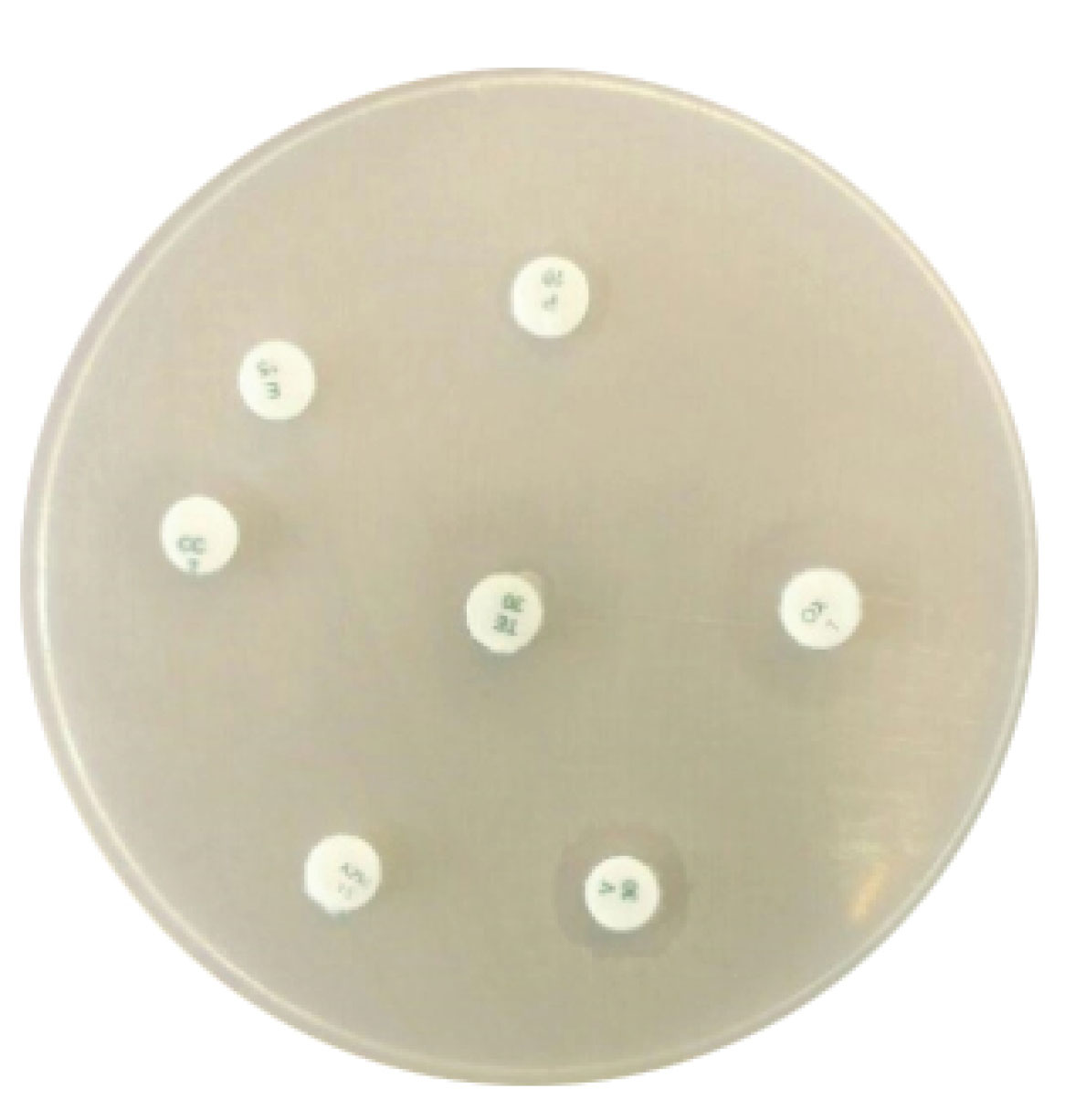
Figure 1.
Antibiotic Resistance Pattern of MRSA Isolates by Disc Diffusion Method to Seven Antibiotics.
.
Antibiotic Resistance Pattern of MRSA Isolates by Disc Diffusion Method to Seven Antibiotics.
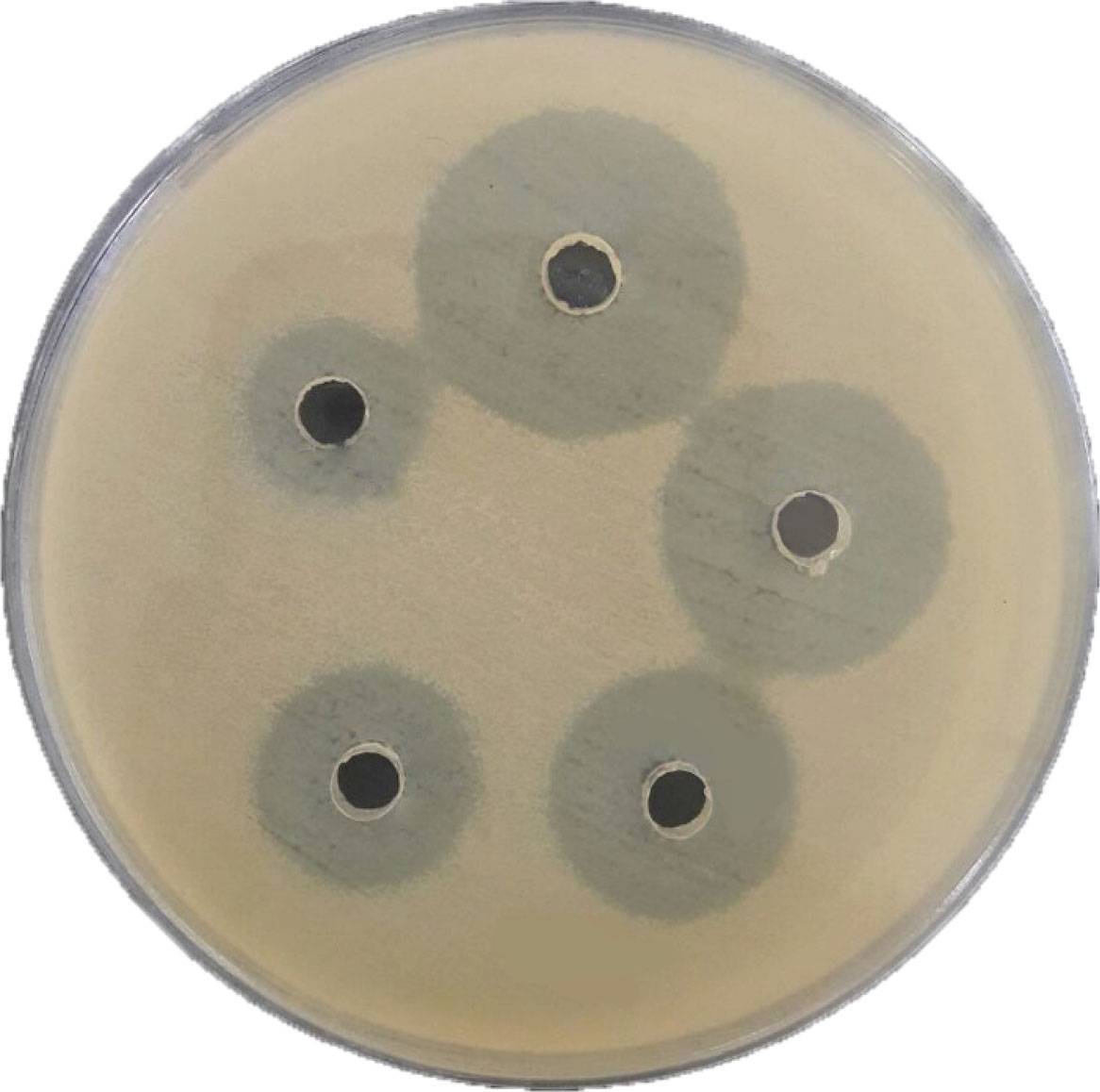
Figure 2.
The Effect of Different Concentrations of AgNPs on MRSA Isolates by Agar Well Diffusion Method.
.
The Effect of Different Concentrations of AgNPs on MRSA Isolates by Agar Well Diffusion Method.
Table 1.
Means of Inhibition Growth Diameter (mm) Obtained by Agar Well Diffusion Method Using Different Concentrations of AgNPs Against10 MRSA Isolates
|
Bacteria
|
Nanoparticle (mg/mL)
|
|
0.015
|
0.03
|
0.07
|
0.15
|
0.31
|
0.62
|
1.25
|
2.5
|
5
|
10
|
20
|
| MRSA1 |
- |
8 |
10 |
12 |
12 |
15 |
17 |
18 |
20 |
20 |
25 |
| MRSA2 |
8 |
9 |
10 |
12 |
13 |
14 |
14 |
15 |
15 |
20 |
20 |
| MRSA3 |
10 |
10 |
12 |
13 |
14 |
16 |
20 |
23 |
23 |
24 |
24 |
| MRSA4 |
9 |
10 |
11 |
12 |
13 |
15 |
20 |
22 |
24 |
24 |
25 |
| MRSA5 |
10 |
11 |
11 |
13 |
14 |
15 |
18 |
21 |
23 |
24 |
24 |
| MRSA6 |
9 |
10 |
11 |
12 |
15 |
17 |
18 |
20 |
22 |
23 |
23 |
| MRSA7 |
10 |
11 |
12 |
14 |
16 |
16 |
17 |
20 |
23 |
23 |
24 |
| MRSA8 |
10 |
12 |
14 |
16 |
18 |
20 |
23 |
23 |
24 |
26 |
26 |
| MRSA9 |
10 |
11 |
13 |
17 |
18 |
20 |
22 |
24 |
24 |
25 |
25 |
| MRSA10 |
- |
10 |
12 |
13 |
14 |
14 |
16 |
17 |
20 |
24 |
26 |
Dynamic Light Scattering (sometimes referred to as Photon correlation spectroscopy or quasi-elastic light scattering) is a technique for measuring the size of particles typically in the sub-micron region.
d(H) = kT/3ηΠD
where:
d(H) = hydrodynamic diameter
D = translational diffusion coefficient
k = Boltzmann constant
T = absolute temperature
η = viscosity
Any change to the surface of a particle that affects the diffusion speed will correspondingly change the apparent size of the particle. The mean particle size was determined to be 150 nm according to dynamic light scattering which is shown in Figure 3. The SEM image of the AgNPs and the formation of nanoparticles are shown in Figure 4. The results showed that the nanoparticle surface had a relatively good dispersion. The results of microscopic imaging of nanoparticles indicate that the particles were formed well in all laboratory conditions according to Table 2.
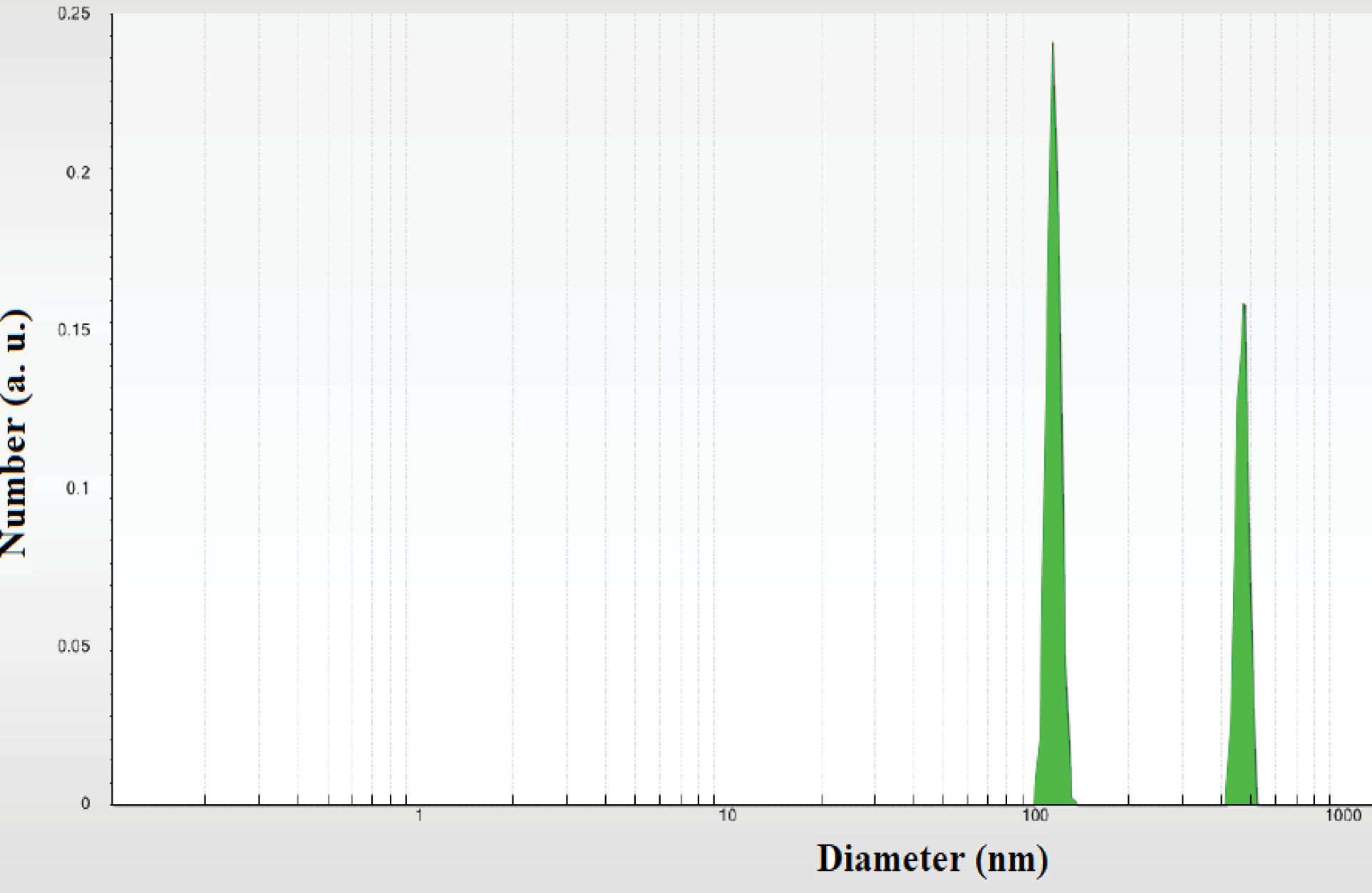
Figure 3.
Dynamic Light Scattering Diagram of Silver Nanoparticles.
.
Dynamic Light Scattering Diagram of Silver Nanoparticles.
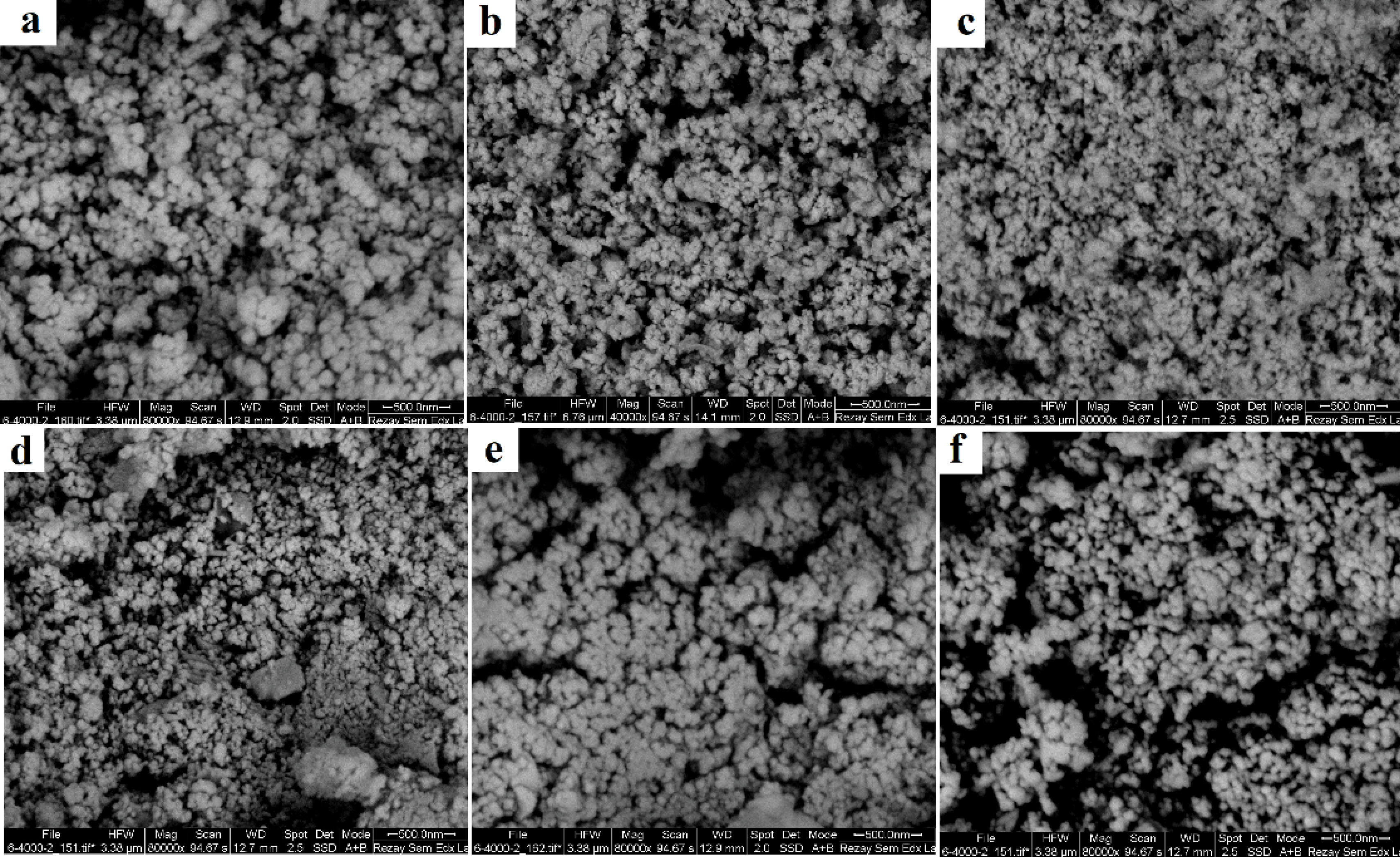
Figure 4.
Scanning Electron Microscopy of Silver Nanoparticles at Different Conditions.
.
Scanning Electron Microscopy of Silver Nanoparticles at Different Conditions.
Table 2.
Laboratory Conditions for Synthesis of Silver Nanoparticles
|
Sample
|
Laboratory condition
|
Morphology
|
| A |
50 watt-5 minutes |
Nanoparticles |
| B |
50 watt-10 minutes |
Nanoparticles |
| C |
50 watt-15 minutes |
Nanoparticles |
| D |
60 watt-5 minutes |
Nanoparticles |
| E |
60 watt-10 minutes |
Agglomeration |
| F |
60 watt-15 minutes |
Agglomeration + nanoparticles |
The XRD pattern of Ag nanocomposites proves the phase formed as a face-centered cubic crystal structure and its lattice parameters. Figure 5 shows the XRD patterns of Ag nanocomposites.
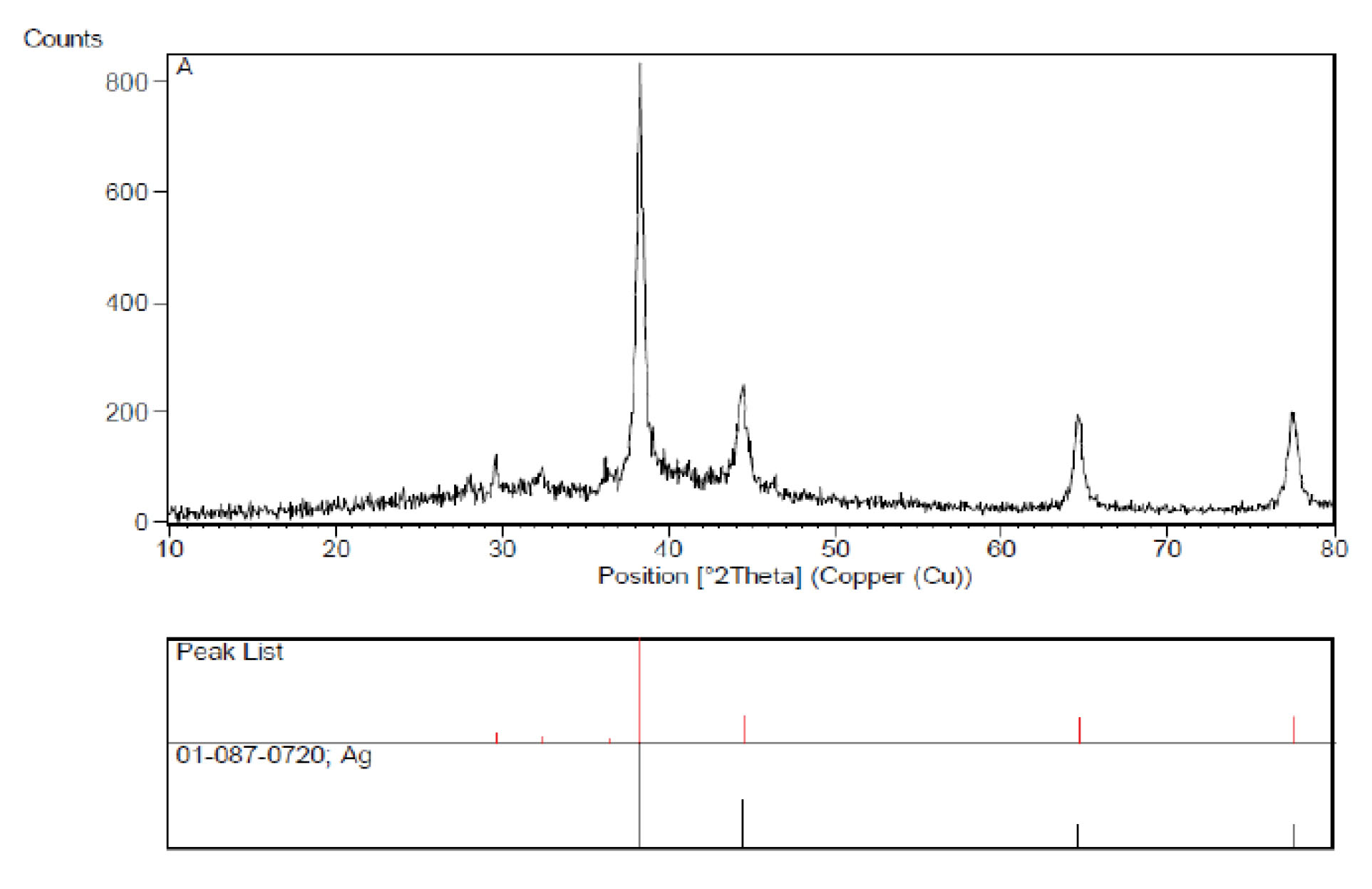
Figure 5.
XRD Patterns of Ag Nanocomposites
.
XRD Patterns of Ag Nanocomposites
Discussion
The antibiotic resistance crisis has become a global concern (12). High mortality rates associated with MRSA infections are the result of the increase in antibiotic resistance via natural selection (13). This investigation aimed to examine the antibacterial activity of AgNPs against MRSA, using in vitro assay. To achieve this goal, we examined 10 clinical MRSA isolates with different concentrations of AgNPs and investigated their effects on their viability and growth. The synthesized AgNPs with a small size and large surface showed beneficial effects even at very low concentrations on the bacteria used. Based on our results, susceptibility to AgNPs depended on the concentration. All MRSA isolates were susceptible to nanoparticles and the MIC and MBC values were found to be 0.015 and 0.07 mg/mL, respectively. The use of silver ions and silver salts as an antimicrobial agent has a long history. Many researchers have studied the antimicrobial effects of AgNPs (14). Zhou et al studied the antibacterial activities of gold and AgNPs against Escherichia coli and bacillus Calmette-Guérin and their results showed that nanoparticles had potential applications as anti-TB compounds (15). In another study, AgNPs synthesized by electrolysis method showed more antibacterial activities against Gram-negative than Gram-positive bacteria (16). Other researchers suggested that yeast and E. coli were inhibited at the low concentration of Ag nanoparticles, whereas the growth-inhibitory effects on S. aureus were mild (17). In another investigation, 100% resistance of A. baumanniito different antibiotics was shown and all isolates (100%) were susceptible to nanosilver (18). AgNPs inhibited the virus from binding to host cells, as demonstrated in vitro (19). Possible mechanisms of AgNPs on bacteria can be attributed to their effects on the respiratory chain and cell division (20).
Conclusions
AgNPs, prepared by the described method, had great potential as antimicrobial agents. Applications of AgNPs based on these findings may lead to valuable discoveries in several fields such as antimicrobial systems and medical devices. The advent of AgNPs as promising antibacterial nanomaterials requires clear and full elucidations of their possible toxicity.
Conflict of Interests
Authors have no conflict of interests to declare.
Acknowledgment
The authors would like to express their thanks to all people who helped with the project. The study was conducted as a part of an MSc thesis at the Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran.
Ethical Approval
Not applicable.
Authors’ Contribution
All authors equally contributed to designing the study, analyzing data, and writing the paper. Further, all authors had primary responsibility for the final content of the manuscript and all authors read and approved the final manuscript.
Funding/Support
This study was financially supported by Islamic Azad University of Kerman, Iran.
References
- Findlater A, Bogoch Bogoch, II II. Human mobility and the global spread of infectious diseases: a focus on air travel. Trends Parasitol 2018; 34(9):772-83. doi: 10.1016/j.pt.2018.07.004 [Crossref] [ Google Scholar]
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000; 406(6797):775-81. doi: 10.1038/35021219 [Crossref] [ Google Scholar]
- Weir E, Lawlor A, Whelan A, Regan F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008; 133(7):835-45. doi: 10.1039/b715532h [Crossref] [ Google Scholar]
- Dibrov P, Dzioba J, Gosink KK, Häse CC. Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob Agents Chemother 2002; 46(8):2668-70. doi: 10.1128/aac.46.8.2668-2670.2002 [Crossref] [ Google Scholar]
- Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 2009; 145(1-2):83-96. doi: 10.1016/j.cis.2008.09.002 [Crossref] [ Google Scholar]
- Mahdieh M, Zolanvari A, Azimee AS, Mahdieh M. Green biosynthesis of silver nanoparticles by Spirulina platensis. Scientia Iranica 2012; 19(3):926-9. doi: 10.1016/j.scient.2012.01.010 [Crossref] [ Google Scholar]
- Nazoori ES, Kariminik A. In vitro evaluation of antibacterial properties of zinc oxide nanoparticles on pathogenic prokaryotes. J Appl Biotechnol Rep 2018; 5(4):162-5. doi: 10.29252/jabr.05.04.05 [Crossref] [ Google Scholar]
- Thakur P, Nayyar C, Tak V, Saigal K. Mannitol-fermenting and tube coagulase-negative staphylococcal isolates: unraveling the diagnostic dilemma. J Lab Physicians 2017; 9(1):65-6. doi: 10.4103/0974-2727.187926 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: CLSI; 2010.
- Baddour MM, AbuElKheir MM, Fatani AJ. Comparison of mecA polymerase chain reaction with phenotypic methods for the detection of methicillin-resistant Staphylococcus aureus. Curr Microbiol 2007; 55(6):473-9. doi: 10.1007/s00284-007-9015-6 [Crossref] [ Google Scholar]
- Valgas C, de Souza SM, Smânia EF, Smânia A Jr. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 2007; 38(2):369-80. doi: 10.1590/s1517-83822007000200034 [Crossref] [ Google Scholar]
- Helan V, Prince JJ, Al-Dhabi NA, Arasu MV, Ayeshamariam A, Madhumitha G. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys 2016; 6:712-8. doi: 10.1016/j.rinp.2016.10.005 [Crossref] [ Google Scholar]
- Martens E, Demain AL. The antibiotic resistance crisis, with a focus on the United States. J Antibiot (Tokyo) 2017; 70(5):520-6. doi: 10.1038/ja.2017.30 [Crossref] [ Google Scholar]
- Mocan L, Matea C, Tabaran FA, Mosteanu O, Pop T, Puia C. Selective in vitro photothermal nano-therapy of MRSA infections mediated by IgG conjugated gold nanoparticles. Sci Rep 2016; 6:39466. doi: 10.1038/srep39466 [Crossref] [ Google Scholar]
- Zhou Y, Kong Y, Kundu S, Cirillo JD, Liang H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnology 2012; 10:19. doi: 10.1186/1477-3155-10-19 [Crossref] [ Google Scholar]
- Theivasanthi T, Alagar M. Anti-bacterial studies of silver nanoparticles. arXiv. 2011. Available from: https://arxiv.org/abs/1101.0348.
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007; 3(1):95-101. doi: 10.1016/j.nano.2006.12.001 [Crossref] [ Google Scholar]
- Niakan S, Niakan M, Hesaraki S, Nejadmoghaddam MR, Moradi M, Hanafiabdar M. Comparison of the antibacterial effects of nanosilver with 18 antibiotics on multidrug resistance clinical isolates of Acinetobacter baumannii. Jundishapur J Microbiol 2013; 6(5):e8341. doi: 10.5812/jjm.8341 [Crossref] [ Google Scholar]
- Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology 2005; 3:6. doi: 10.1186/1477-3155-3-6 [Crossref] [ Google Scholar]
- Klasen HJ. A historical review of the use of silver in the treatment of burns II Renewed interest for silver. Burns 2000; 26(2):131-8. doi: 10.1016/s0305-4179(99)00116-3 [Crossref] [ Google Scholar]