Avicenna Journal of Clinical Microbiology and Infection. 7(4):104-108.
doi: 10.34172/ajcmi.2020.23
Original Article
Antibiotic Resistance Pattern Among Staphylococcus aureus Isolated From Wound Cultures in Burn Patients: A Five-Year Study
Bahram Askarpour 1  , Alireza Sedaghat 2, Nazanin Hazrati 1, Ali Ahmadabadi 3, Masoud Youssefi 4, Majid Khadem-Rezaiyan 5, *
, Alireza Sedaghat 2, Nazanin Hazrati 1, Ali Ahmadabadi 3, Masoud Youssefi 4, Majid Khadem-Rezaiyan 5, *  , Nooshin Abdollahpour 6
, Nooshin Abdollahpour 6
Author information:
1Medical doctor, Mashhad University of Medical Sciences, Mashhad, Iran.
2Department of Anesthesiology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3Surgical Oncology Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4Antimicrobial Resistance Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
5Department of Community Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
6Department of Biology, Young Researchers and Elite Club, Islamic Azad University-Mashhad Branch, Mashhad, Iran.
*
Corresponding author: Majid Khadem-Rezaiyan, Clinical Research Development Unit of Akbar Hospital, Fakuri Boulevard, Mashhad, Iran. Tel: +985138461816; Email:
khademrm@mums.ac.ir
Abstract
Background: Burn remains a globally significant life-threatening problem, especially in developing countries, and infection is considered as a major complication among burn patients. The rate of antibiotic-resistant bacteria isolated from burn patients has demonstrated a significant increase. In this regard, this study aimed to determine the antibiotic resistance pattern in Staphylococcus aureus isolated from patients’ burn wound infections.
Methods: All available wound cultures of burn patients admitted to the burn unit of Emam-Reza hospital/ Mashhad, northeast Iran from March 2012 to March 2017 were included in this retrospective study. Then, the resistance of isolated S. aureus strains against 25 different antibiotic disks was studied based on the aim of the study.
Results: Overall, 1973 patients were admitted, out of whom 4758 swab samples were taken from them. Out of 3188 micro-organisms isolated from burn wound cultures, 185 (5.8%) cases were S. aureus. Based on the results, the highest susceptibility rates were related to vancomycin (98.8%), cefazolin (72%), ciprofloxacin (75%), and gentamicin (74.6%).
Conclusions: In general, vancomycin, cefazolin, and ciprofloxacin appeared to be the most effective agents among all tested antibiotics for S. aureus. The extensive use of antibiotics in treating infections has resulted in the emergence of resistant strains. Routine microbiological surveillance and careful in vitro testing before antibiotic use may help in the prevention of the ever-increasing antibiotic-resistant pathogens in burn infections.
Keywords: Antibiotic susceptibility, Staphylococcus aureus, Burn infection
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
One of the most challenging worldwide public health problems is managing burn wounds, particularly in developing countries with weaker wound care strategies (1). Microorganisms have the opportunity to enter in a suppressed immune system in burn patients as a result of the disrupted skin barrier and organ dysfunction (2-4).
The bacterial infection is a leading cause of morbidity and mortality in hospitalized patients with burn wounds (1,2). The rate of death related to infections is 75% compared to osmotic shock and hypovolemia in burn patients (5). According to previous research on the bacteria spectrum, its pattern has changed during the past ten years (6). The most frequent bacteria isolated from burn patients were Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, and various coliform bacilli. S. aureus remains a significant cause of infection in burn injuries (5,7). In addition, the emergence of antimicrobial resistance microorganisms results in the failure management of wound infections in treatments and require specialized management (8).
Given the above-mentioned explanations, our study sought to evaluate the antimicrobial resistance pattern among S. aureus strains isolated from burn wounds in a burn unit in Mashhad, Iran. Data from this study can be a helpful guide for the future antimicrobial therapy of burn patients and the review of hospital wound care strategies.
Methods
A retrospective computer database from all available wound cultures of burn patients during March 2012-2017 (5-years) was included in this analysis from the burn unit of Emam-Reza hospital, Mashhad, Iran.
During patients’ hospital stay in the burn intensive care unit (BICU) and the burn ward, samples were taken from burn wounds for microbiological tests. Further, culture and sensitivity tests were undertaken at the central microbiology laboratory of Imam Reza Hospital by the microbiologist. All isolated S. aureus samples were tested against twenty-four antibiotic disks based on the usual protocols in our hospital. The antibiotics were cefazolin, ciprofloxacin, cefepime, clindamycin, erythromycin, tetracycline, penicillin G, vancomycin, ampicillin, gentamicin, amikacin, colistin, imipenem, ceftriaxone, cotrimoxazole, piperacillin/tazobactam, meropenem, ceftazidime, tobramycin, levofloxacin, cefotaxime, cefixime, and oxacillin. All disks were purchased from Rosco Diagnostica, Taastrup, Denmark (www.rosco.dk). The disks were chosen for each sample based on the available disks at the time of admission or the particular demand of the physician.
The plates were preserved at 35ºC for 24 hours in an incubator. After the appearance of colonial growth, Gram-staining and catalase tests were used to identify Staphylococcus spp. Additional tests applied to isolate S. aureus were mannitol salt agar, DNase, and coagulase. Moreover, the resistance pattern of S. aureus against 24 different antibiotics was tested by the Kirby-Bauer method according to the Clinical and Laboratory Standards Institute (CLSI) protocol (9). The sensitivity pattern was identified and classified under susceptible (S) and resistant (R) groups. The quality control for antibiotic susceptibility test was based on laboratory standard protocols. Furthermore, a standard strain of S. aureus with the number of ATCC 29213 was used for the quality control of antibiotic susceptibility.
The vancomycin resistance report in S. aureus by the disk diffusion method is an initial screening. Our reference for resistance determination was the diameter of the inhibition zone which was based on the manufacturer’s instruction. According to CLSI instructions, vancomycin-resistant cases should be confirmed with minimal inhibitory concentration (MIC). As a result, vancomycin-resistant cases are not definite positive, which can be due to the laboratory assay error.
The statistical analysis was performed using SPSS software, version 24 for Windows. Descriptive analysis was performed, including median and inter-quartile range (25%-75%), and numeric data were summarized as means or medians depending on normality. Eventually, associations between categorical variables were tested by Pearson chi-square or Fisher exact test, and P<0.05 was considered statistically significant.
Results
In general, 4758 swab samples were taken among 1973 burn patients who were admitted during the 5 years of assessment. A total of 3188 bacterial strains were isolated, and 185 (5.8%) of them were identified as S. aureus.
Among S. aureus infected patients, participants’ mean (SD, median and interquartile range) age was 20.8 (21,16,2-35) years, and the majority of patients were men (73, 61%).
All samples were obtained from burn wounds.Resistance and susceptibility rates to various antibiotics are described in Table 1. Based on the results, vancomycin was the most effective antibiotic against S. aureus infection while erythromycin, tetracycline, penicillin G, ceftriaxone, and oxacillin were found to have the highest resistance among the tested drugs during 5 years.
Table 1.
The Resistance and Susceptibility Rates of Various Antibiotics
|
Antibiotics
|
Resistance
|
Susceptibility
|
| Amikacin |
6 (54.5) |
5 (45.5) |
| Ampicillin |
3 (75.0) |
1 (25.0) |
| Cefazolin |
7 (28.0) |
18 (72.0) |
| Cefepime |
18 (47.4) |
20 (52.6) |
| Cefixime |
0 |
1 (100.0) |
| Cefotaxime |
17 (37.8) |
28 (62.2) |
| Cefoxitin |
26 (28.9) |
64 (71.1) |
| Ceftazidime |
1 (25.0) |
3 (75.0) |
| Ceftriaxone |
8 (57.1) |
6 (42.9) |
| Ciprofloxacin |
26 (25.0) |
78 (75.0) |
| Clindamycin |
54 (31.4) |
118 (68.6) |
| Colistin |
3 (27.3) |
8 (72.7) |
| Cotrimoxazole |
50 (35.7) |
90 (64.3) |
| Erythromycin |
88 (50.6) |
86 (49.4) |
| Gentamicin |
33 (25.4) |
97 (74.6) |
| Imipenem |
6 (24.0) |
19 (76.0) |
| Levofloxacin |
2 (66.7) |
1 (33.3) |
| Meropenem |
1 (100.0) |
0 |
| Oxacillin |
36 (97.3) |
1 (2.7) |
| Penicillin G |
53 (91.4) |
5 (8.6) |
| Piperacillin/Tazobactam |
8 (80.0) |
2 (20.0) |
| Tetracycline |
13 (59.1) |
9 (40.9) |
| Tobramycin |
2 (66.7) |
1 (33.3) |
| Vancomycin |
2 (1.2) |
170 (98.8) |
Note. Data are represented as frequency (%).
All microbiologic samples were collected from patients in the BICU and the burn ward, and the frequency of S. aureus was 13 (8%) and 172 (92%) in BICU and the burn ward, respectively. Figure 1 shows the comparison of S. aureus resistance patterns to different antibiotics in the BICU and burn ward. There were no significant differences between the two wards in terms of antibiotic resistance.
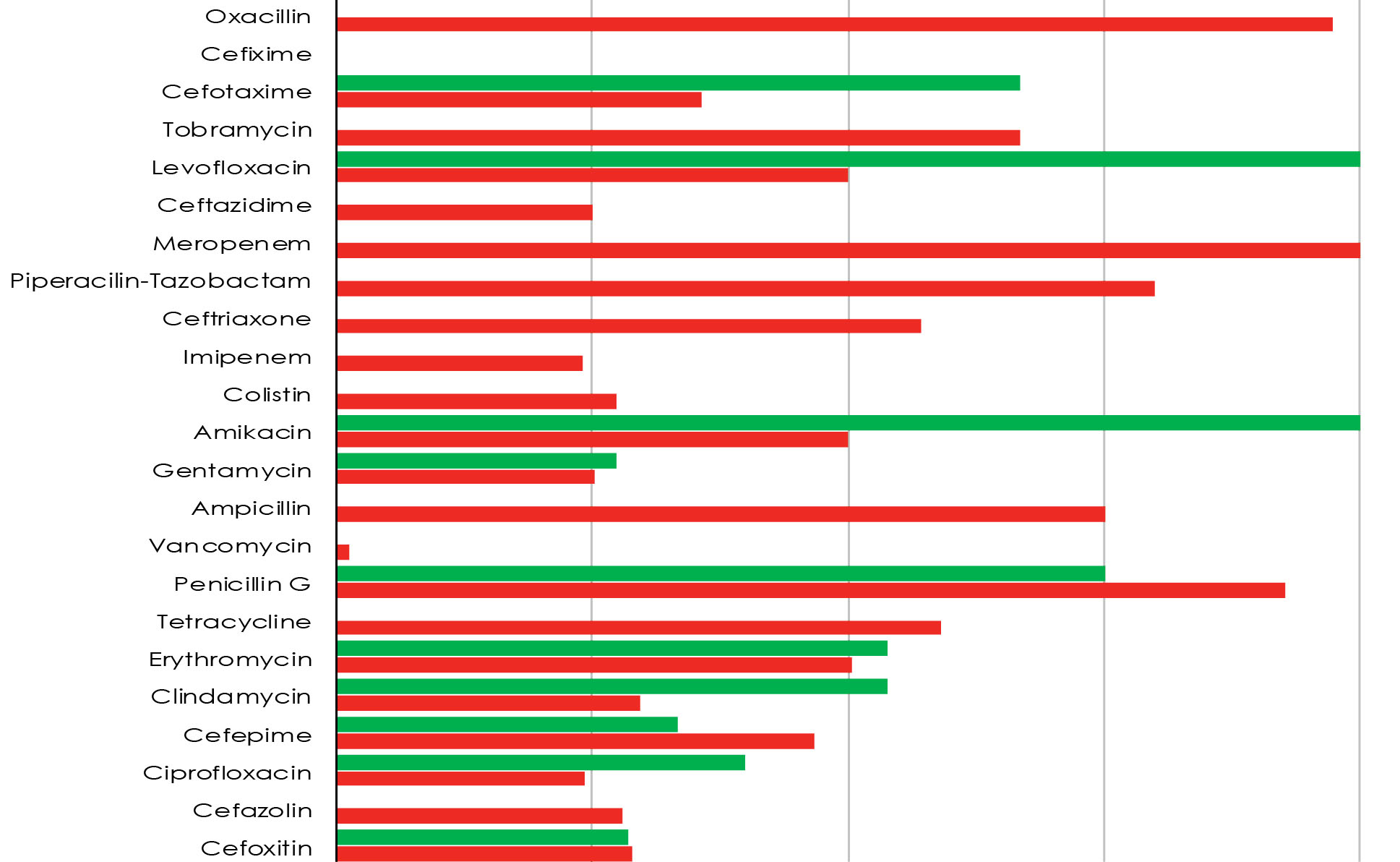
Figure 1.
The Resistance Pattern of Staphylococcus aureus to Various Antibiotics in Burn ICU and Burn Ward. Note. ICU: Intensive care unit.
.
The Resistance Pattern of Staphylococcus aureus to Various Antibiotics in Burn ICU and Burn Ward. Note. ICU: Intensive care unit.
The distribution of the resistance pattern from 2012 to 2017 is illustrated in Figure 2. In these 5 years, the cefoxitin resistant rate decreased from 18% to 6% and represented a statistically significant difference (P = 0.002). Further, resistance to gentamicin reduced from 43.3% to 12% with a significant difference (P= 0.05). Although a reduction in the rate of resistance to erythromycin was observed (64% to 30%), these changes were just in a statistically significance threshold (P = 0.06).
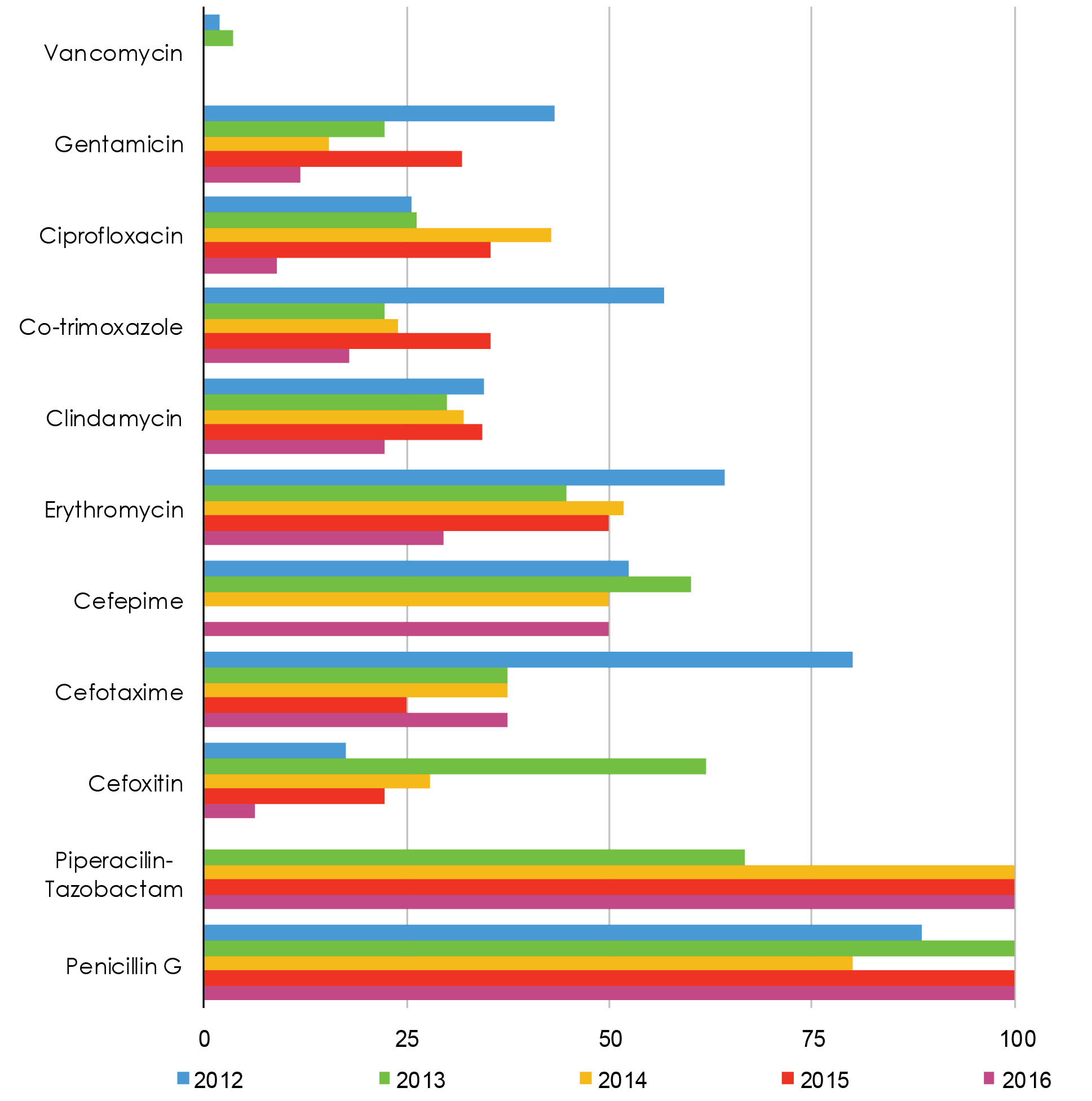
Figure 2.
Resistance Pattern of Staphylococcus aureus to Various Antibiotics During 2012-2017 in Burn Patients. Note. ICU: Intensive care unit.
.
Resistance Pattern of Staphylococcus aureus to Various Antibiotics During 2012-2017 in Burn Patients. Note. ICU: Intensive care unit.
Discussion
Burn patients are at the risk of bacterial infections during their hospitalization, and infections are prone to become complicated because of their compromised immune system. Among our patients, S. aureus was one of the most common isolated organisms, which is in line with the findings of other researches (7,10,11). S. aureus is important among gram-positive organisms because of complications that it causes in burn patients, putting them in hazardous conditions. Along with toxins such as hemolysin, leukotoxin, exfoliative toxin, and toxic shock syndrome toxin, S. aureus with the help of lipase, protease, and hyaluronidase enzymes can damage the patient’s tissues and cause the toxic-shock syndrome. These factors can endanger the health of burn patients who already suffer from hypovolemic shock.
It is also one of the most important causes of bacteremia, mortal septicemia, and endocarditis (12-14). Fournier et al reported that S. aureus carriage was found as a potent predictor of early-onset Pneumonia (15). Other dominant organisms found in burn patients in other studies were Pseudomonas aeruginosa, Klebsiella spp., and Proteus spp. (16,17).
The available antibiotics currently used as the empirical treatment for S. aureus are vancomycin, clindamycin, and fluoroquinolone family (e.g., ciprofloxacin and the like), and it seems to be an appropriate treatment. In case of facing high resistance to any of these antibiotics in the future, this empirical treatment should be changed and replaced with other effective antibiotics. Furthermore, these antibiotics can be chosen based on the laboratory resistance pattern, patient conditions, other applied drugs, and the availability and cost-effectiveness of antimicrobial choices. Clinicians make such decisions for each patient. Moreover, the infection control committee of the hospital provides effective policies that are renewed every couple of years to help clinicians prescribe the most effective antibiotics. For example, Zorgani et al (18) recommended that tigecycline and linezolid are good choices for treating methicillin-resistant S. aureus.
Vancomycin, with its high sensitivity (98.8%), is the only available choice in resistant S. aureus, and the emergence of vancomycin-resistant strains is an alarming health threat (2,7,10,18,19). To avoid creating vancomycin resistance S. aureus, prescribing this antibiotic should only be preserved for treating multidrug-resistant strains.
Based on the results of the study by Rahimipour et al(20), the two reported vancomycin resistance samples of our study were probably due to a technical problem. Nonetheless, the confirmation of S. aureus resistance to vancomycin needs conducting a thorough genetic study and checking the presence of mecA and vanA genes based on molecular techniques, but that of our study was only a phenotypic type.
In our study, S. aureus was resistant to piperacillin, which contradicts with the result of the study by Rezaei et al (21) that was done in 2009 at the same hospital (80.0% vs. 31.6%). Several steps can be taken to decrease or prevent this increasing resistance rate to antibiotics, including using reliable antibiotic prescription guidelines such as Infectious Diseases Society of America (IDSA), using the correct dose and the optimal duration for treatment, changing empirical antibiotics to definitive as soon as possible, and following the hospital infection control guidelines. Additionally, another step is to consult with a team consisting of a clinical microbiologist, an infectious disease specialist, and a clinical pharmacist for choosing the right antibiotic. It is not just about seeking their help in choosing the best antibiotic. More precisely, they can even help in decreasing drug interactions and their adverse effects.
The reviewing of 11 antibiotics during five years demonstrated decreasing resistance to gentamicin, ciprofloxacin, cotrimoxazole, clindamycin, vancomycin, cefoxitin, erythromycin, and cefotaxime, which may be due to an appropriate prescription of antibiotics by clinicians and proper infection control of burn wards in recent years.
Staphylococcus aureus infections in burn wards are different in comparison to community-acquired infections regarding resistance patterns to antibiotics. Oxacillin is a good example as it is used in other wards for the treatment of S. aureus infections with good results. However, in our study, S. aureus showed a high resistant rate to this antibiotic (97.3%). Although S. aureus represented a high resistant rate to some antibiotics such as piperacillin (80%), using t test and MIC may prove that they still can be effective in higher doses. Given that this was a retrospective epidemiological study, we were unable to study methicillin-resistant S. aureus strains.
Conclusions
Among all tested antibiotics, vancomycin, cefazolin, and ciprofloxacin were the most effective agents against S. aureus. The widespread use of antibiotics in the treatment of bacterial infections has led to the emerging of resistant strains. Routine microbiological surveillance and careful in-vitro testing before antibiotic use and strict adherence to hospital antibiotic policy may help prevent antibiotic-resistant pathogens in burn infections.
Conflict of Interests
None.
Acknowledgements
The authors would like to thank the help of the Clinical Development Research Unit of Akbar Hospital in preparing this manuscript.
Ethical Approval
This study was approved by Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1397.124).
Authors’ Contribution
Study design: AS, NH, AA, MY, and MKR; Data gathering: BA, NH, AA, and NA; Data analysis: AA, MY, and MKR; Manuscript drafting: BA, AS, NH, MY, and MKR; Final approval: BA, AS, NH, AA, MY, MKR, and NA.
Funding/Support
This study was supported by Vice Chancellor for Research of Mashhad University of Medical Sciences.
References
- Babakir-Mina M, Othman N, Najmuldeen HH, Noori CK, Fatah CF, Perno CF. Antibiotic susceptibility of vancomyin and nitrofurantoin in Staphylococcus aureus isolated from burnt patients in Sulaimaniyah, Iraqi Kurdistan. New Microbiol 2012; 35(4):439-46. [ Google Scholar]
- Rastegar Lari A, Bahrami Honar H, Alaghehbandan R. Pseudomonas infections in Tohid Burn Center, Iran. Burns 1998; 24(7):637-41. doi: 10.1016/s0305-4179(98)00090-4 [Crossref] [ Google Scholar]
- Namvar AE, Khodaei F, Bijari A, Lari AR. Detection of integrons and Staphylococcal Cassette Chromosome (SCCmec) types in Staphylococcus aureus isolated from burn and non-burn patients. Int J Health Sci (Qassim) 2015; 9(4):440-5. [ Google Scholar]
- Parhizgari N, Khoramrooz SS, Malek Hosseini SA, Marashifard M, Yazdanpanah M, Emaneini M. High frequency of multidrug-resistant Staphylococcus aureus with SCCmec type III and Spa types t037 and t631 isolated from burn patients in southwest of Iran. Apmis 2016; 124(3):221-8. doi: 10.1111/apm.12493 [Crossref] [ Google Scholar]
- Alebachew T, Yismaw G, Derabe A, Sisay Z. Staphylococcus aureus burn wound infection among patients attending Yekatit 12 hospital burn unit, Addis Ababa, Ethiopia. Ethiop J Health Sci 2012; 22(3):209-13. [ Google Scholar]
- Wang LF, Li JL, Ma WH, Li JY. Drug resistance analysis of bacterial strains isolated from burn patients. Genet Mol Res 2014; 13(4):9727-34. doi: 10.4238/2014.January.22.10 [Crossref] [ Google Scholar]
- Amissah NA, van Dam L, Ablordey A, Ampomah OW, Prah I, Tetteh CS. Epidemiology of Staphylococcus aureus in a burn unit of a tertiary care center in Ghana. PLoS One 2017; 12(7):e0181072. doi: 10.1371/journal.pone.0181072 [Crossref] [ Google Scholar]
- Sedaghat A, Khadem-Rezaiyan M, Ahmadabadi A, Abbaspour H, Youssefi M, Shirzad MM. Antibacterial resistance pattern of Acinetobacter baumannii in burn patients in Northeast of Iran. Jundishapur J Microbiol 2019; 12(10):e94668. doi: 10.5812/jjm.94668 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). CLSI Standards: Guidelines for Health Care Excellence. Available from: https://clsi.org/standards/. Accessed February 5, 2020.
- Bayram Y, Parlak M, Aypak C, Bayram I. Three-year review of bacteriological profile and antibiogram of burn wound isolates in Van, Turkey. Int J Med Sci 2013; 10(1):19-23. doi: 10.7150/ijms.4723 [Crossref] [ Google Scholar]
- Rastegar Lari AR, Alaghehbandan R, Akhlaghi L. Burn wound infections and antimicrobial resistance in Tehran, Iran: an increasing problem. Ann Burns Fire Disasters 2005; 18(2):68-73. [ Google Scholar]
- Edwards-Jones V, Dawson MM, Childs C. A survey into toxic shock syndrome (TSS) in UK burns units. Burns 2000; 26(4):323-33. doi: 10.1016/s0305-4179(99)00142-4 [Crossref] [ Google Scholar]
- de Macedo JLS, Rosa SC, Castro C. Sepsis in burned patients. Rev Soc Bras Med Trop 2003; 36(6):647-52. doi: 10.1590/s0037-86822003000600001 [Crossref] [ Google Scholar]
- Regules JA, Glasser JS, Wolf SE, Hospenthal DR, Murray CK. Endocarditis in burn patients: clinical and diagnostic considerations. Burns 2008; 34(5):610-6. doi: 10.1016/j.burns.2007.08.002 [Crossref] [ Google Scholar]
- Fournier A, Voirol P, Krähenbühl M, Bonnemain CL, Fournier C, Dupuis-Lozeron E. Staphylococcus aureus carriage at admission predicts early-onset pneumonia after burn trauma. Eur J Clin Microbiol Infect Dis 2017; 36(3):523-8. doi: 10.1007/s10096-016-2828-0 [Crossref] [ Google Scholar]
- Ozumba UC, Jiburum BC. Bacteriology of burn wounds in Enugu, Nigeria. Burns 2000; 26(2):178-80. doi: 10.1016/s0305-4179(99)00075-3 [Crossref] [ Google Scholar]
- Soleymanzadeh-Moghadam S, Azimi L, Amani L, Rastegar Lari A, Alinejad F, Rastegar Lari A. Analysis of antibiotic consumption in burn patients. GMS Hyg Infect Control 2015; 10:Doc09. doi: 10.3205/dgkh000252 [Crossref] [ Google Scholar]
- Zorgani AA, Elahmer O, Abaid A, Elaref A, Elamri S, Aghila E. Vancomycin susceptibility trends of methicillin-resistant Staphylococcus aureus isolated from burn wounds: a time for action. J Infect Dev Ctries 2015; 9(11):1284-8. doi: 10.3855/jidc.6976 [Crossref] [ Google Scholar]
- Kaushik R, Kumar S, Sharma R, Lal P. Bacteriology of burn wounds--the first three years in a new burn unit at the Medical College Chandigarh. Burns 2001; 27(6):595-7. doi: 10.1016/s0305-4179(01)00023-7 [Crossref] [ Google Scholar]
- Rahimipour F, Roodbari F, Ghazvini K, Youssefi M. VanA and MecA genes in Staphylococcus aureus isolates in North-Eastern Iran. Acta Microbiol Bulg 2016; 32(4):232-6. [ Google Scholar]
- Rezaei E, Safari H, Naderinasab M, Aliakbarian H. Common pathogens in burn wound and changes in their drug sensitivity. Burns 2011; 37(5):805-7. doi: 10.1016/j.burns.2011.01.019 [Crossref] [ Google Scholar]