Avicenna Journal of Clinical Microbiology and Infection. 7(4):124-128.
doi: 10.34172/ajcmi.2020.27
Original Article
A Comparative Study of Potassium Hydroxide Wet Mount, Calcofluor White Staining and Culture for the Diagnosis of Keratomycosis
Pallati Alekhya 1, *  , C. Aruna Sunder 2, Prathiba 3
, C. Aruna Sunder 2, Prathiba 3
Author information:
1Department of Microbiology, Government Medical College, Mahabubnagar, Telangana, India.
2Department of Microbiology, Government Medical College, Nizambad, Telangana, India.
3Department of Microbiology, Gandhi Medical College, Secunderabad, Telangana, India.
*
Corresponding author: Pallati Alekhya, Department of Microbiology, Government Medical College, Mahabubnagar, Telangana, India. Tel: +91-8801350380, +91-9951796963; Email:
chakrambala88@gmail.com
Abstract
Background: The incidence of keratomycosis has increased dramatically in recent years. Early diagnosis and treatment of keratomycosis are important in preventing further complications. Direct microscopic techniques are time-saving for diagnosing keratomycosis when compared to culture methods. This study was carried out to determine the sensitivities of potassium hydroxide (KOH) wet mount, Gram stain and Calcofluor white (CFW) stain plus KOH wet mount by taking culture as the gold standard.
Methods: Corneal scrapings were collected from 150 clinically suspected patients with keratomycosis. Demographic profile was collected and analyzed.
Results: Of these patients, 67.33% were male, 24% were in the age group of 51-60 years, 70% were ruralresidents, 44% were agricultural workers, and 60% presented with a history of corneal trauma. Laboratory investigations have revealed that 29.33% (44 cases) were culture positive. In other words, Fusarium spp. was isolated in 17 cases, Aspergillus spp. in 14 cases, phaeoid fungi in 3 cases and unidentified fungi in 10 cases. The positivity of CFW stain plus KOH wet mount, KOH wet mount, and Gram stain was 30%, 23.3%, and 20%, respectively. Sensitivities of CFW stain plus KOH wet mount, KOH wet mount, and Gram stain were 79.55%, 54.55%, and 47.62%, respectively.
Conclusion: Post-investigative analysis has revealed that CFW stain plus KOH wet mount was better than KOH wet mount alone in demonstrating fungal pathogens. Therefore, early diagnosis of keratomycosis by meticulous examination of corneal scrapings by direct microscopy specifically using CFW stain plus KOH wet mount and institution of antifungal therapy may limit ocular morbidity and disastrous sequelae among these patients.
Keywords: Calcofluor white stain, Potassium hydroxide wet mount, Keratomycosis, Direct microscopy, Grams stain
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Microorganisms including bacteria, fungi, viruses, and parasites penetrate deep into the corneal layers, resulting in inflammation causing keratitis (1,2). Keratomycosis represents 30%-40% of culture-positive infectious keratitis (3). Moreover, fungi have replaced bacteria as the predominant cause of infectious keratitis in developing countries. Epidemiology is complicated and encompasses a wide variety of infectious eye diseases because the prevalence of corneal blindness varies from country to country and even from one population to another, depending on many factors, such as availability and general standards of eye care (4).
Laboratory diagnosis is essential for the accurate diagnosis of etiological agents. Prompt and effective treatment not only slows the progression of the disease but also results in early healing of the ulcer. False negative diagnosis not only delays the specific antifungal therapy but also injudicious use of medication leads to the rapid growth of organisms (5).
This study was undertaken at a regional institute of ophthalmology:
(i) To identify the fungal etiology of corneal ulcer by direct microscopy using Calcofluor white (CFW) staining, potassium hydroxide (KOH) wet mount, Gram stain, and culture on Sabouraud dextrose agar (SDA).
(ii) To evaluate the sensitivity, specificity, and predictive value of CFW staining and KOH wet mount.
Methods
A total of 150 patients of all age groups and both genders attending the outpatient department, who were clinically suspected of having keratomycosis by the ophthalmologist, were included in the study. Informed consent was obtained from all the participants. The demographic profile of the patients including age, gender, occupation, community, signs, and symptoms was evaluated.
Inclusion Criteria
Clinically suspected cases of keratomycosis, on the basis of at least two of the following criteria:
-
Patients with a history of trauma to the eye with vegetable matter or organic matter
-
Patients with clinical signs and symptoms of fungal corneal ulcer
-
Ulcer with irregular and feathery margins
-
Ulcer with satellite lesion
-
Presence of an endothelial plaque, fibrinoid aqueous reaction, and hypopyon formation
-
Dry looking ulcer
-
Pigmented ulcers
Exclusion Criteria
-
Patients already on antifungal therapy.
-
Clinically suggestive of bacterial or viral corneal ulcer
-
Other extra ocular infections
Specimen Collection
Under aseptic conditions, after instillation of 4% lignocaine, corneal scrapings were obtained using a Bard-Parker knife (No. 15) to debride material from the base and edges of the ulcerated part of the cornea (6-8).
Specimen Processing
Fungal elements were detected in the clinical specimen by direct microscopic examination of material from lesion. Corneal scrapings were placed on 2 slides for preparing 10% KOH wet mount (Himedia Laboratories L.B.S Marg, Mumbai, India) and Gram stain (Himedia Laboratories L.B.S Marg, Mumbai, India). After reporting KOH wet mount, one drop of CFW (CFW) comprising 1 g/L Calcofluor White M2R and 0.5 g/L Evans blue (Sigma-Aldrich, St. Louis, MO, USA) was then added to one edge of the cover slip and a filter paper was placed at the opposite edge to draw the stain over the smears between the slide and cover slip.
The scraping material obtained from leading edge and base of the ulcer was inoculated directly on to the surface of solid media such as SDA (Himedia Laboratories L.B.S Marg, Mumbai, India) and incubated in biological oxygen demand incubator (Bionics Scientific Technologies (P) Ltd. Sindhora Kalan, Delhi) at 25°C.
Compound microscope (Olympus CX23 – Olympus Medical Systems India Private Limited, Gurgaon, Delhi) was used for KOH wet mount and Gram stain smears. CFW plus KOH smear was visualized under a fluorescence microscope (LB-244, Labomed, Inc. Los Angeles, USA). In KOH wet mount (Figure 1A), fungi were reported as refractile filaments, in CFW plus KOH (Figure 1B) as apple green filaments and in Gram stain as Gram-positive granular fungal filaments or Gram-positive yeast cells (Figure 1C).
Culture
Fungi on SDA were identified by a combination of:
-
Growth rate
-
Colonial morphological features: color of colony (on obverse and reverse side), colony topography, colony texture, aerial and submerged hyphae
-
Microscopic features: identification of filamentous fungi was based on microscopic features like mycelium, conidium and relationship between hyphae and fruiting bodies. Slide cultures were used for the observation of conidiogenesis of filamentous fungi. Germ tube test and urease test were used for the identification of yeast.
Microbial culture was considered positive if:
-
There was semi-confluent growth at the site of inoculation on solid medium
-
It was consistent with clinical signs.
-
Smear results were consistent with culture.
Sensitivity (true positive rate), specificity (true negative rate), positive predictive value, negative predictive value, and accuracy of the staining methods were calculated using the standard statistical formulae.
Statistical analysis was performed using OpenEpi (version 3.01). The results obtained from direct microscopy of the smear samples were compared with those of fungal culture using the McNemar χ2test with respect to sensitivity and specificity. A P value of less than 0.05 was considered statistically significant.
Results and Discussion
In this study, male predominance was seen (males: 67.33% and females: 32.66%). This is in line with all the studies in which keratomycosis was investigated. The most affected age group was 51-60 years (24.0%). This is in accordance with the study of Srinivasan et al (9) (23.04%). According to studies by Deshpande and Koppikar (10) (79%), Kumari et al (11) (77%), Gopinathan et al (12) (64.4%), Bharathi et al (13) (66.85%), Chander et al (14) (47.14%), and the present study (81.99%), most of the affected individuals are in the age group of 21-60 years (Figure 1).
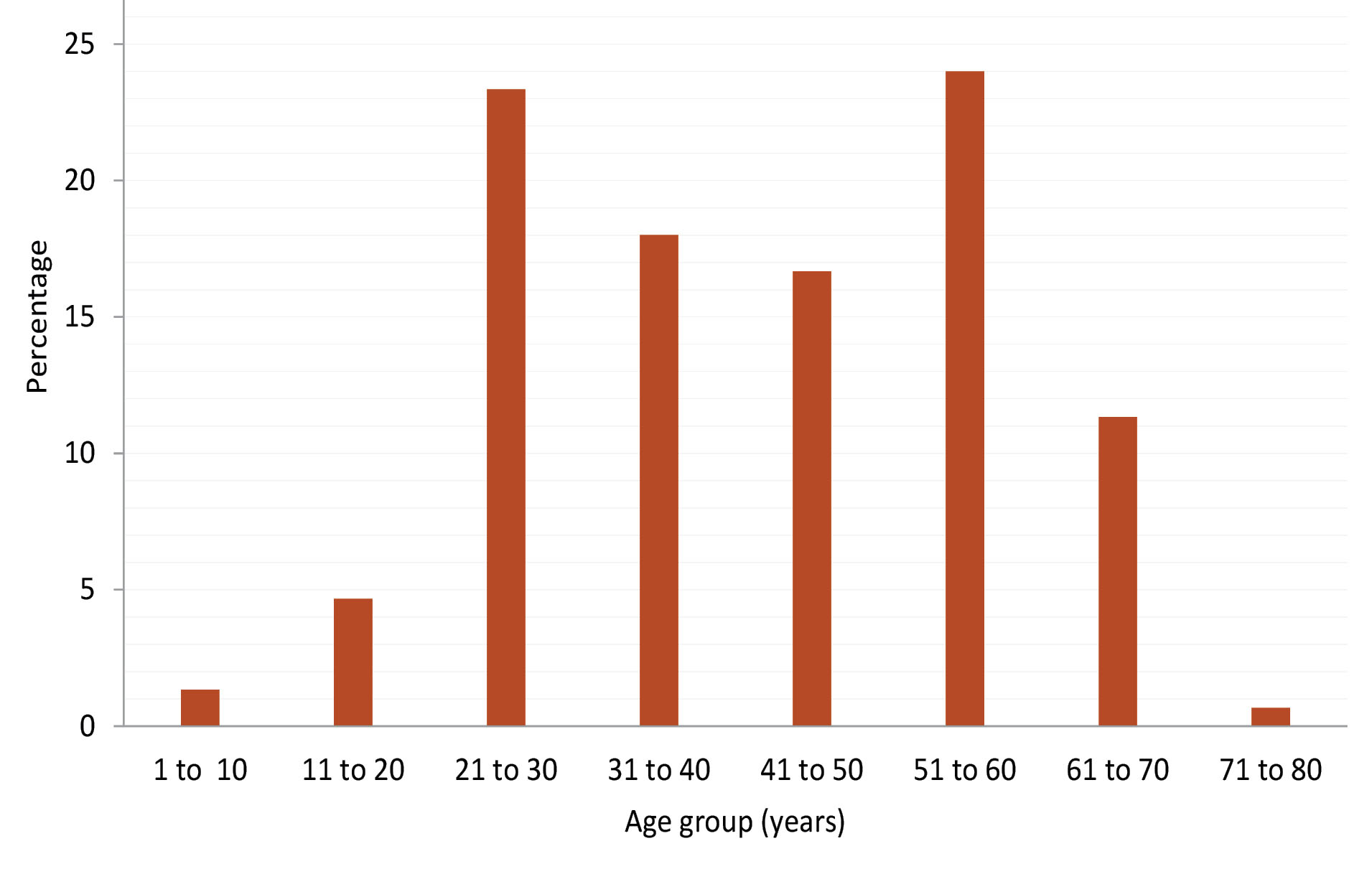
Figure 1.
Frequency of Keratomycosis by Age Group.
.
Frequency of Keratomycosis by Age Group.
Most of the suspected cases were from rural backgrounds (70%), which is in line with other studies (13-15). Out of 150 suspected cases, 66 (44%) were agriculture workers, 32 (21.33%) were laborers, 18 (12%) were households, 7 (4.6%) were students, 3 (2%) were garden workers, and 15 (10%) were people in other occupations. This is in accordance with the studies conducted by other researchers (9,16,17). The results of studies by Riaz et al (18) (Domestic group: 25.6%) and Jacob et al (19) (Housewife: 33.3%) are not in line with the present study.
In the present study, the most common predisposing factor associated with suspected keratomycosis was corneal trauma (60 %) (Table 1).
Table 1.
Predisposing Factors Associated With Keratomycosis
|
Predisposing Factor
|
|
Number Of Cases
|
Percent
|
| Vegetable matter trauma |
Thorn |
3 |
2 |
| Stick |
18 |
12 |
| Flower |
1 |
0.6 |
| Others |
24 |
16 |
| Foreign body trauma |
Stone |
7 |
4.6 |
| Dust |
13 |
8.6 |
| Cement |
3 |
2 |
| Others |
21 |
14 |
| Diabetes mellitus |
|
6 |
4 |
| Ocular surgery |
|
6 |
4 |
| Steroid therapy |
|
2 |
1.3 |
| Others |
|
58 |
38.66 |
This is in accordance with studies conducted by Reddy et al (16) (52.8%) and Anusuya et al (20) (62.96%). Shokohi et al (17) reported most common predisposing factor as use of antimicrobials (22.7%), compared to corneal trauma (9.09%). It is well documented that corticosteroids may well potentiate these commensal fungi to pathogenicity (21). Agarwal et al (22) provided experimental proof of producing corneal ulcer after the injection of fungi and showed that corticosteroids enhance the virulence.
Clinical symptoms associated with keratomycosis are pain, photophobia, and decreased vision. The slit lamp examination reveals shaggy edged ulcer with satellite lesion, marked hypopyon, and endothelial plaque (24). In the present study, 16.6% of cases had stromal infiltrate, 4% had pigmented ulcer, 53.3% had conjunctival congestion, 46.6% had epithelial defect, and 46% showed hypopyon. Verma et al (25) reported that the corneal ulcers were associated with stromal infiltrate (43.47%), satellite lesions (13.04%), feathered margins (8.69%), hypopyon (60.86%), conjunctival congestion (56.52%), epithelial defect (43.47%), non-reactive pupils (30.43%), and scleral congestion (26.08%).
Total fungal culture positivity in this study was 29.33% (Figure 2). This is in accordance with the studies by Saha et al (26) (25.6%) and Khorgade et al (27) (23.8%). However, Gopinathan et al (12) and Jacob et al (19) reported fungal culture positivity of 61.53%, 94.5%, and 62%, respectively. This variable incidence suggests the importance of ecological differences in temperature, humidity of the place, number of eyes investigated, and the methods employed.
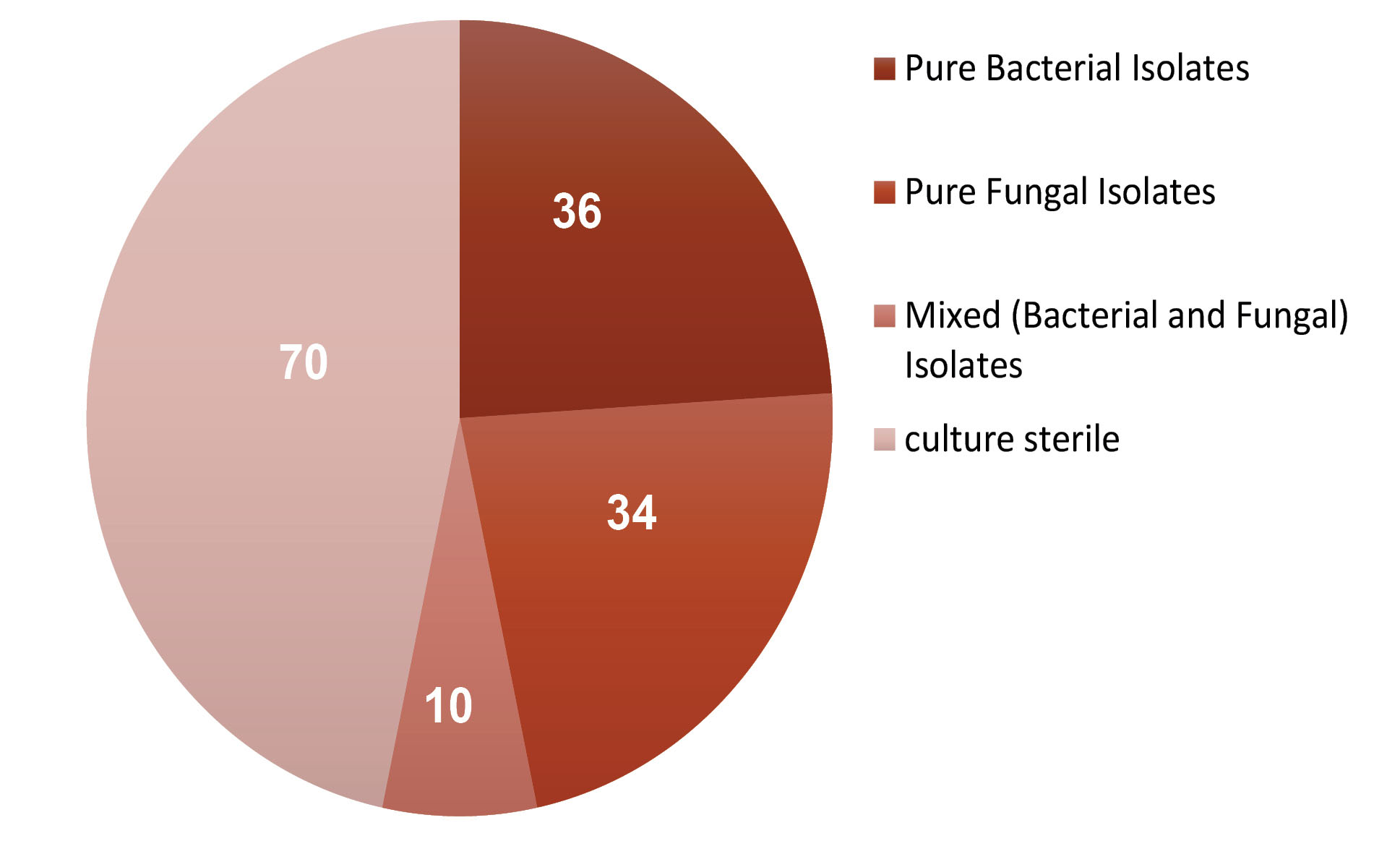
Figure 2.
Incidence of Various Microbial Isolates.
.
Incidence of Various Microbial Isolates.
In the present study, Fusarium spp. was isolated from 38.64% of the cases and Aspergillus spp. was isolated from 31.82% of the cases (Figure 3). This is in line with other similar studies (9,12,13,20). However, in the studies conducted by other researchers, Aspergillus spp. was the most common isolate (3,14,19,29-32). While Jampala et al (33) reported Candida spp. as the most common isolate. In the present study, yeast was not isolated; however, a study conducted by Prathiba et al (34) at the same regional institute reported a prevalence of 1% for Candida.
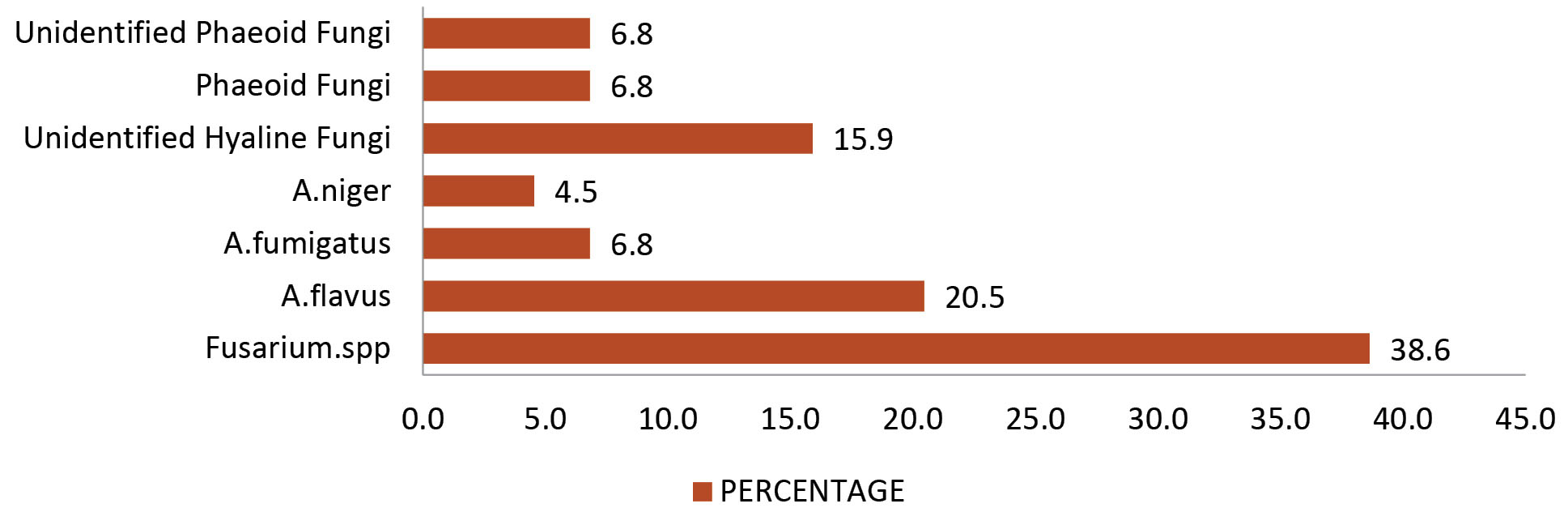
Figure 3.
Etiological Agents of Keratomycosis.
.
Etiological Agents of Keratomycosis.
Isolation is considered a definitive method of diagnosis of keratomycosis. Culture on SDA was considered as the gold standard in many studies. However, reports suggest that direct microscopy with KOH wet mount when correlated with clinical presentation can reveal more cases than culture on SDA alone.
In the present study, the sensitivity using KOH wet mount was 54.55% (Table 2), in a study conducted by Jampala et al (33), the sensitivity of KOH wet mount was reported to be 33.33%. Positivity of CFW plus KOH wet mount in the present study is in line with a study conducted by Shokohi et al (17) (31.81%) and the sensitivity of CFW plus KOH wet mount is in line with the study of Vemuganti et al (35) (84.6%).
Table 2.
Comparison of the Results of Fungal Culture and Direct Microscopic Techniques for Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and Accuracy
|
|
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
| KOH wet mount |
54.55% |
89.62% |
68.57% |
82.61% |
79.33% |
| CFW plus KOH wet mount |
79.55% |
90.57% |
77.78% |
91.43% |
87.33% |
| Gram stain |
47.62% |
90.38% |
66.67% |
81.03% |
78.08% |
P value = 0.0025, P<0.05 is considered statistically significant.
The positivity of CFW plus KOH wet mount (30%) was the highest in the present study when compared to culture (29.33%), KOH wet mount (23.3%), and Gram stain (20%). This is in line with the results of studies conducted by other researchers (17,36-38).
Using KOH wet mount in combination with Gram stain smears resulted in an increase in positivity. In this study, 9 cases were KOH wet mount negative and gram stain positive and 10 cases were KOH positive and Gram negative. However, all the 19 cases were CFW plus KOH wet mount positive. This discrepancy in the results may be due to the quantity of the sample taken and the amount of fungal material present in sample. Other researchers did not analyze such a combination. Most of the laboratories in developing countries like India do not have expensive laboratory equipment to analyze samples using CFW plus KOH wet mount. In such setups, a simple KOH wet mount in combination with Gram stain can lead to a diagnosis which is as accurate as CFW plus KOH wet mount.
Conclusions
Keratomycosis continues to be an important cause of ocular morbidity, mostly in the individuals inhabiting rural areas and involving in outdoor and agricultural activities. Young male adults affected in these circumstances are often the breadwinners of their family and blindness in them leads to grave economic consequences. By performing direct microscopy using CFW plus KOH wet mount, treatment was initiated earlier (by 2-5 days) which helped in preventing complications in the patients. The sensitivity and specificity of CFW plus KOH wet mount were higher compared to those of KOH alone. The collection of corneal scrapings is an invasive procedure which increases risk of developing complications in the patients. Further, advancements like in vivo fluorescent imaging technology can be compared with direct microscopy as it is a non-invasive and rapid procedure.
Conflict of Interests
None.
Acknowledgments
We acknowledge Dr. Vinod Kumar (Superintendent, Sarojini Devi eye hospital, Hyderabad, India) for permitting us to conduct the study and Dr. Manoj Arvind (Associate Professor, Social and Preventive medicine, Government general hospital, Mahabubnagar) for statistical support for the study. We acknowledge Mrs. Saritha (Microbiology Lab Technician, Sarojini Devi eye hospital) and Mr. Srinivas (Microbiology Lab Technician, Sarojini Devi eye hospital) for preparation of media and all other Ophthalmologists at Sarojini Devi Eye Hospital for providing the clinical samples.
Ethical Approval
This prospective study was approved by the Ethics Committee of Osmania Medical College. It was conducted from December 2015 to May 2016 at Sarojini Devi Eye Hospital, Hyderabad, Telangana, India.
Authors’ Contribution
PA: Concept and design of the study, acquisition, analysis and interpretation of data.
CAS: Final approval and monitoring of the study.
P: Critical revision of the study
Funding/Support
None.
References
- Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep 2013; 7(3):209-18. doi: 10.1007/s12281-013-0150-110.1007/s12281-013-0150-1 [Crossref] [ Google Scholar]
- Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Shivkumar C, Palaniappan R. Epidemiology of bacterial keratitis in a referral centre in south India. Indian J Med Microbiol 2003; 21(4):239-45. [ Google Scholar]
- Gupta A, Capoor MR, Gupta S, Kochhar S, Tomer A, Gupta V. Clinico-demographical profile of keratomycosis in Delhi, North India. Indian J Med Microbiol 2014; 32(3):310-4. doi: 10.4103/0255-0857.136582 [Crossref] [ Google Scholar]
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001; 79(3):214-21. [ Google Scholar]
- Chandra A, Gupta MK, Prakash D, Tilak R, Maurya OPS. Diagnosis of keratomycosis: an update. Int J Curr Res 2013; 5(11):3474-9. [ Google Scholar]
- Larone DH. Medically Important Fungi: A Guide to Identification. 5th ed. Washington, DC: ASM Pres; 2011.
- Zhang W, Yang H, Jiang L, Han L, Wang L. Use of potassium hydroxide, Giemsa and calcofluor white staining techniques in the microscopic evaluation of corneal scrapings for diagnosis of fungal keratitis. J Int Med Res 2010; 38(6):1961-7. doi: 10.1177/147323001003800609 [Crossref] [ Google Scholar]
- Park K. Park’s Textbook of Preventive and Social Medicine. 20th ed. Jabalpur: M/S Banarsidas Bhanot; 2009.
- Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol 1997; 81(11):965-71. doi: 10.1136/bjo.81.11.965 [Crossref] [ Google Scholar]
- Deshpande SD, Koppikar GV. A study of mycotic keratitis in Mumbai. Indian J Pathol Microbiol 1999; 42(1):81-7. [ Google Scholar]
- Kumari N, Xess A, Shahi SK. A study of keratomycosis: our experience. Indian J Pathol Microbiol 2002; 45(3):299-302. [ Google Scholar]
- Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 2002; 21(6):555-9. doi: 10.1097/00003226-200208000-00004 [Crossref] [ Google Scholar]
- Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis A three-year study. Indian J Ophthalmol 2003; 51(4):315-21. [ Google Scholar]
- Chander J, Singla N, Agnihotri N, Arya SK, Deep A. Keratomycosis in and around Chandigarh: a five-year study from a north Indian tertiary care hospital. Indian J Pathol Microbiol 2008; 51(2):304-6. doi: 10.4103/0377-4929.41700 [Crossref] [ Google Scholar]
- Mallareddy US, Katay P, Anke G. A study of fungal etiology of infective keratitis. Int J Curr Microbiol Appl Sci 2015; 4(8):664-70. [ Google Scholar]
- Reddy PS, Satyendran OM, Satapathy M, Kumar HV, Reddy PR. Mycotic keratitis. Indian J Ophthalmol 1972; 20(3):101-8. [ Google Scholar]
- Shokohi T, Nowroozpoor-Dailami K, Moaddel-Haghighi T. Fungal keratitis in patients with corneal ulcer in Sari, Northern Iran. Arch Iran Med 2006; 9(3):222-7. [ Google Scholar]
- Riaz Q, Fawwad U, Bhatti N, ur Rehman A, ul Hasan M. Epidemiology of microbial keratitis in a tertiary care center in Karachi. Pak J Ophthalmol 2013; 29(2):94-9. doi: 10.36351/pjo.v29i2.360 [Crossref] [ Google Scholar]
- Jacob JM, Goudinho SJ. Comparison of the results of KOH wet mount method and the SDA culture in the etiological diagnosis of corneal ulcers. Int J Med Appl Sci 2014; 3(1):185-94. [ Google Scholar]
- Anusuya DD, Ambica R, Nagarathnamma T. The epidemiological features and laboratory diagnosis of keratomycosis. Int J Biol Med Res 2013; 4(1):2879-83. [ Google Scholar]
- Vajpayee RB, Angra SK, Sandramouli S, Honavar SG, Chhabra VK. Laboratory diagnosis of keratomycosis: comparative evaluation of direct microscopy and culture results. Ann Ophthalmol 1993; 25(2):68-71. [ Google Scholar]
- Agarwal LP, Malik SR, Mohan M, Khosla PK. Mycotic corneal ulcers. Br J Ophthalmol 1963; 47(2):109-15. doi: 10.1136/bjo.47.2.109 [Crossref] [ Google Scholar]
- El-Tahtawi NFAO. Epidemiological characteristics and laboratory diagnosis of fungal keratitis in patients with corneal ulcer in Riyadh, Saudi Arabia. Clin Med Res 2015; 4(6):214-20. [ Google Scholar]
- Chander J. Textbook of Mycology. 3rd ed. Mehta Publishers; 2009. p. 400-405.
- Verma S, Sharma V, Kanga A, Sharma R, Angrup A, Mokta K. Current spectrum of oculomycosis in North India: a 5-year retrospective evaluation of clinical and microbiological profile. Indian J Med Microbiol 2016; 34(1):72-5. doi: 10.4103/0255-0857.174104 [Crossref] [ Google Scholar]
- Saha S, Banerjee D, Khetan A, Sengupta J. Epidemiological profile of fungal keratitis in urban population of West Bengal, India. Oman J Ophthalmol 2009; 2(3):114-8. doi: 10.4103/0974-620x.57310 [Crossref] [ Google Scholar]
- Khorgade RR, Gaikwad AA, Nilekar SL, Kulkarni DM. Mycotic keratitis in patients attending a tertiary care hospital. Int J Curr Microbiol Appl Sci 2015; 4(9):428-37. [ Google Scholar]
- Ibrahim MM, Vanini R, Ibrahim FM, Fioriti LS, Furlan EM, Provinzano LM. Epidemiologic aspects and clinical outcome of fungal keratitis in southeastern Brazil. Eur J Ophthalmol 2009; 19(3):355-61. doi: 10.1177/112067210901900305 [Crossref] [ Google Scholar]
- Arora U, Gill PK, Chalotra S. Fungal profile of keratomycosis. Bombay Hosp J 2009; 51(3):325-7. [ Google Scholar]
- Tilak R, Singh A, Maurya OP, Chandra A, Tilak V, Gulati AK. Mycotic keratitis in India: a five-year retrospective study. J Infect Dev Ctries 2010; 4(3):171-4. doi: 10.3855/jidc.309 [Crossref] [ Google Scholar]
- Rautaraya B, Sharma S, Kar S, Das S, Sahu SK. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol 2011; 11:39. doi: 10.1186/1471-2415-11-39 [Crossref] [ Google Scholar]
- Sathyanarayan MS, Sonth SB, Surekha YA, Mariraj J, Krishna S. Epidemiology and aetiological diagnosis of keratomycosis in a tertiary care hospital in north Karnataka. Int J Cur Res Rev 2013; 5(6):92-7. [ Google Scholar]
- Jampala S, Gopinathan A, Aparna Aparna, Nair D, Dinesh KR, Radhakrishnan A. Epidemiological and microbiological profile of infective keratitis in a tertiary care centre, South India. Asian J Biomed Pharm Sci 2014; 4(37):44-51. doi: 10.15272/ajbps.v4i37.616 [Crossref] [ Google Scholar]
- Taruni P, Rani VS, Sunder A. Mycotic keratitis in and around Hyderabad. J Evol Med Dent Sci 2015; 4(51):8913-7. [ Google Scholar]
- Vemuganti GK, Naidu C, Gopinathan U. Rapid detection of fungal filaments in corneal scrapings by microwave heating-assisted Grocott’s methenamine silver staining. Indian J Ophthalmol 2002; 50(4):326-8. [ Google Scholar]
- Sahay R, Kumar A, Kumar J. Mycotic corneal ulcer in North India. IOSR J Dent Med Sci 2015; 14(4):45-8. [ Google Scholar]
- Satpathi P, Satpathi S. Study of microbial keratitis in central India. J Infect Dev Ctries 2012; 6(3):295-8. doi: 10.3855/jidc.1929 [Crossref] [ Google Scholar]
- Vadivoo NS, Sujatha K, Sridevi NVK, Sharda R, Niranjana M. Ten year study of fungal keratitis-tertiary care hospital. National Journal of Basic Medical Sciences (NJBMS) 2012; 3(2):149-53. [ Google Scholar]