Avicenna Journal of Clinical Microbiology and Infection. 8(4):123-129.
doi: 10.34172/ajcmi.2021.23
Original Article
Antimicrobial Potential of Titanium Dioxide Nanoparticles in Urinary Tract Infections: An Experimental Study on the Growth Inhibitory Activity and Biofilm Inhibition
Sabar Jabbar Shawkat 1  , Khosrow Chehri 1, *
, Khosrow Chehri 1, * 
Author information:
1Department of Biology, Faculty of Sciences, Razi University, Kermanshah, Iran
*
Corresponding author: Khosrow Chehri, Department of Biology, Faculty of Sciences, Razi University, Kermanshah, Iran, Email:
khchehri@gmail.com
Abstract
Background: T Microorganisms cause many diseases for the human body such as urinary tract infection and, therefore, it is highly important to eliminate and control them. Bacterial resistance to different types of antibiotics was increased and it is necessary to find alternative agents to eliminate these microbes.
Methods: This study aimed was to evaluate the antimicrobial effect of different concentrations of titanium dioxide nanoparticles (TiO2 NPs) on some gram-positive bactria, gram-negative bacteria, and Candida albicans. TiO2 NPs were synthesized using the chemical methods, coated with carboxymethyl cellulose (CMC) and prepared in different concentrations (0.098, 0.196, 0.392, 0.784, 1.568, and 3.136 mg/mL). Eventually, a minimum inhibitory concentration (MIC) and a minimum biofilm inhibitory concentration (MBIC) were applied to investigate the effect of TiO2 NPs on microorganisms.
Results: According to the study results, the MICs of TiO2 NPs were found to be 1.489, 1.208, and 1.166 mg/ mL for Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae as the Gram-negative bacteria, respectively; and they were discovered to be 0.512, 0.830, and 0.707 mg/mL for Streptococcus pneumoniae, Staphylococcus aureus, and Staphylococcus epidermidis as the Gram-positive bacteria, respectively. As for C. albicans, as the yeast strain, MIC was 0.253 mg/ mL. The MBIC of more than 90% of TiO2 NPs was 6.25 mg/mL for both Gram-negative and Gram-positive bacterial types and 1.562 mg/mL for C. albicans.
Conclusions: It was concluded that TiO2 NPs were effective antimicrobial agents for Gram-positive bacteria, Gram-negative bacteria, and C. albicans, but their inhibitory effect on yeast was greater than that of bacteria.
Keywords: Titanium dioxide nanoparticles, Gram-positive bacteria, Gram-negative bacteria, Yeast, Biofilm
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
The urinary system is one of the major vital systems in the human body, which is responsible for purifying the blood from toxic materials and returning clean blood to the circulatory system. This system, referred to as the renal system, includes the bladder, ureters, kidneys, and urethra. Microorganisms cause many infectious diseases for the human body, especially for the urinary tract system; therefore, it is essential to eliminate and control them. The emergence of antibiotic-resistant microorganisms has been increased recently, and this increase necessitates finding alternative agents to eliminate these microbes (1). Today, biofilms have become a major medical problem due to their high resistance to the immune system, as well as their resistance to antimicrobial medicine and other disinfectants (2). There are different microorganisms capable of forming a biofilm on different surfaces which can accumulate on medical devices and cause specific problems in treatment by colonization and infection (3). Most of the gram-positive bacteria, gram-negative bacteria, and yeast in nature make up the biofilm. Biofilms can cause many hospitalized infections, indicating their important role in developing infectious diseases (4). Several strains of microbial pathogens can grow in biofilm structures. These structures can be developed by one species of microbe or a mixture of several microorganisms. Biofilms are the source of many resistant systemic infections such as urinary tract, ear, tooth, catheter, and other infections (5). Therefore, identifying factors that can inhibit the formation of biofilms has recently received extensive attention from scientists (6), including nanoparticles (NPs). The microorganisms have a high potential for adapting to new environments and antibiotics. The development in nanotechnology has provided a competitive condition against the adaptability characteristics of microorganisms and limited their growth in new media and, consequently, prevented their infections (7). The application of some nano-sized materials has shown that they have a significant effect on microbe cells (8). Many nanomaterials, such as zinc, silver, and, Copper have been used to eliminate microorganisms. Haghi et al used titanium dioxide (TiO2) NPs in dental manufacturing and showed that this NP had highly effective properties against yeast and bacteria causing teeth calcification (9). TiO2 has received special attention in these studies for its high photocatalytic properties (10). Some of the NPs’ features that make them superior to other NPs include their high chemical resistance, non-toxicity, long service life, availability, and low cost (11). TiO2 is widely used in many industries including water purification as well as paints, plastics, paper, and inks manufacturing industries (12). TiO2 NPs have been applied extensively for killing different groups of microorganisms including bacteria, fungi, and viruses (13). This study, therefore, aimed to synthesize TiO2 NPs and measure the antibacterial and antifungal effect of different concentrations of them on gram-positive bacteria, gram-negative bacteria, and C. albicans isolated from infected urine samples.
Materials and Methods
Bacterial Strains
To conduct the present experimental study, three strains of Gram-positive bacteria including Streptococcus pneumoniae PTCC 1240, Staphylococcus aureus PTCC 1431, and Staphylococcus epidermidis PTCC 1114, three Gram-negative bacteria including Escherichia coli PTCC 1399, Pseudomonas aeruginosa PTCC 1690,and Klebsiella pneumoniae PTCC 1290, as well as Candida albicans ATCC 10231 were all purchased from the Iranian Research Organization for Science and Technology.
Sample Collection
As for the sample collection, 60 urine samples (46 male and 14 female cases) were collected from the subjects with typical symptoms of a urinary tract infection. The subjects whose ages ranged from 1.5 to 72 years had been referred to Mohamad Kermanshahi Hospital in Kermanshah, Iran.It should be noted that this study lasted for six months. The men and women referring for the test were advised to clean the genital area before collecting the urine sample, discard the first part of the urine, and ccollect a sample of urine (mid-stream) in the container without stopping the process of urination. Patients’ complete information was written on the test tubes. The samples were stored in special containers and their lids were closed and transferred to the university laboratory as soon as possible. Midstream urine samples were quickly cultured on the agar media.
The Identification of Microorganisms
For detecting microorganisms in urine samples, a loop full of samples was shed into EMB, blood agar, and potato dextrose agar and incubated at 37°C overnight. After 24 hours, individual colonies were separated and identified based on the gram-staining, morphological characteristics, and biochemical characters. To detect the gram-negative bacteria, standard biochemical tests were used, including methyl red, citrate, indole, Voges-Proskauer, H2S production, oxidase, urea hydrolysis, and gas production, and to detect the Gram-positive bacteria, catalase, coagulase, hemolysis, motility, oxidase, DNase, and mannitol tests. Yeast isolates were identified by the germ tube test and cultured on CHROM agar media. Three strains of gram-positive bacteria including S. pneumoniae, S. aureus, and S. epidermidis, as well as three gram-negative bacteria including E. coli, P. aeruginosa, K. pneumoniae, and Candida albicans were isolated (Tables 1, 2, and 3) in this experimental study (14,15). A microbial solution was obtained from each strain according to the 0.5 McFarland standard (1×108 colony forming unit/milliliter for the bacterial strain and 3×106 CFU/mL for yeast) to perform the antimicrobial assays (16).
Table 1.
Differential Tests for Gram-negative Bacteria
|
Biochemical Tests
|
Gram-negative Bacteria
|
|
E. coli
|
P. aeruginosa
|
K. pneumoniae
|
| Gram-staining |
Negative |
Negative |
Negative |
| EMB culture |
The green metallic sheen |
Pink to purple in color |
The absence of color |
| TSI |
Acid/Acid |
K/ - |
Acid/Acid |
| Indole |
Positive |
Negative |
Negative |
| Motility |
Positive |
Positive |
Negative |
| Citrate |
Negative |
Positive |
Positive |
| Urea |
Negative |
Negative |
Positive |
| Lysine |
Negative |
No reaction |
Positive |
| MR |
Positive |
Negative |
Negative |
| Phenyl alanine |
Negative |
Negative |
Negative |
Note. EMB: Eosin methylene blue; TSI: Triple sugar iron; MR: Methyl red.
Table 2.
Differential Tests for Gram-positive Bacteria
|
Biochemical Tests
|
Gram-positive Bacteria
|
|
S. aureus
|
S. epidermidis
|
S. pneumoniae
|
| Gram-staining |
Positive |
Positive |
Positive |
| Catalase |
Positive |
Positive |
Negative |
| Coagulase |
Positive |
Negative |
Negative |
| Mannitol |
Positive |
Negative |
Negative |
| Oxidase |
Negative |
Negative |
Negative |
| DNase |
Positive |
Negative |
Positive |
| Hemolysis |
Positive |
Negative |
α |
| Motility |
Negative |
Negative |
Negative |
Table 3.
Differential Tests for Candida albicans
|
Biochemical Tests
|
Candida albicans
|
| CHROM agar |
Light to medium green colonies |
| Germ tube |
Positive |
TiO2 NP Preparation and Coating
In order to carry out this experiment, 5 mL of titanium isopropoxide was added to 8 mL deionized water, and then the precipitation of hydrous TiO2 and alkoxide was hydrolyzed as well. The resulting solution was kept completely and mixed by persistent stirring at 40°C for half an hour. Finally, the white sediment of TiO2 NPs was created at the bottom of the tube and then it was separated by centrifuge, washed multiple times with methanol and deionized water, and dried at 80°C in an oven for 12 hours (17,18). To prepare the photocatalytic NPs, the synthesized TiO2 NPs were first poured into a sterilized dish and an ultraviolet lamp with a wavelength of 350 nm was placed inside a flask. Then, the output of ultraviolet rays was measured using a digital device. The measured value was 56 µW/m2. The UV lamp was then immersed in the NP solution for 60 minutes. Carboxymethyl cellulose (CMC) was used in this research to coat and improve the presentation of the TiO2 NPs to the target microorganisms as well as to protect the nano agent from degradation (19). TiO2 NPs were dispersed in 2% CMC and sonicated for 3 hours. For preparing 2% of CMC, 2 g of CMC was mixed with deionized water through mechanical stirring for 12 hours until a perfect dissolution was performed. The morphological and structural characteristics of these TiO2 were analyzed using X-ray diffraction, X-ray microanalysis, transmission, and scanning electron microscopes. TiO2 NPs used in this study were prepared at a concentration of 25 mg/mL. NPs were diluted (in DMSO-5%) in different concentrations to detect the lethal dose (LD-50). The LD-50 of TiO2 NP responsible for the 50% death of the tested mice (n= 4 per group) was evaluated by intraperitoneal injection with three concentrations of 0.15 μg/kg (5 mg/mL), 0.3 μg/kg (10 mg/mL), and 0.45 μg/kg (15 mg/mL). Mice were monitored for body temperature, weight, mortality rate, diarrhea, drowsiness, and seizures for 2 weeks. The results of this experiment demonstrated that TiO2 NPs at these concentrations stimulated no lethal activity after 2 weeks.
Disk Diffusion Method
For determining the antimicrobial effect of nanoparticles, the disk diffusion method was used. Briefly, the Muller-Hinton agar (MHA) medium was prepared and then the plates were inoculated with the sterile cotton swab of microorganisms. The stocks of various concentrations of TiO2 NP were prepared using dimethyl sulfoxide 2% (DMSO, there was no antimicrobial effect in this dilution), and then 6 mm diameter sterile paper disk, Whatman sterilized filter paper, was used to make the disks, which were impregnated with different concentration of NPs, dried under the laminar hood and sterile condition, and placed on MHA plates. The plates were incubated at 37ºC for 24 hours. The diameter of the inhibition zone, measured as a clear area around the disk, was calculated by a ruler (19). Antibiotic standards adopted in this study were purchased from Padtan Teb Company.
Minimum Inhibitory Concentrations Determination
The antimicrobial effects of TiO2 NPs were investigated by using the micro-dilution method. In this method, 100 µm of Muller-Hinton broth medium was added to all wells. The NPs were prepared in seven concentrations (0.098, 0.196, 0.392, 0.784, 1.568, and 3.136 mg/mL). The first well in each row was filled with 100 µL of the highest concentration of TiO2 NPs, and 100 µL of this NP was transferred to the second well, and this process was continued to the 8th well. The 8th well was adopted to control the growth of microorganisms. Finally, microplates were inoculated for 24 hours at 37°C. After that optical density (OD) of these plates was read at 600 nm and inhibitory concentration was reported in the presence of NPs (20).
Minimum Biofilm Inhibitory Concentration (MBIC) Determination
For the determination of anti-biofilm effects of nanoparticles, trypticase soy broth (TSB) medium was supplemented with 1% glucose. Different concentrations of TiO2 NPs were added into the well of 96-well microplates (0.784, 1.568, 3.136, 6.272, and 12.544 mg/mL). The 24-hours microbial suspension was inoculated in the wells. The microplate was inoculated at 37° C for 24 hours (21). The cultures were then aspirated and the wells were washed three times with phosphate-buffered saline. Next, the plates were air-dried overnight and stained with 0.1% crystal violet. The OD of the wells was measured at 600 nm using the ELISA reader device.
Results
Disk Diffusion Method
MHA was used for antimicrobial susceptibility testing of the mentioned TiO2 NPs. Anti-bacterial disks of the CMC-coated TiO2 NP were evaluated for inhibition of microbial growth of the UTI-causing bacterial agents. In this step, concentrations from 1.568, 3.136, 6.272, and 12.544 mg/mL were used. The zone of bacterial growth inhibition was inspected after planting the discs on the Muller-Hinton media. Evaluating the zone of inhibition after 24 hours showed that the prepared nano-agent was effectively capable of suppressing the bacterial growth on all UTI-causing bacteria at all different TiO2 concentrations. In Table 4, the diameter of the zone of inhibition on the bacterial species was represented in millimeters. The results of the disk diffusion method using standard antibiotic disks demonstrated that E. coli had the highest resistance to antibiotics ampicillin, while cephalothin and cotrimoxazole had the highest sensitivity to antibiotics gentamicin and amikacin. P. aeruginosa exhibited the highest resistance to antibiotics ciprofloxacin and the highest sensitivity to gentamicin. Klebsiella represented the highest resistance to antibiotics ampicillin and the highest sensitivity to antibiotics gentamicin and amikacin. S. pneumonia had the highest resistance to antibiotics penicillin and the highest sensitivity to antibiotics erythromycin. S. aureus developed the highest resistance to antibiotics ceftriaxone and cefixime, as well as the highest sensitivity to antibiotics cotrimoxazole. S. epidermis had the highest resistance to antibiotics oxacillin and the highest sensitivity to antibiotics cephalothin.
Table 4.
Antimicrobial effect of Different Concentrations of Titanium Dioxide Nanoparticles by the Disk Diffusion Method
|
Microorganisms
|
Concentration (mg/mL)
|
|
3.136
|
6.272
|
12.544
|
|
E. coli
|
10±0.7 |
10.5±0.8 |
11.7±0.4 |
|
P. aeruginosa
|
9.6±0.7 |
9.6±0.7 |
10.3±0.6 |
|
K. pneumoniae
|
9.5±0.4 |
10.1±0.7 |
11.2±0.2 |
|
S. aureus
|
10.1±0.1 |
10.8±0.9 |
12.2±0.6 |
|
S. epidermidis
|
10.4±0.9 |
10.9±0.5 |
12.1±0.4 |
|
S. pneumoniae
|
0 |
9.6±0.7 |
10.3±0.6 |
|
C. albicans
|
11.1±1.2 |
11.4±0.9 |
12.5±1.2 |
Characteristics of TiO2 NPs
Details of this component are TiO2, anatase, average particle size of 10-25 nm, the specific surface area of 200-240 m2/g, pH rate of 6-6.5, white color, purity of > 99%, bulk density of 0.24 g/cm3, true density of 3.9 g/cm3, loss of weight in drying of 4.17%, and loss of weight on ignition equal to 8.24% (Figures 1-3).
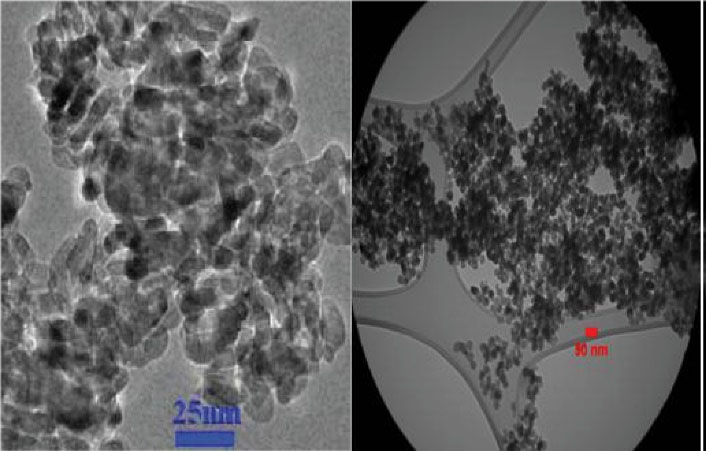
Figure 1.
SEM (left) and TEM (right) Imaging of TiO2 NPs. Note. SEM: Scanning electron microscopy; TEM: Transmission electron microscopy; TiO2 NPs: Titanium dioxide nanoparticles. The TEM image shows that loaded NPs could efficiently internalize into the target cells.
.
SEM (left) and TEM (right) Imaging of TiO2 NPs. Note. SEM: Scanning electron microscopy; TEM: Transmission electron microscopy; TiO2 NPs: Titanium dioxide nanoparticles. The TEM image shows that loaded NPs could efficiently internalize into the target cells.
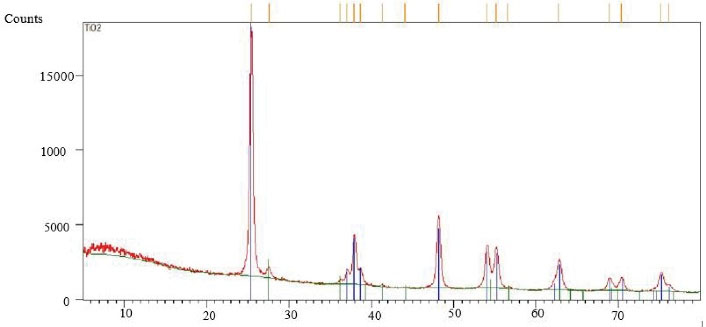
Figure 2.
EDX Characteristics of the TiO2 Nanoparticles. Note. EDX: X-ray microanalysis; TiO2: Titanium dioxide nanoparticles.
.
EDX Characteristics of the TiO2 Nanoparticles. Note. EDX: X-ray microanalysis; TiO2: Titanium dioxide nanoparticles.
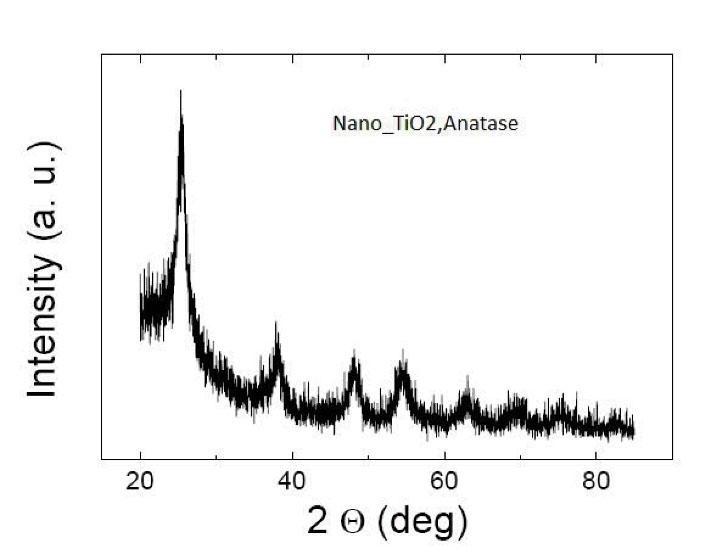
Figure 3.
XRD Characteristics of TiO2 Nanoparticles. Note. XRD: X-ray diffraction; TiO2: Titanium dioxide nanoparticles.
.
XRD Characteristics of TiO2 Nanoparticles. Note. XRD: X-ray diffraction; TiO2: Titanium dioxide nanoparticles.
Minimum Inhibitory Concentration Results
In this test, the MIC of nanoparticles was determined by the microdilution method for S. pneumoniae, S. aureus, S. epidermidis, E. coli, P. aeruginosa, K. pneumoniae, and C. albicans. Microbial growth rates in the presence of different concentrations of TiO2 NPs were calculated in 600 nm OD (Tables 5-7). For MBC detection, two wells before and after the MIC well cultured on Mueller Hinton agar media.Table 8 presents the MIC test results for the microbial strains in the current study. As shown in the table, these particles were capable of producing an acceptable inhibitory effect on all examined groups of bacteria and fungi. The result revealed that the MICs of TiO2 NPs for E. coli, P. aeruginosa, and K. pneumoniae, as Gram-negative bacteria, were 1.489, 1.208, and 1.166 mg/mL, respectively. The corresponding values for S. pneumoniae, S. aureus, and S. epidermidis as gram-positive bacteria were 0.512, 0.830, and 0.707 mg/mL, respectively. Finally, this value was 0.253 mg/mL for C. albicans, as the yeast strain. The cultured plates of microbes representing no microbial growth were considered MBC/MFC (Table 8). Thus,TiO2 NPs are effective antimicrobial agents for gram-positive, Gram-negative bacteria, and C. albicans, but their inhibitory effect on yeast was greater than bacteria.
Table 5.
Gram-Negative Bacteria Growth Rate in the Presence of Different Concentrations of Titanium Dioxide Nanoparticles in 600 nm Optical Density
|
Concentration (mg/mL)
|
Gram-Negative Bacteria
|
|
E. coli
|
P. aeruginosa
|
K. pneumoniae
|
| 0 (Control) |
0.727±0.005 |
0.601±0.001 |
0.855±0.003 |
| 0.098 |
0.572±0.014 |
0.579±0.003 |
0.712±0.005 |
| 0.196 |
0.485±0.004 |
0.438±0.003 |
0.643±0.004 |
| 0.392 |
0.356±0.003 |
0.219±0.011 |
0.442±0.004 |
| 0.784 |
0.233±0.004 |
0.157±0.001 |
0.342±0.002 |
| 1.568 |
0.098±0.003 |
-0.013±0.002 |
0.103±0.002 |
| 3.136 |
-0.033±0.001 |
-0.029±0.006 |
-0.005±0.003 |
Table 6.
Gram-positive Bacteria Growth Rate in the Presence of Different Concentrations of Titanium Dioxide Nanoparticles in 600 nm Optical Density
|
Concentration (mg/mL)
|
Gram-Positive Bacteria
|
|
S. aureus
|
S. epidermidis
|
S. pneumoniae
|
| 0 (Control) |
0.718±0.002 |
0.851±0.004 |
0.632±0.004 |
| 0.098 |
0.706±0.003 |
0.535±0.003 |
0.544±0.003 |
| 0.196 |
0.691±0.001 |
0.465±0.007 |
0.508±0.005 |
| 0.392 |
0.654±0.005 |
0.236±0.005 |
0.363±0.005 |
| 0.784 |
0.384±0.005 |
0.204±0.003 |
0.227±0.003 |
| 1.568 |
-0.029±0.002 |
0.012±0.003 |
0.096±0.004 |
| 3.136 |
-0.037±0.001 |
-0.015±0.003 |
0.039±0.004 |
Table 7.
Yeast Growth Rate in the Presence of Different Concentrations of Titanium Dioxide Nanoparticles in 600 nm Optical Density
|
Concentration (mg/mL)
|
Candida albicans
|
| 0 (Control) |
0.571±0.008 |
| 0.098 |
0.552±0.002 |
| 0.196 |
0.365±0.004 |
| 0.392 |
0.044±0.005 |
| 0.784 |
-0.011±0.003 |
| 1.568 |
-0.036±0.003 |
| 3.136 |
-0.039±0.002 |
Table 8.
MIC of Titanium Dioxide Nanoparticles Against Yeast, Gram-Negative, and Gram-Positive Bacteria
|
Microorganisms
|
MIC (mg/mL)
|
MBC/MFC (mg/mL)
|
|
E. coli
|
1.489 |
3.136 |
|
P. aeruginosa
|
1.208 |
3.136 |
|
K. pneumoniae
|
1.166 |
3.136 |
|
S. aureus
|
0.830 |
1.568 |
|
S. epidermidis
|
0.707 |
1.568 |
|
S. pneumoniae
|
0.512 |
1.568 |
|
C. albicans
|
0.253 |
0.784 |
Note. MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration; MFC: Minimum fungicidal concentration.
Minimum Biofilm Inhibitory Concentration
The results of biofilm inhibition assay using CMC coated TiO2 NPs on 96-well plates showed that these NPs are completely capable of suppressing biofilm formation, and preventing further growth of the microbial biofilm of S. pneumoniae, S. aureus, S. epidermidis, E. coli, P. aeruginosa, K. pneumoniae, and C. albicans. The test results indicated that in the absence of the TiO2 NPs, strong biofilms will form on the plates, but adding the particles would strongly inhibit biofilm formation and maturation. TiO2 demonstrated that it could effectively inhibit the growth of all isolated bacterial strains at concentrations of 25-0.39 mg/mL. Based on the result in Tables 9-11, the MBIC of more than 90% of TiO2 NPs was 6.25 mg/mL for both Gram-negative and Gram-positive bacterial types and 1.562 mg/mL for C. albicans.
Table 9.
Percentage of Gram-Negative Biofilm Inhibition in Different Concentrations of Titanium Dioxide Nanoparticles in 600 nm Optical Density
|
Concentration (mg/mL)
|
Gram-Negative Bacteria
|
|
E. coli
|
P. aeruginosa
|
K. pneumoniae
|
| 1.568 |
47.3 |
21.62 |
48.56 |
| 0.784 |
51.08 |
24.60 |
65.29 |
| 1.568 |
69.29 |
71.02 |
74.77 |
| 3.136 |
80.82 |
80.07 |
89.89 |
| 6.272 |
92.41 |
91.12 |
93.29 |
| 12.544 |
100 |
100 |
100 |
Table 10.
Percentage of Gram-Positive Biofilm Inhibition in Different Concentrations of Titanium Dioxide Nanoparticles in 600 nm Optical Density
|
Concentration (mg/mL)
|
Gram-positive Bacteria
|
|
S. aureus
|
S. epidermidis
|
S. pneumoniae
|
| 1.568 |
23.01 |
48.81 |
53.10 |
| 0.784 |
45.71 |
63.26 |
73.11 |
| 1.568 |
74.81 |
70.13 |
77 |
| 3.136 |
81.12 |
80.79 |
83.87 |
| 6.272 |
92.82 |
100 |
100 |
| 12.544 |
99.76 |
100 |
100 |
Table 11.
Percentage of Yeast Biofilm Inhibition in Different Concentrations of Titanium Dioxide Nanoparticles in 600 nm Optical Density
|
Concentration (mg/mL)
|
Candida albicans
|
| 1.568 |
57.24 |
| 0.784 |
84.02 |
| 1.568 |
90.38 |
| 3.136 |
100 |
| 6.272 |
100 |
| 12.544 |
100 |
Discussion
In recent years, physicians and microbial scientists have found that the causative agents of microbial infections are becoming resistant to common antimicrobial medicine. On the other side, by the acceleration of science and a better understanding of new materials with the potential for treating infection, many have focused on studying the antimicrobial properties of these materials in order to find new substitutions for ordinary antibiotics (22). As previously stated, biofilms are a major cause of infectious diseases and could increase the tolerance of bacteria inside them toward different antibiotics (23). Therefore, finding a drug that could penetrate into the biofilm and destroy them, would be helpful for effectively battling infections. Inhibition of biofilm-associated diseases requires incredible attention since this structure has high levels of resistance to antibiotics (24). Several studies recommended combination therapy as the treatment of choice in biofilm-associated infections while considering macrolides as one of the first selected antibiotics, but many of these microbial agents of biofilm construction have become resistant to them over time. Biofilms can be up to multiple-fold more resistant to antimicrobial agents compared to planktonic forms due to various mechanisms (25). The current time could be considered as the age of nanotechnology for its fascinating applications and encouraging results of their use for improving different aspects of human life. An understanding of NPs affecting the critical causative agent of microbial infections allows us to gain a mechanistic understanding of toxicity and guides us toward designing rules for creating safe antimicrobial nanomaterials (26). As mentioned earlier, an NP is a small-sized particle with a dimension of less than 100 nm. NP studies are a field of intensive research interest due to a broad diversity of potential applications in electronic, biomedical, and optical fields. These particles have the capacity to attach and penetrate into bacterial cells, disrupt the bacterial membrane, and interact with chromosomal DNA (27). The purpose of this research was to provide new insights about using CMC-coated photocatalytic TiO2 NPs as antimicrobial agents against different gram-positive, gram-negative bacteria, and C. albicans.
In this study, the antimicrobial effect of CMC-coated photocatalytic TiO2 NPs was investigated on seven common agents of many infections, including S. pneumoniae, S. aureus, S. epidermidis, E. coli, P. aeruginosa, K. pneumoniae, and C. albicans. The antibacterial effects of these materials were evaluated using the microdilution method. The biofilm inhibition effects of TiO2-coated NPs were determined as well. The results of our test indicated that strong biofilms will form on the plates in the absence of the TiO2 NPs, but adding the particles would strongly inhibit biofilm formation and maturation. The result showed that the MICs of TiO2 NPs for E. coli, P. aeruginosa, and K. pneumoniae, as the Gram-negative bacteria, were 1.489, 1.208, and 1.166 mg/mL, respectively. Further, these values were 0.512, 0.830, and 0.707 mg/mL for S. pneumoniae, S. aureus, and S. epidermidis as the Gram-positive bacteria, respectively. Moreover, it was equal to 0.253 mg/mL for C. albicans as the yeast strain. The MBIC of more than 90% of TiO2 NPs was 6.25 mg/mL for both Gram-negative and Gram-positive bacterial types and 1.562 mg/mL for C. albicans. In their study, Jalvo et al focused on measuring the antibiofilm and antimicrobial properties of functionalized surfaces coated by photocatalytic TiO2 NPs against Pseudomonas putida and S. aureus. They presented that the antimicrobial activity of TiO2 led to wide cover damage. The decrease of cell inviability was more than 99.9% for TiO2 NPs on the glass level (21). In another study, Verdier et al examined the antimicrobial effect of photocatalytic TiO2 alone or in coatings on E. coli bacterium and measured various types of parameters that significantly influence the antimicrobial activity (28). Similarly, Lavaee et al reported the results of investigating the antibacterial and biofilm inhibition activity of silver, TiO2, and iron NPs. They aimed at comparing the antibacterial effects of erythromycin, clindamycin, chlorhexidine, penicillin, vancomycin, and tetracycline with TiO2, iron, and Ag NPs and consider the synergistic antimicrobial and biofilm inhibition effects of these NPs in pathogenic and standard species of Streptococcus sanguinis and Streptococcus mutans. Based on their findings, the use of NPs alone showed a higher MIC than using them synergistically. The most efficient synergistic resolving agent was the one containing Ag, TiO2, and Fe3O4 (29). In another study, Vincent et al evaluated the efficacy of TiO2 and zinc oxide nanomaterials against biofilm-producing and metallo beta-lactamase P. aeruginosa aiming at determining the effect of TiO2 and zinc oxide NPs on biofilm-producing and metallo beta-lactamase P. aeruginosa. These nanomaterials demonstrated perceptible activity at whole experimental concentrations, and it was revealed that TiO2 and zinc oxide nanomaterials may serve as promising antimicrobial agents in this regard (30).
Conclusions
Overall, the results of the current study demonstrated that TiO2 NPs are effective antimicrobial agents for E. coli, P. aeruginosa, and K. pneumoniae, as Gram-negative bacteria, as well as S. pneumoniae, S. aureus, and S. epidermidis, as Gram-positive bacteria, and C. albicans, as the yeast strain, and repressed biofilm composition for the first time. In addition, the findings confirmed that the efficiency for the inhibition of both microbial and fungi biofilms is of great importance for these particles and they could be used against a diversity of infections. Although the results of the present study seem to be successful, further studies are required so that to consider these agents for clinical applications.
Acknowledgements
This paper is extracted from the MSc thesis of the first author submitted to the Department of Biology, Faculty of Science, Razi University (67149-67346), Kermanshah, Iran. The authors acknowledge the personnel of the laboratory of microbiology for technical assistance.
Conflict of Interests
None declared.
Ethical Approval
In this experimental study, Ethical principles were considered in relation to the proposed work.
Financial Support
This study was supported by intramural funds.
References
- Jang HD, Kim SK, Kim SJ. Effect of particle size and phase composition of titanium dioxide nanoparticles on the photocatalytic properties. J Nanopart Res 2001; 3(2):141-7. doi: 10.1023/A:1017948330363 [Crossref] [ Google Scholar]
- Talpaert MJ, Balfour A, Stevens S, Baker M, Muhlschlegel FA, Gourlay CW. Candida biofilm formation on voice prostheses. J Med Microbiol 2015; 64(Pt 3):199-208. doi: 10.1099/jmm.0.078717-0 [Crossref] [ Google Scholar]
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 2016; 14(9):563-75. doi: 10.1038/nrmicro.2016.94 [Crossref] [ Google Scholar]
- Butterfield PW, Bargmeyer AM, Camper AK, Biederman JA. Modified enzyme activity assay to determine biofilm biomass. J Microbiol Methods 2002; 50(1):23-31. doi: 10.1016/s0167-7012(02)00005-2 [Crossref] [ Google Scholar]
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev 2004; 17(2):255-67. doi: 10.1128/cmr.17.2.255-267.2004 [Crossref] [ Google Scholar]
- Habimana O, Steenkeste K, Fontaine-Aupart MP, Bellon-Fontaine MN, Kulakauskas S, Briandet R. Diffusion of nanoparticles in biofilms is altered by bacterial cell wall hydrophobicity. Appl Environ Microbiol 2011; 77(1):367-8. doi: 10.1128/aem.02163-10 [Crossref] [ Google Scholar]
- Nana A, Nelson SB, McLaren A, Chen AF. What’s new in musculoskeletal infection: update on biofilms. J Bone Joint Surg Am 2016; 98(14):1226-34. doi: 10.2106/jbjs.16.00300 [Crossref] [ Google Scholar]
- Lin X, Li J, Ma S, Liu G, Yang K, Tong M. Toxicity of TiO2 nanoparticles to Escherichia coli: effects of particle size, crystal phase and water chemistry. PLoS One 2014; 9(10):e110247. doi: 10.1371/journal.pone.0110247 [Crossref] [ Google Scholar]
- Haghi F, Haghi M, Maadi H, Charkhiyan H, Ghahramani A. The antibacterial effect of TiO2 nanoparticles on pathogenic strain of Escherichia coli, in vitro. In: The First International Congress of Medical Bacteriology. Tabtiz University of Medical Sciences; 2011.
- Jeena V, Robinson RS. Green oxidations: titanium dioxide induced tandem oxidation coupling reactions. Beilstein J Org Chem 2009; 5:24. doi: 10.3762/bjoc.5.24 [Crossref] [ Google Scholar]
- Enyashin AN, Seifert G. Structure, stability and electronic properties of TiO2 nanostructures. Phys Status Solidi B 2005; 242(7):1361-70. doi: 10.1002/pssb.200540026 [Crossref] [ Google Scholar]
- Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 2003; 21(1):41-6. doi: 10.1038/nbt764 [Crossref] [ Google Scholar]
- Gupta K, Singh RP, Pandey A, Pandey A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S aureus P aeruginosa and E coli. Beilstein J Nanotechnol 2013; 4:345-51. doi: 10.3762/bjnano.4.40 [Crossref] [ Google Scholar]
- Costa AR, Silva F, Henriques M, Azeredo J, Oliveira R, Faustino A. Candida clinical species identification: molecular and biochemical methods. Ann Microbiol 2010; 60(1):105-12. doi: 10.1007/s13213-009-0007-6 [Crossref] [ Google Scholar]
- Franco-Duarte R, Černáková L, Kadam S, Kaushik KS, Salehi B, Bevilacqua A. Advances in chemical and biological methods to identify microorganisms-from past to present. Microorganisms 2019; 7(5):130. doi: 10.3390/microorganisms7050130 [Crossref] [ Google Scholar]
- Weinstein MP, Lewis JS 2nd. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol 2020; 58(3):e01864-19. doi: 10.1128/jcm.01864-19 [Crossref] [ Google Scholar]
- Buraso W, Lachom V, Siriya P, Laokul P. Synthesis of TiO2 nanoparticles via a simple precipitation method and photocatalytic performance. Mater Res Express 2018; 5(11):115003. doi: 10.1088/2053-1591/aadbf0 [Crossref] [ Google Scholar]
- Lachom V, Poolcharuansin P, Laokul P. Preparation, characterizations and photocatalytic activity of a ZnO/TiO2 nanocomposite. Mater Res Express 2017; 4(3):035006. doi: 10.1088/2053-1591/aa60d1 [Crossref] [ Google Scholar]
- Olajuyigbe OO, Afolayan AJ. In vitro antibacterial and time-kill assessment of crude methanolic stem bark extract of Acacia mearnsii de wild against bacteria in shigellosis. Molecules 2012; 17(2):2103-18. doi: 10.3390/molecules17022103 [Crossref] [ Google Scholar]
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008; 3(2):163-75. doi: 10.1038/nprot.2007.521 [Crossref] [ Google Scholar]
- Jalvo B, Faraldos M, Bahamonde A, Rosal R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J Hazard Mater 2017; 340:160-70. doi: 10.1016/j.jhazmat.2017.07.005 [Crossref] [ Google Scholar]
- Penesyan A, Gillings M, Paulsen IT. Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules 2015; 20(4):5286-98. doi: 10.3390/molecules20045286 [Crossref] [ Google Scholar]
- Al-Badr A, Al-Shaikh G. Recurrent urinary tract infections management in women: a review. Sultan Qaboos Univ Med J 2013; 13(3):359-67. doi: 10.12816/0003256 [Crossref] [ Google Scholar]
- Musk DJ Jr, Hergenrother PJ. Chemical countermeasures for the control of bacterial biofilms: effective compounds and promising targets. Curr Med Chem 2006; 13(18):2163-77. doi: 10.2174/092986706777935212 [Crossref] [ Google Scholar]
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003; 2(2):114-22. doi: 10.1038/nrd1008 [Crossref] [ Google Scholar]
- Renn O, Roco MC. Nanotechnology and the need for risk governance. J Nanopart Res 2006; 8(2):153-91. doi: 10.1007/s11051-006-9092-7 [Crossref] [ Google Scholar]
- Kamaruzzaman NF, Tan LP, Mat Yazid KA, Saeed SI, Hamdan RH, Choong SS. Targeting the bacterial protective armour; challenges and novel strategies in the treatment of microbial biofilm. Materials (Basel) 2018; 11(9):1705. doi: 10.3390/ma11091705 [Crossref] [ Google Scholar]
- Verdier T, Coutand M, Bertron A, Roques C. Antibacterial activity of TiO2 photocatalyst alone or in coatings on E coli: the influence of methodological aspects. Coatings 2014; 4(3):670-86. doi: 10.3390/coatings4030670 [Crossref] [ Google Scholar]
- Lavaee F, Ghapanchi J, Motamedifar M, Sorourian S. An in vitro analysis of the effects of iron sulfate and iron acetate on Streptococcus mutans. J Dent Biomater 2018; 5(1):528-32. [ Google Scholar]
- Vincent MG, John NP, Narayanan PM, Vani C, Murugan S. In vitro study on the efficacy of zinc oxide and titanium dioxide nanoparticles against metallo beta-lactamase and biofilm producing Pseudomonas aeruginosa. J Appl Pharm Sci 2014; 4(7):41-6. doi: 10.7324/japs.2014.40707 [Crossref] [ Google Scholar]