Avicenna Journal of Clinical Microbiology and Infection. 7(1):1-7.
doi: 10.34172/ajcmi.2020.01
Original Article
Efficacy of Some Antibiotics and Essential Oils Against Acinetobacter baumannii: An in Vitro Study
Mazen Safi , *  , Laila Al-Hallab , Rasha Al-Abras , Marwa Khawajkiah , Heba Kherbik , Ayman AL-Mariri
, Laila Al-Hallab , Rasha Al-Abras , Marwa Khawajkiah , Heba Kherbik , Ayman AL-Mariri 
Author information:
Molecular Biology and Biotechnology Department, Atomic Energy Commission of Syria, Damascus, Syria
*
Corresponding author: Mazen Safi, Atomic Energy Commission of Syria, P.O. Box 6091, Damascus, Syria, Tel: +963.11.213580, Fax: +963.11.6112289, Email:
ascientific34@aec.org.sy
Abstract
Background and Objective: Acinetobacter baumannii is considered as a main opportunistic pathogen in hospitals and exhibit high resistance against most antibiotic groups. The aim of this study was to evaluate the efficacy of some antibiotics and essential oils against this bacterium, in vitro.
Materials and Methods: Two hundred and one clinical samples were collected from the Children’s Hospital of Damascus. The polymerase chain reaction was conducted to identify the genus and type of bacteria. Finally, the minimum inhibitory concentrations of several antibiotics and essential oils, including Thymus syriacus, Origanum syriacum, Citrus aurantium, Cinnamomum verum, Syzygium aromaticum, Cupressus macrocarpa, Myristica fragrans, Biota orientalis, and Zingiber officinale, were investigated on Luria-Bertani broth agar.
Results: Fifty-nine isolates of A. baumannii were identified and the results showed that the DNA fragments of 16S rRNA and the blaOXA-51 _ like gene were approximately equal to 280 bp and 350 bp, respectively. In addition, most effective antibiotics against 50% of bacteria in each isolate of A. baumannii were rifampicin, linezolid, and levofloxacin whereas most effective essential oils included Cupressus macrocarpa, Citrus aurantium, Myristica fragrans, and Biota orientalis.
Keywords: Acinetobacter baumannii, Essential oils, Fluoroquinolones, Drug therapy, Drug resistance
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Acinetobacter baumannii (A. baumannii ) is a bacterium that is classified as opportunistic pathogens in hospitals, especially in immunocompromised patients and children. Regarding their role as pathogens, some studies in the last two decades have shown their involvement in many human infections, especially in intensive care units (1,2). A. baumanniicolonizes the skin and upper respiratory tracts. It is isolated from urine, sputum, blood, and feces. In addition, it is usually found in hospitals on different surfaces. In other words, they are isolated from different locations within the hospitals (e.g., air, water faucets, bedsides, gloves, and catheters). Historically, this type of infection is associated with war-wounded due to the direct contamination of wounds in the surrounding environment. For example, it was the most isolated Gram-negative bacterium from the wounds of those wounded in the Vietnam War, as well as the case with those wounded in the US war in Iraq. Recent reports indicate an increased incidence of septicemia in military hospital patients (2). A. baumanniiexhibits high resistance against most antibiotic groups because it owns genes that encode inhibitory enzymes (3). For example, carbapenem-resistant A. baumannii strains show high resistance to most antibiotics, particularly beta-lactamase groups (4), because these strains own blaOXA-51-like and blaOXA-23-like genes which encode enzymes that inhibit the action of these antibiotics (5). Further, other studies demonstrated that most clinical isolates of A. baumanniiwere resistant to most cephalosporins (6), and this bacterium was also completely resistant to aztreonam, cefotaxime in addition to amoxicillin-clavulanic acid combination (7).
Essential oils and plant extracts are used as new sources of antibacterial and antimicrobial agents in many fields (8), which include food preservation (9), pharmaceuticals, alternative medicine, and natural treatments (10-11).
Furthermore, many plant extracts and oils are commonly used as medicinal plants in Syria for several purposes, especially for respiratory and gastrointestinal disorders. Considering the above-mentioned explanations, this study aimed to conduct a survey on the antibacterial activity of several antibiotics and essential oils, in vitro, against the isolates of A. baumannii obtained from children.
Materials and Methods
Identification of Bacteria
Two hundred and one samples were collected from Children’s Hospital of Damascus, Damascus, Syria from different sources (e.g., skin abscesses, bronchial secretions, urine, pharyngeal smears, and blood) during May-December 2016.
First, the samples were cultured on peptone water for 3 hours at 37 °C. Then, 10 µL of the primary culture were taken and added to 5 mL of Luria-Bertani (LB) broth culture medium for bacterial multiplication, followed by incubating the bacterial fluid at 37 °C for 18 hours. Subsequently, according to (12), a swab of the bacterial fluid was transplanted onto the solid culture medium LB agar or/and selective media (Herellae Agar and Leeds Acinetobacter Agar). All used media were purchased from Himedia, India.
DNA Isolation and Amplification by Polymerase Chain Reaction (PCR)
The isolation of DNA was carried out using the method of cetyltrimethylammonium bromide (13). The final extracted DNA was re-suspended in the Tris-EDTA (TE) buffer and the concentration was read using 2 µL of the sample in the nanodrop machine by the TE buffer as blank. Then, the concentration was made up to 100 ng/mL in each sample and stored at -20 °C until use.
Next, specific primers were used to amplify the 16S rRNA with a pair of primers including the 17 nucleotide forward primer of F16S (5ꞌ- TTTAAGCGAGGAGGAGG-ꞌ3) and the 18-nucleotide reverse primer of R16S (5ꞌ-ATTCTACCATCCTCTCCC-ꞌ3) (14). The primers of 16S rRNA yielded PCR products equal to 280 bp. According to (15), the blaOXA-51_like gene was amplified with a pair of specific primers encompassing the 20-nucleotide forward primer of Fbla (5ꞌ-TAATGCTTTGATCGGCCTTG-ꞌ3) and the 20- nucleotide reverse primer of Rbla: (5ꞌ-TGGATTGCACTTCATCTTGG-ꞌ3). The primers of the blaOXA-51_like gene gave a PCR product equal to 350 bp. The following reaction mixture contained 200 ng of bacterial DNA, 10 µmol of each forward and reverse primers, and the PCR mixture (3 mM of magnesium sulfate, 1X binding buffer, 0.2 mM dNTPs, and 1UTaq polymerase) taking into account the mixing of the reaction tube content well after the addition of each one of these substances. Finally, distilled water was supplemented until reaching a final volume of 25 µL. The essential steps of PCR are scheduled in Table 1.
Table 1.
Polymerase Chain Reaction (PCR) Steps
|
PCR Step
|
|
Temperature
|
Duration
|
| Initial denaturation |
|
95 °C |
5 minutes |
| 37 cycles of amplification |
DNA denaturation |
95 °C |
30 seconds |
| DNA annealing |
55 °C |
30 seconds |
| DNA extension |
72 °C |
60 seconds |
| Final extension |
|
72 °C |
10 minutes |
Note. DNA: Deoxyribonucleic acid.
PCR products were loaded on the 1.5% agarose gel and a 100 bp molecular weight DNA ladder was used for the validation of the length of the amplified products (Bio-Rad, USA; UV Tec GmbH, Germany).
Essential Oil Extraction
The samples of 100 g of wild thyme (Thymus syriacus ), Origanum syriacum, Citrus aurantium, cinnamon (Cinnamomum verum), Syzygium aromaticum, Cupressus macrocarpa, Myristica fragrans, Biota orientalis, and ginger (Zingiber officinale) were collected during the flowering season from different regions in Syria or purchased from local markets (Table 2). Then, they were air-dried (hydro-steam distillation) away from sunlight, and finally, grinded by an electrical mill. Moreover, the essential oils were extracted using a Clevenger-type apparatus according to the European pharmaceutical instruction method. The Clevenger-type apparatus was connected to a condenser and a cold water recycling device. Next, distilled water was added by the 1/10 volume to volume, and each sample was distilled for 2 hours. The floating essential oil was filtered through anhydrous sodium sulphate to dry the yielded essential oils, which were approximately about 1.6% for T. syriacus, O. syriacum, C. Macrocarpa, and B. orientalis, and about 0.6% for C. aurantium, C. verum, S. aromaticum, M. fragrans, and Z. officinale. Eventually, the essential oils were collected in sealed dark glass bottles and kept in the fridge until use (16). For the antimicrobial activity test, several dilutions of the oils were done using dimethyl sulfoxide.
Table 2.
Plants, Along With Their Families and Collection Sites
|
Scientific Name
|
Plant Family
|
Collection Site
|
Altitude (m)
|
Extracted Part
|
|
Thymus syriacusBoiss.
|
Lamiaceae |
Alsoja mountain |
840 |
Aerial parts |
|
Citrus aurantium L.
|
Rutaceae |
Latakia |
300 |
Peels |
|
Cinnamomum verum L.
|
Lauraceae |
Market |
|
Barks |
|
Origanum syriacum L.
|
Lamiaceae |
Alsoja mountain |
840 |
Aerial parts |
|
Cupressus macrocarpa L.
|
Cupressaceae |
Market |
|
Leaves |
|
Syzygium aromaticum L.
|
Myrtaceae |
Market |
|
Leaves |
|
Myristica fragransHaultt.
|
Myristicaceae |
Market |
|
Leaves |
|
Biota orientalis L.
|
Cupressaceae |
Market |
|
Seeds |
|
Zingiber officinaleRosc.
|
Zingiberaceae |
Market |
|
Rhizomes |
Antibiotic Susceptibility Determination by Disk Diffusion
The isolates were grown in LB medium at 37 °C for 22 hours. Final inoculum bacterial numbers were adjusted to 1.5*108 CFU/mL. A total of 0.1 mL of bacterial suspension was poured on each plate containing LB agar. Then, the lawn culture was prepared by sterile cotton swab and allowed to remain in contact for 1 minute. Additionally, the sterile antibiotic disks were placed on lawn cultures, followed by incubating Petri dishes at 37 °C for 24 hours and measuring the inhibition zone around each disk. All antibiotic disks were purchased from Himedia, India and contained imipenem (10 µg), meropenem (10 µg), cefprozil (30 µg), cefotaxime (30 µg), ceftazidime (30 µg), ciprofloxacin (5 µg), ofloxacin (5 µg), levofloxacin (5 µg), doxycycline (30 µg), amoxicillin + clavulanic acid (30 µg), tobramycin (10 µg), oxacillin (1 µg), rifampicin (5 µg), linezolid (30 µg), and azithromycin (15 µg).
Antibiotic Minimum Inhibitory Concentration (MIC) Determination
Antibiotics susceptibility was estimated by using 96-well plates (TPP, Switzerland) according to the well broth microdilution method. The two-fold dilution of each antibiotic was executed in LB broth® (Acumedia, Michigan, USA). Next, plate wells were inoculated with 1×106 CFU of bacteria (0.2 mL final volume) and incubated at 37 °C for 24 hours. In addition, MIC50 and MIC90 were interpreted as the lowest concentration that inhibited 50% or 90% of the visual growth of bacteria, respectively. Further, the MIC testing was performed according to the recommendations of the Clinical and Laboratory Standards Institute (17). The absorbance was determined at 590 nm (Thermo-Lab Systems Reader, Finland). Investigated antibiotics were imipenem (Sigma, St. Louis, USA), meropenem (Supelco, Merck, Germany), cefprozil (Bristol-Myers Squibb, New-York, USA), cefotaxime (Sigma), ceftazidime (Sigma), ciprofloxacin (Bayer, Istambul, Turkey), ofloxacin (Sigma), levofloxacin (Sigma), and doxycycline (Sigma, St. Louis, USA). The other antibiotics included amoxicillin + clavulanic acid (Sigma, St. Louis, USA), tobramycin (Sigma, St. Louis, USA), oxacillin (Thermo Scientific, Oxoid Germany), rifampicin (Sigma), linezolid (Thermo Scientific, Oxoid Germany), azithromycin (Thermo Scientific, Oxoid, Germany), cefoperazone (Sigma, St. Louis, USA), sulbactam (Supelco, Merck, Germany), and amikacin (Sigma).
Essential Oil MIC Determination
The microdilution broth susceptibility assay was used in this regard (18). Furthermore, the serial dilutions of each essential oil were prepared in the LB broth medium in 96-well microtiter plates using a range of concentrations for each essential oil from 0.02 to 5.26 μL/mL. Next, 100 μL of freshly grown bacteria, standardized until achieving a bacterial number of 1×106 CFU/mL in the LB broth, was added to each well, followed by doing positive and negative controls. Then, the plate was incubated for 24 hours at 37 ˚C by continuing the process of shaking. Finally, the lowest concentration inhibiting 50% or 90% of visual growth was recorded and interpreted as MIC50 and MIC90, respectively.
Results
Identification of Bacteria
The results showed that 67 isolates (out of 201 samples) were positive when cultured on selective media (42%).
PCR Results
DNA isolation, PCR, and electrophoresis on the agarose gel were carried out and the results revealed that 59 (88%) isolates were identified as Acinetobacter genus (280 bp) and baumannii type (350 bp). Figure 1 shows the results of DNA fragment migration on the agarose gel.
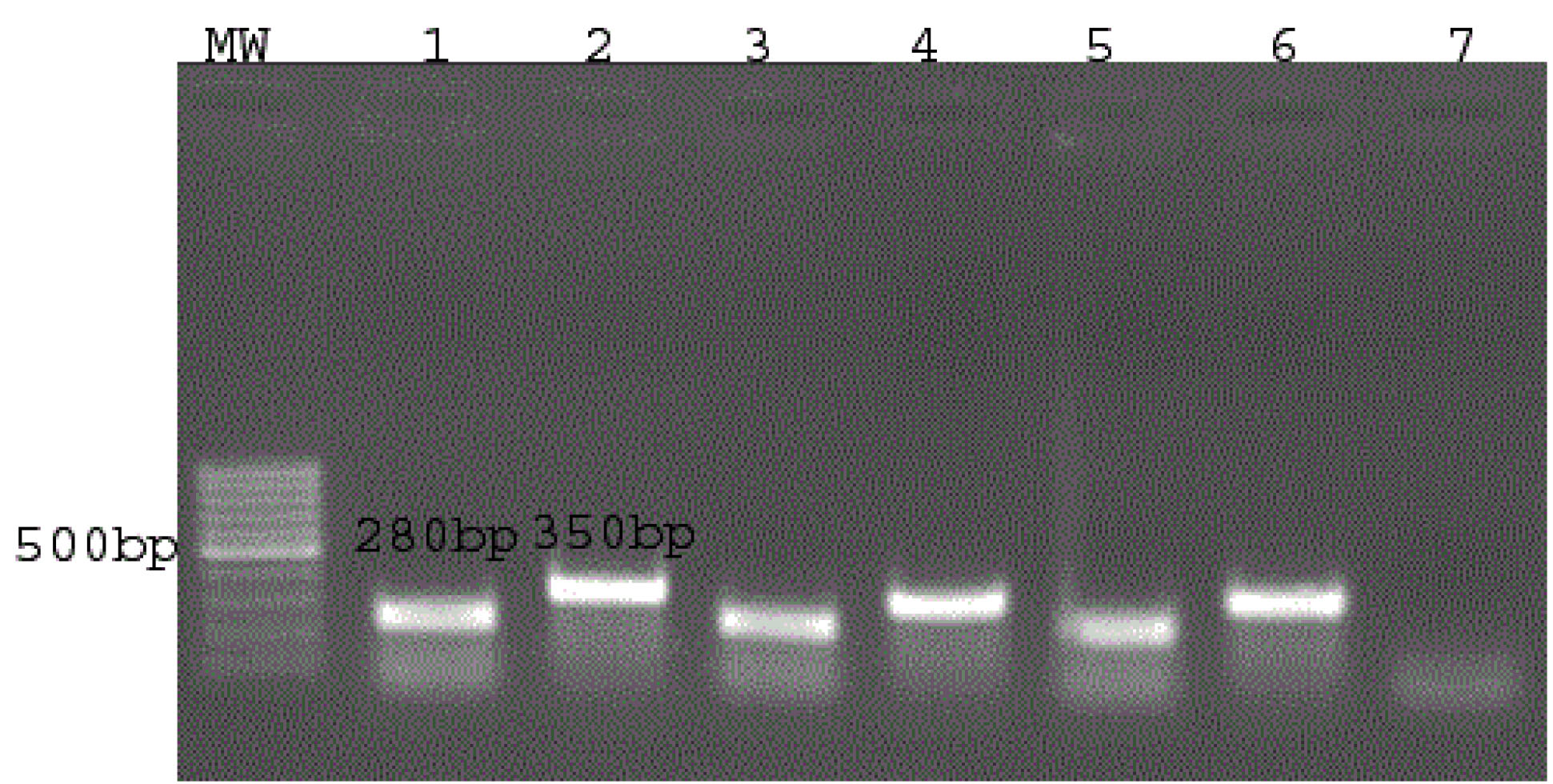
Figure 1.
DNA Fragment Electrophoresis on Agarose Gel
Note. MW: 100 bp molecular weight; DNA ladder bands 1 and 2 display positive control (for genus and type). In addition, bands 3-6 represent two selective isolates, and band 7 indicates the negative control.
.
DNA Fragment Electrophoresis on Agarose Gel
Note. MW: 100 bp molecular weight; DNA ladder bands 1 and 2 display positive control (for genus and type). In addition, bands 3-6 represent two selective isolates, and band 7 indicates the negative control.
Antibiotic Disk Diffusion Susceptibility Test
Based on the data in Figure 2, almost all used antibiotics had moderate to low effects against 50 tested isolates and the most effective antibiotics were rifampicin, levofloxacin, linezolid, and imipenem.
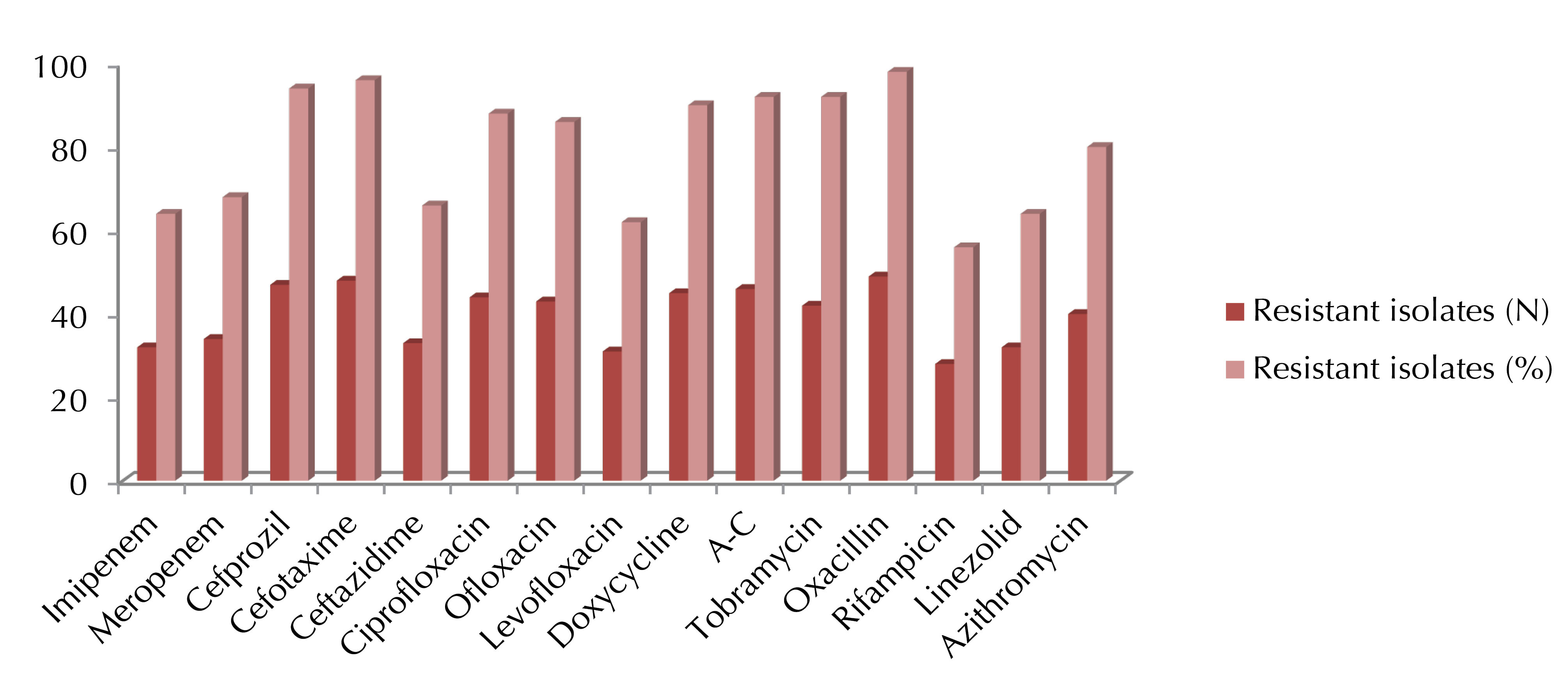
Figure 2.
Disk Diffusion Susceptibility of Different Antibiotics Against A. baumannii. A-C = Amoxicillin + Clavulanic Acid
.
Disk Diffusion Susceptibility of Different Antibiotics Against A. baumannii. A-C = Amoxicillin + Clavulanic Acid
Antibiotic Minimum Inhibitory Concentration (MIC) Test
The results (Table 3) showed that most effective antibiotics against 50 isolates of A. baumannii were rifampicin (MIC50 = 4 µL/mL), linezolid (MIC50 = 8 µL/mL), and levofloxacin (MIC50 = 16 µL/mL) and the best antibiotic combination was cefoperazone/sulbactam + rifampicin (MIC50 = 4 µL/mL).
Table 3.
Minimum Inhibitory Concentration (MIC) of Different Antibiotics Against A. baumannii
|
Antibiotic
|
Number of Resistant Isolates (%)
|
MIC
50
|
MIC
90
|
| Imipenem (IP) |
46 (92%) |
64 |
128 |
| Meropenem |
46 (92%) |
64 |
128 |
| Ceftezole |
50 (100%) |
>256 |
>256 |
| Cefprozil |
50 (100%) |
>256 |
>256 |
| Cefotaxime |
50 (100%) |
>256 |
>256 |
| Ceftazidime |
46 (92%) |
64 |
128 |
| Ciprofloxacin |
45 (90%) |
32 |
128 |
| Ofloxacin |
45 (90%) |
32 |
128 |
| Levofloxacin |
40 (80%) |
16 |
32 |
| Doxycycline |
44 (88%) |
32 |
128 |
| AC |
50 (100%) |
>256 |
>256 |
| Tobramycin |
46 (92%) |
32 |
128 |
| Oxacillin |
50 (100%) |
>256 |
>256 |
| Rifampicin |
2 (4%) |
4 |
8 |
| Linezolid |
2 (4%) |
8 |
16 |
| Azithromycin |
43 (86%) |
32 |
64 |
| CPS |
43 (86%) |
32 |
64 |
| CPS + rifampicin |
2 (4%) |
4 |
8 |
| CPS + Ciprofloxacin |
44 (88%) |
32 |
32 |
| CPS + Amikacin |
46 (92%) |
64 |
128 |
| CPS + Tobramycin |
45 (90%) |
32 |
64 |
| CPS + Ceftazidime |
44 (88%) |
32 |
64 |
| IP + Rifampicin |
44 (88%) |
32 |
64 |
| IP + Ciprofloxacin |
45 (90%) |
16 |
64 |
| IP + Amikacin |
46 (92%) |
64 |
128 |
| IP + Tobramycin |
45 (90%) |
32 |
64 |
| IP + Ceftazidime |
44 (88%) |
32 |
64 |
Note. AC: Amoxicillin + Clavulanic acid; CPS: Cefoperazone + Sulbactam combination.
Essential Oil MIC Test
Based on the results (Table 4), all the applied essential oils were able, within the range of used concentrations, to inhibit 50% of the bacteria in each isolate. However, only some of these oils, especially those of Cupressus macrocarpa, Citrus aurantium, Myristica fragrans, and Biota orientaliswere able to inhibit 90% of the bacteria in each isolate.
Table 4.
Minimum Inhibitory Concentration (MIC) of Different Essential Oils Against A. baumannii
|
Essential Oil
|
MIC
50 (µL/mL)
|
MIC
90 (µL/mL)
|
|
Cinnamomum verum
|
0.08 |
1.28 |
|
Syzygium aromaticum
|
0.04 |
1.28 |
|
Cupressus macrocarpa
|
0.08 |
0.32 |
|
Origanum syriacum
|
0.08 |
1.28 |
|
Citrus aurantium
|
0.08 |
0.64 |
|
Thymus syriacus
|
0.04 |
- |
|
Zingiber officinale
|
0.64 |
- |
|
Myristica fragrans
|
0.01 |
0.64 |
|
Biota orientalis
|
0.16 |
0.64 |
Discussion
Diseases caused by A. baumannii demonstrate a real threat to human health in developing countries, especially in children and in places of armed conflicts. The unacceptably high rates of infection or a significant increase in the number of cases of any epidemic disease strongly led to greater efforts to prevent it, either by following health guidelines or conducting vaccination campaigns. The previous research has long focused on the importance of infectious disease occurrence data (19). Nonetheless, standards adopted in the diagnosis of infectious diseases in developing countries still largely depend on clinical symptoms and signs more than laboratory analysis or pathological anatomy (20-21). Thus, the determination of the incidence rate of any disease, in which clinical symptoms are not specific, represents a major challenge in the chase of effective control and infectious disease prevention in developing countries. Despite the significant development in recent years, developing countries are still suffering from many problems in terms of infrastructure, funding sources, and trained personnel that are capable of executing a rapid detection of such diseases (22-23).
The antimicrobial resistance of A. baumannii against some antibiotics is related to the secretion of genes encoding enzymes which have the ability to inhibit the activity of several antibiotics (4). This property is mainly responsible for the emergence of resistance to several types of drugs such as tobramycin and fluoroquinolones (i.e., ciprofloxacin, levofloxacin, and ofloxacin). In this study, the resistance rates against these antibiotics were elevated and several ratios were recorded (i.e., 92%, 90%, 90%, and 80%, respectively) by using the minimum inhibitory concentration (MIC) method. Othman et al (24) revealed the presence of moderate resistance against tobramycin and ciprofloxacin in isolates from Tunisian hospitals (45% and 36%, respectively). On the other hand, the MIC of ciprofloxacin and levofloxacin in this study was significantly higher compared to the other studies (25-26).
Fonseca et al (5) showed that the emergence of carbapenem-resistant isolates is responsible for the emergence of carbapenem- (imipenem and meropenem) and rifampicin-resistant bacteria. In our study, the percentage of rifampicin-resistant isolates was very low (4%) by using the MIC method with the MIC90 of less than 8 µg/mL. On the contrary, the percentage of imipenem- and meropenem-resistant isolates was 92% against both antibiotics. Considering international publications, in this study, the MIC of rifampicin was higher compared with the study of Aranda et al (25). However, a relatively lower MIC of imipenem was reported in several previous studies as 2 µg/mL (27), 8 µg/mL (28), and 16 µg/mL (29) compared to this study.
Beta-lactamase enzymes produced by the A. baumannii strains are responsible for the emergence of penicillin and cephalosporin resistance. In our study, it was clear that almost all studied isolates were resistant to these two groups. Accordingly, significant resistance was observed to all cephalosporin used in this work with a value of MIC90 exceeding 128 µg/mL, which is consistent with the results of previous studies using cefuroxime (27,29) while it contradicts the values revealed by other studies using cefazolin (27-28).
Drugs such as sulbactam, colistin, imipenem, and rifampicin have been used in the treatment of A. baumannii (30-32). The combination of several drug groups such as colistin-rifampicin, colistin-imipenem, imipenem-rifampicin, and cefoperazone/sulbactam-imipenem showed better efficacy against these bacteria (33-35). Thus, it is clear that the combination of several types of antibiotic groups may be the appropriate alternative treatment for these bacteria. In this context, Tunyapanit et al (36) found that the proportion of isolates resistant to cefoperazone/sulbactam combination did not exceed 3% with the MIC90 of less than 2 µg/mL, which is absolutely inconsistent with the results of our study where the proportion of isolates resistant to this combination was 86% with the MIC90 of more than 64 µg/mL. Conversely, our finding concurs with that of this study which proved that cefoperazone/sulbactam-rifampicin combination is the best pharmacological combination in terms of both sensitivity and the MIC value. Finally, although linezolid is normally used against Gram-positive bacteria, it showed a good synergistic effect against A. baumannii in combination with colistin (37-38). The present study evaluated the effect of linezolid against A. baumannii and revealed good results in this regard.
Recently, plant extracts have been developed and used in several fields such as natural antioxidants or antimicrobial agents (39-40). The antibacterial mechanisms of natural compounds found in herbs and spices were discussed as well (41).
Most applied plants in this study are used in traditional medicine in all Syrian regions in order to treat many disorders, especially respiratory and gastrointestinal diseases. Therefore, it was possible to investigate the efficacy of these plants against A. baumannii. All studied essential oils were able to inhibit 50% of the bacteria in each isolate. However, only some of these essential oils had the ability to inhibit 90% of bacteria, especially the essential oils of Cupressus macrocarpa, Citrus aurantium, Myristica fragrans, and Biota orientalis and the MIC90 values for these essential oils were 0.32, 0.64, 0.64, and 0.64 µg/mL, respectively.
There are only a few studies concerning the role of essential oils and plant extracts in the treatment of antibiotic-resistant A. baumannii. For instance, Miyasaki et al (42) showed that most of the de-tanninized parts of the Scutellaria baicalensis have a good effect against these bacteria (MIC90 = 128 µg/mL). Karaman et al (43) also demonstrated that the methanolic extract of Juniperus oxycedrus L. has a good effect against A. Baumannii and many other bacteria. In addition, Intorasoot et al (44) found that the extracts of Cinnamomum verum, Syzygium aromaticum, and Ocimumbasilicum Linn. volatile oils had an effective minimum bactericidal concentration against A. baumannii (MBC90 = 0.5, 1, and 2 µg/mL, respectively). Furthermore, Kumar et al (45) reported the essential oils of Citrus maxima and Citrus aurantifolia showed potential antimicrobial properties against A. baumannii. However, KumariPushpa et al (45) concluded that the secondary metabolites of Myristica fragrans Houtt can be an indispensable source of antimicrobial compounds against A. baumannii. In addition, Ehretia microphylla and Piper betle L leaf extracts showed a good inhibitory effect against A. baumannii(47-48). On the other hand, according to Duraipandiyan et al (49), the ethyl acetate extract of Toddaliaasiatica represented high efficacy against these bacteria (MIC = 125 µg/mL). Finally, Saghi et al (50) found good efficacy of Origanum syriacum, Thymus syriacus, and Satureja extracts against A. baumannii (MIC = 2.6, 0.44, and 0.3 µg/mL, respectively).
Conclusion
In general, A. baumannii strains were resistant to most antibiotic groups and alternative therapies. Rifampicin, linezolid, and levofloxacin were the most effective antibiotics against these bacteria using the minimum inhibitory concentration method whereas cefoperazone/sulbactam-rifampicin was the best antibiotic combination. Eventually, all essential oils used in this study were able to inhibit 50% of these bacteria while only some of these oils showed the ability to inhibit 90% of these bacteria.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgments
The authors would like to thank the Director General of the Atomic Energy Commission of Syria and the Head of the Molecular Biology and Biotechnology Department for their support.
Ethical Statement
Ethical permission was obtained from the Ethical Committee of Damascus Children Hospital.
Authors’ Contribution
We confirm that the manuscript, as well as the order of authors listed in the manuscript, has been contributed, reviewed and approved by all named authors.
Funding/Support
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed Consent
The verbally informed consent was obtained from the children’s parents.
References
- Tiwari V, Vashistt J, Kapil A. Comparative Proteomics of inner membrane fraction from carbapenem-resistant Acinetobacterbaumannii with a Reference Strain. PLoS One 2012; 7(6):e39451. doi: 10.1371/journal.pone.0039451. [Crossref] [ Google Scholar]
- Kempf M, Rolain JM, Diatta G. Carbapenem Resistance and Acinetobacterbaumannii in Senegal: The Paradigm of a Common Phenomenon in Natural Reservoirs. PLoS One 2012; 7(6):e39495. doi: 10.1371/journal.pone.0039495. [Crossref] [ Google Scholar]
- Eveillard M, Kempf M, Belmonte O. Reservoirs of Acinetobacterbaumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis 2013; 17(10):e802-805. [ Google Scholar]
- Revathi G, Siu LK, Huang LY. first report of NDM-1-producing Acinetobacterbaumannii in East Africa. Int J Infect Dis 2013; 17(12):e1255-1258. [ Google Scholar]
- Fonseca EL, Scheidegger E, Freitas FS, et al. Carbapenem-resistant Acinetobacterbaumannii from Brazil: role of carOalleles expression and bla OXA-23 gene. BMC Microbiol 2013; 13: 245. PMCID: PMC4228306. PMID: 24195496. 10.1186/1471-2180-13-245
- Rezaee MA, Pajand O, Nahaei MR. Prevalence of Ambler class A beta-lactamases and ampC expression in cephalosporin-resistant isolates of Acinetobacterbaumannii. DiagnMicrobiol Infect Dis 2013; 76(3):330-334. [ Google Scholar]
- Al-Agamy MH, Khalaf NG, Tawfick MM. Molecular characterization of carbapenem-insensitive Acinetobacterbaumannii in Egypt. Int J Infect Dis 2014; 22:49-54. [ Google Scholar]
- Mitscher LA, Drake S, Gollapudi SR. A modern look at folkloric use of anti-infective agents. J Nat Prod 1987; 50:1025-1040. [ Google Scholar]
- Faid M, Bakhy K, Anchad M. Physicochemical and microbiological characterizations and preservation with sorbic acid and cinnamon. J Food Prod 1995; 58:547-550. [ Google Scholar]
- Lis-Balchin M, Deans SG. Bioactivity of selected plant essential oils against Listeria monocytogenes. J ApplBacteriol 1997; 82:759-762. [ Google Scholar]
- Milhau G, Valentin A, Benoit F. In vitro antimicrobial activity of eight essential oils. J Essent Oil Res 1997; 9:329-333. [ Google Scholar]
- Jawad A, Hawkey PM, Heritage J. Description of Leeds Acinetobacter Medium, a New Selective and Differential Medium for Isolation of Clinically Important Acinetobacter spp, and Comparison with Herellea Agar and Holton’s Agar. J ClinMicrobiol 1994; 32(10):2353-2358. [ Google Scholar]
- Rajeh A, Al-Achkar K, Al-Mariri A. Role of Polymerase Chain Reaction (PCR) in the detection of antibiotic-resistant Staphylococcus aureus. The Egyptian Journal of Medical Human Genetics 2014; 15(3):293-298. [ Google Scholar]
- Vanbroekhoven K, Ryngaert A, Wattiau P. Acinetobacter diversity in environmental samples assessed by 16s rRNA gene PCR-DGGE fingerprinting. FEMS MicrobiolEcol 2004; 50(1):37-50. [ Google Scholar]
- Woodford NMJ, Ellington JM, Coelho JF. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 2006; 27:351-353. [ Google Scholar]
- Al-Mariri A. Safi M In Vitro Antibacterial Activity of Several Plant Extracts and Oils against Some Gram-Negative Bacteria. IJMS 2014; 39(1):36-43. [ Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. 6th ed. Wayne: National Committee for Clinical Laboratory Standards; 2003.
- Koneman EW, Allen SD, Janda WM. Color Atlas and Textbook of Diagnostic Microbiology Philadelphia. Philadelphia: Lippincott-Raven Publishers; 1997. p. 785-856.
- Giesecke J. Modern infectious disease epidemiology. 2nd ed. New York (NY): Oxford University Press; 2002. P. 268.
- John TJ, Samuel R, Balraj V. Disease surveillance at district level: a model for developing countries. Lancet 1998; 352:58-61. [ Google Scholar]
- Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis 2001; 7:302-305. [ Google Scholar]
- De Salazar L. Building capacity for risk factor surveillance in developing countries: a new approach. SozPraventivmed 2005; 50(Suppl 1):S33-37. [ Google Scholar]
- Oum S, Chandramohan D, Cairncross S. Community- based surveillance: a pilot study from rural Cambodia. Trop Med Int Health 2005; 10:689-697. [ Google Scholar]
- Ben Othman A, Burucoa C, Battikh H. Comparison of Acinetobacterbaumannii multidrug resistant isolates obtained from French and Tunisian hospitals. J BacteriolParasitol 2011; 2:106. doi: 10.4172/2155-9597.1000106 [Crossref] [ Google Scholar]
- Aranda JC, Bardina C, Beceiro A. AcinetobacterbaumanniiRecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J Bacteriol 2011; 193(15):3740-3747. [ Google Scholar]
- McCracken M, Decorby M, Fuller J. Identification of multidrug- and carbapenem-resistant Acinetobacterbaumannii in Canada: results from CANWARD 2007. J AntimicrobChemother 2009; 64(3):552-555. [ Google Scholar]
- Koeleman JG, Stoof J, Van Der Bijl MW. Identification of epidemic strains of Acinetobacterbaumannii by integrase gene PCR. J ClinMicrobiol 2001; 39(1):8-13. [ Google Scholar]
- Sevillano E, Fernandez E, Bustamante Z. Emergence and clonal dissemination of carbapenem-hydrolysing OXA-58-producing Acinetobacterbaumannii isolates in Bolivia. J Med Microbiol 2012; 61(pt.1):80-84. [ Google Scholar]
- Adams MD, Goglin K, Molyneaux N. Comparative genome sequence analysis of multidrug-resistant Acinetobacterbaumannii. J Bacteriol 2008; 190(24):8053-8064. [ Google Scholar]
- Jamulitrat S, Thongpiyapoom S, Suwalak N. An outbreak of imipenem-resistant Acinetobacterbaumannii at Songklanagarind Hospital: the risk factors and patient prognosis. J Med Assoc Thai 2007; 90:2181-2191. [ Google Scholar]
- Li J, Nation RL, Owen RJ. Antibiograms of multidrug-resistant clinical Acinetobacterbaumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Cli Infect Dis 2007; 45:594-598. [ Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacterbaumannii: emergence of a successful pathogen. CliMicrobiol Rev 2008; 21:538-582. [ Google Scholar]
- Timurkaynak F, Can F, Azap OK. In vitro activities of non-traditional antimicrobials alone and in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacterbaumannii isolated from intensive care units. Int J Antimicrob Agents 2006; 27:224-228. [ Google Scholar]
- Tripodi MF, Durante-Mangoni E, Fortunato R. Comparative activities of colistin, rifampicin, imipenem and sulbactam/ampicillin alone and in combination against epidemic multidrug-resistant Acinetobacterbaumannii strains producing OXA-58 carbapenemases. Int J Antimicrob Agents 2007; 30:537-540. [ Google Scholar]
- Kiratisin P, Apisarnthanarak A, Kaewdaeng S. Synergistic activities between carbapenems and other antimicrobial agents against Acinetobacterbaumannii including multidrug-resistant and extensively drug-resistant isolates. Int J Antimicrob Agents 2010; 36:243-246. [ Google Scholar]
- Tunyapanit W, Pruekprasert P, Laoprasopwattana K. Antimicrobial susceptibility of Acinetobacterbaumannii isolated from hospital patients. Science Asia 2014; 40:28-34. [ Google Scholar]
- Armengol E, Asuncion T, Vinas M. When Combined with Colistin, an Otherwise Ineffective Rifampicin–Linezolid Combination Becomes Active in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacterbaumannii. Microorganisms 2020; 8(1):86-93. [ Google Scholar]
- Bin L, Youning L, Xiuzhen D. Colistin and anti-Gram-positive bacterial agents against Acinetobacterbaumannii. Rev Soc Bras Med Trop 2014; 47(4):451-456. [ Google Scholar]
- Basaga H, Tekkaya C, Acikel F. Antioxidative and free radical scavenging properties of rosemary extract. LebensmWissTechnol 1997; 30:134-142. [ Google Scholar]
- Hsieh PC, Mau JL, Huang SH. Antimicrobial effect of various combinations of plant extracts. Food Microbiol 2001; 18:35-43. [ Google Scholar]
- Brul B, Coote P. Preservative agents in foods mode of action and microbial resistance mechanisms. Int J Food Microbiol 1999; 50:1-17. [ Google Scholar]
- Miyasaki y, Rabenstein JD, Rhea J. Isolation and Characterization of Antimicrobial Compounds in Plant Extracts against Multidrug-Resistant Acinetobacterbaumannii. Plos One 2013; 8(4):1594-1601. [ Google Scholar]
- Karaman İ, ŞahinF ŞahinF, Güllüce M. Antimicrobial activity of aqueous and methanol extracts of Juniperusoxycedrus L. Journal of Ethnopharmacology 2003; 85(2-3):231-235. [ Google Scholar]
- Intorasoot I, Chornchoem P, Sookkhee S. Bactericidal activity of herbal volatile oil extracts against multidrug-resistant Acinetobacterbaumannii. Journal of Intercultural Ethnopharmacology 2017; 6(2):218-222. [ Google Scholar]
- Anil Kumar C, Aruna Lakshmi K, Venkata Kumar C. Comparative study of antimicrobial activity of essential oils of selected plants of Rutaceaeand TLC bioautographic studies for detection of bioactive compounds. The Journal of essential oil research 2015; 27(1):9-16. [ Google Scholar]
- KumariPushpa Rani TP, Sundar SK, Vijayalakshmi BA. GC MS analysis and antibacterial activity of Myristicafragrans seed extracts against lower respiratory tract pathogen acinetobacterbaumanii. Asian Journal of Pharmaceutical and Clinical Research 2014; 7(3):126-129. [ Google Scholar]
- Valle DL jr, Andrade JI, Puzon JJM. Antibacterial activities of ethanol extracts of Philippine medicinal plants against multidrug-resistant bacteria. Asian Pacific Journal of Tropical Biomedicine 2015; 5(7):532-540. [ Google Scholar]
- Valle DL jr, Cabrera EC, Puzon JJM. Antimicrobial activities of methanol, ethanol and supercritical CO2 extracts of Philippine Piper betle L on clinical isolates of Gram Positive and Gram Negative bacteria with transferable multiple drug resistance. PLoS ONE 2016 Jan 7; 11(1):e0146349. doi: 10.1371/journal.pone.0146349. [Crossref] [ Google Scholar]
- Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddaliaasiatica (L) Lam. Journal of Ethnopharmacology 2009; 123(3):494-498. [ Google Scholar]
- Saghi H, Bahador A, Dastjerdi FA. Antibacterial Effects of Herbal Compounds against Acinetobacterbaumannii Isolated from Hospital of Tehran, Iran. Glob J Infect Dis Clin Res 2015; 1(1):18-20. [ Google Scholar]