Avicenna Journal of Clinical Microbiology and Infection. 7(4):129-134.
doi: 10.34172/ajcmi.2020.28
Review Article
Clinical Effectiveness of Ertapenem Versus Piperacillin/Tazobactam in Patients With Mild to Moderate Intraabdominal Infections: A Systematic Review and Meta-analysis of Randomized Controlled Trials
Mehran Pezeshki 1  , Fatemeh Soleymani 1, Meysam Seyedifar 1, *
, Fatemeh Soleymani 1, Meysam Seyedifar 1, *
Author information:
1Pharmaceutical Management and Economics Research Center, The Institute of Pharmaceutical Sciences, Tehran University of Medical Sciences, Tehran, Iran.
*
Corresponding author: Meysam Seyedifar, Department of Pharmacoeconomics, 4th Floor, Faculty of Pharmacy, 16 Azar Street, Tehran, Iran. Tel: 09122781827, Email:
seyedifar@gmail.com
Abstract
Purpose: This study aimed to search for randomized clinical trials evaluating the clinical effectiveness of ertapenem compared to piperacillin/tazob actam in adult patients with mild to moderate intra-abdominal infections.
Design: A literature review was performed in PubMed, Scopus, Google Scholar, and Cochrane databases in order to find articles published up to April 2019. Then, the pairwise method was used to compare the difference between the mean score of the clinical effectiveness of these two interventions before and after the intervention by the means of a non-direct method (the comparison of drugs with each other).
Results: The analysis of 4 studies involving 767 patients in the ertapenem group and 728 patients in the piperacillin/tazobactam group showed that ertapenem can be 3% more effective than piperacillin/tazobactam (Weighted mean differences = 3.02, confidence interval (0.79-6.84) although the difference was insignificant (I-squared = 0.0%, P=0.98).
Conclusions: In general, the findings demonstrated that there is no significant difference in the clinical effectiveness of ertapenem in comparison with piperacillin/tazobactam in adult patients with mild to moderate intra-abdominal infections.
Keywords: Ertapenem, Piperacillin/Tazobactam, Intra-abdominal infections
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Introduction
The peritoneum is a crucial membrane that lines the abdominal cavity and covers most of the intra-abdominal organs. Unlike uncomplicated intra-abdominal infections, complicated ones extend beyond the site of infection and may cause peritonitis or abscess formation, requiring surgical interventions (1,2).
Intra-abdominal infections have different classifications such as primary (or spontaneous), secondary (due to the inflammation and infection of intra-abdominal organs), and tertiary (or status and permanent) peritonitis. In another classification, these infections are either localized or generalized, which is consistent with abscess formation and peritonitis, respectively (3-5).
Before the 1930s, the mortality rate of intra-abdominal infections was high (more than 90%). After the introduction and use of surgeries as a common intervention and the advent of novel anesthesia methods, this rate decreased to less than 40%. In the last decade, management and treatment of peritonitis profoundly changed because of an increase in the pathologic and microbiologic knowledge of peritonitis and the introduction of new antibiotics. In addition, the advent of simple abdominal radiography, diagnostic ultrasound, a computerized tomography scan, magnetic resonance imaging, and nuclear medicine helped an accurate diagnosis of the infected site and the etiology of peritonitis. Thus, intra-abdominal infections can now be managed and controlled more feasibly (6,7). The predominant bacteria involved in mild to moderate intra-abdominal infections are coliforms including (mainly Escherichia coli, Klebsiella spp., Proteus spp., and Enterobacter spp.) streptococci, enterococci, and anaerobic bacteria. In most series, dominant isolates are Bacteroides fragilis and E. coli (8).
There are various symptoms associated with intra-abdominal infections, including abdominal muscle rigidity, abdominal tenderness, abdominal pain, systemic infection or inflammation symptoms (mild to severe), and septic shock (9,10).
It is noteworthy that the infection source control and timely administration of antibiotic therapy are crucial for the management of patients with intra-abdominal infections (11).
Ertapenem is a carbapenem antibiotic with a narrower spectrum in comparison with imipenem and meropenem thus it can minimize the risk of developing resistance to carbapenems and can be used in the Iranian antimicrobial stewardship program. Further, the administration of ertapenem once daily, intravenously or intramuscularly, may be more convenient, safe, economic, and suited for use in the inpatient setting (12,13).
Due to outdated clinical trials and the lack of meta-analyses on the effectiveness of ertapenem and piperacillin/tazobactam in patients with mild to moderate intra-abdominal infections, this systematic review and meta-analysis aimed to investigate information from much more recent studies. The results of this study can help health care policymakers deciding on whether to include ertapenem in the National Medication Formulary of Iran.
Methods
All the outlined procedures in this review were done based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Figure 1) (14).
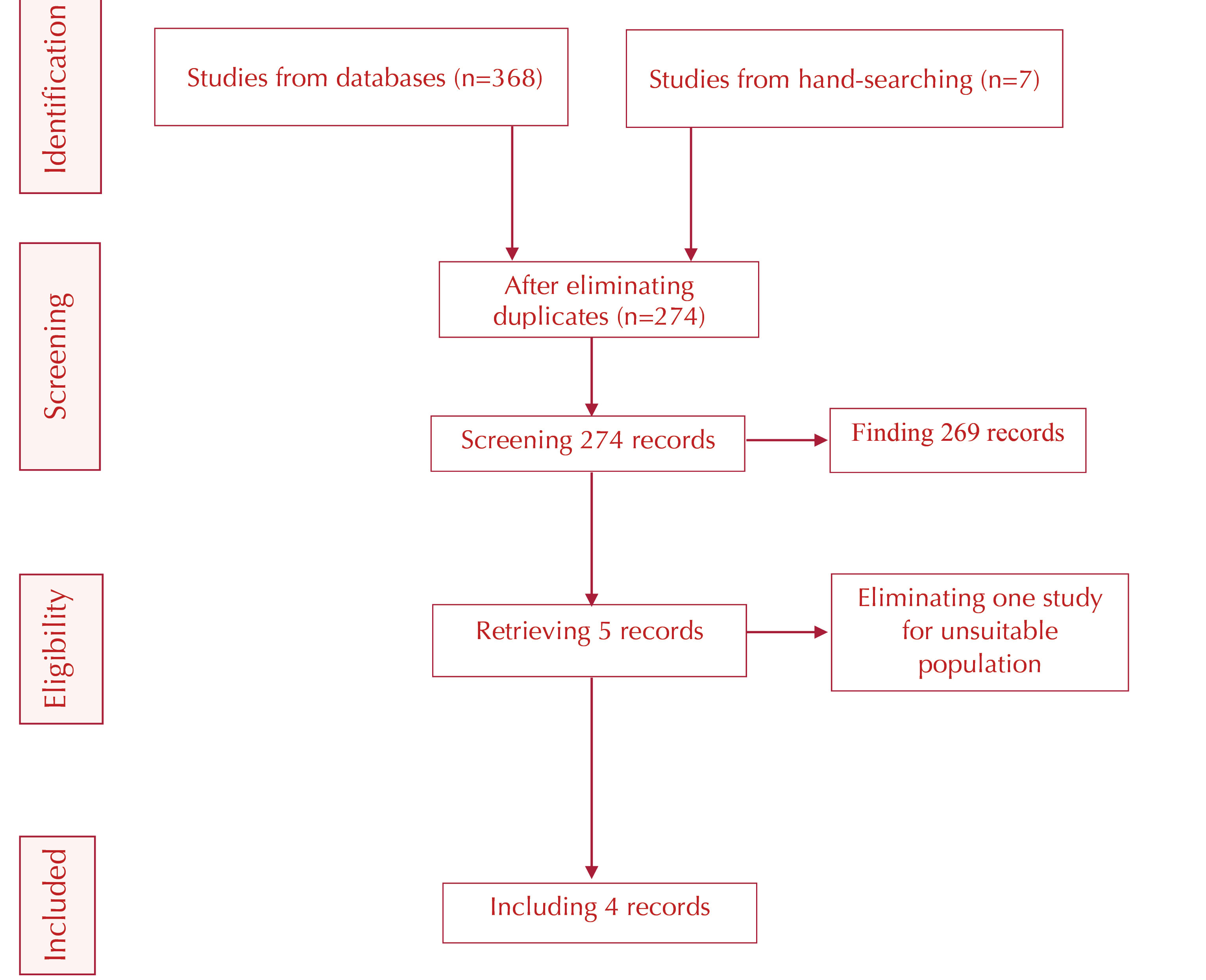
Figure 1.
Screening Flow Chart Based on the PRISMA Standard.
.
Screening Flow Chart Based on the PRISMA Standard.
In this study, the PICO model was used, which stands for the population (adult patients with mild to moderate intra-abdominal infections), intervention (ertapenem regimen), comparison (piperacillin/tazobactam regimen), and outcomes (clinical effectiveness reported in randomized controlled trials).
The study was performed in two steps. First, the related literature was searched with related keywords using the PubMed MeSH tool in order to ensure the lack of similar recent studies. Subsequently, a structured study question was extracted and used to define keywords, possible combinations, and search strategies. Then, the literature was systematically reviewed again in relevant databases.
The clinical effectiveness of ertapenem and piperacillin/tazobactam was reviewed in the second step. In this step, the analysis of effectiveness was run using the data obtained from the systematic review previously conducted in the first step.
Search Strategy
Several databases were systematically searched, including PubMed, Cochrane Library, Scopus, and Google Scholar in order to find articles published from January 2000 to April 2019 using the following keywords:
1. “Intra-abdominal infections [Title/Abstract]” OR “Intraabdominal infections [Title/Abstract]” OR “Complicated intra-abdominal infections [Title/Abstract]” OR ” “Complicated intraabdominal infections [Title/Abstract]” OR “IAI [Title/Abstract]” OR “CIAI [Title/Abstract]” OR “Peritonitis [Title/Abstract].”
AND 2. “Ertapenem [Title/Abstract]” OR “Invanz [Title/Abstract]” OR “Ertopenem [Title/Abstract]”
AND 3. “Piperacillin and Tazobactam [Title/Abstract]” OR “Piperacillin/Tazobactam [Title/Abstract]” OR “Zosyn [Title/Abstract]” OR “Tazocin [Title/Abstract]”.
For each database, appropriate methods were applied, including MeSH keywords. Then, the reference lists of identified clinical trials and review articles were checked to increase sensitivity. A manual search of relevant websites was carried out, and all identified articles were imported into EndNote software (version X8, Thomson Reuters, New York). After the elimination of duplicated studies, the remaining articles were screened by titles, abstracts, and full texts according to our inclusion and exclusion criteria.
Study Selection
The inclusion criteria for including studies were study population (adult patients with mild to moderate intra-abdominal infection), intervention (ertapenem medication), comparator (piperacillin/tazobactam medication), outcome (clinical effectiveness, and study design (parallel and crossover clinical trials).
On the other hand, exclusion criteria included study population (studies on non-human species and diseases other than intra-abdominal infection), intervention (using ertapenem in combination with other medications that can influence the results and using medications other than ertapenem), comparator (using medications other than piperacillin/tazobactam), outcome (studies with no report on the improvement of patients on antibiotics medication and studies reporting other outcomes such as the microbiological effects of antibiotic therapy), and study design (types of studies other than clinical trials and those studies using inappropriate methods and having biases).
Two reviewers separately screened titles and abstracts based on eligibility criteria, and any disagreements were resolved through discussions between the two reviewers.
Data Gathering and Extraction
After assessing the quality of clinical trials, an extraction data form was designed based on previous review articles, and the Cochrane extraction form was also used for data gathering. In addition, two separate authors extracted data from the included articles. Further, the extracted data included study specifications (i.e., design, duration of intervention, and duration of follow-up), participant’s specifications (i.e., numbers, ages, and gender), intervention specifications (i.e., ertapenem and piperacillin/tazobactam doses), and measured outcomes (i.e., clinical effectiveness).
Quality Assessment
Two separate reviewers assessed the quality of randomized clinical trials with the Cochrane Collaboration tool (15). It is a procedure to assess the quality of clinical trials based on the following criteria: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of the outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Then, each trial was rated as low risk, unclear, or high risk. Any disagreements in scoring were resolved through discussions between the two reviewers. Cochrane risk of the bias of the included studies showed in Table 1.
Table 1.
Summarized Clinical Effectiveness From Clinical Trial Studies
|
Author
|
Success of Ertapenem (%)
|
Success of PT (%)
|
| Namias (16) |
89.6 |
86.2 |
| Pena (17) |
98.2 |
96.4 |
| Solomkin (18) |
79.3 |
76.2 |
| Tellado (19) |
86.4 |
82.4 |
Note. PT: Piperacillin/Tazobactam.
Results
Study Selection and Data Extraction
The specifications of the included studies in this meta-analysis are presented in Table 2. All 4 studies were published between 2000 and 2019. Furthermore, clinical effectiveness was reported in these studies, and ertapenem and piperacillin/tazobactam were administered with a dose of 1 g every 24 hours and 3.375 g every 6 hours via intravenous infusion, respectively. Moreover, interventions lasted for 7-10 days, and studies were performed in different countries with a sample size of 120-350 participants.
Table 2.
Cochrane Risk of the Bias of the Included Studies
|
First Author
|
Sequence Generation
|
Allocation Concealment
|
Blinding of Participants and Personnel
|
Blinding of Outcome Assessment
|
Incomplete Outcome Data
|
Selective Outcome Reporting
|
Other Potential Threats to Validity
|
| Namias (16) |
L |
L |
L |
L |
L |
L |
U |
| Dela Pena (17) |
U |
L |
U |
U |
L |
L |
U |
| Solomkin (18) |
L |
L |
L |
L |
L |
L |
U |
| Tellado (19) |
U |
U |
L |
U |
L |
L |
U |
Note. L: Low risk of bias; H; High risk of bias; U: Unknown risk of bias.
Data Synthesis and Analysis
From 374 identified articles in our search, 100 cases were duplicate. The remaining 274 articles were screened, and five articles were retrieved based on titles and abstracts. According to the inclusion criteria, the full texts of these five studies were reviewed, and only four of them proved to be eligible for the meta-analysis.
Effectiveness Comparison
Based on the analysis of four studies including 767 patients in the Ertapenem group and 728 patients in the piperacillin/tazobactam group, the observed clinical improvement after the administration of ertapenem was 3% more than that of piperacillin/tazobactam (Weighted mean differences = 3.02, confidence interval = 0.79-6.84). Although no significant heterogeneity was detected (I-square = 0.0%, P = 0.98), there was no significant difference between the two groups (P = 0.12). The results are shown in Figure 2.
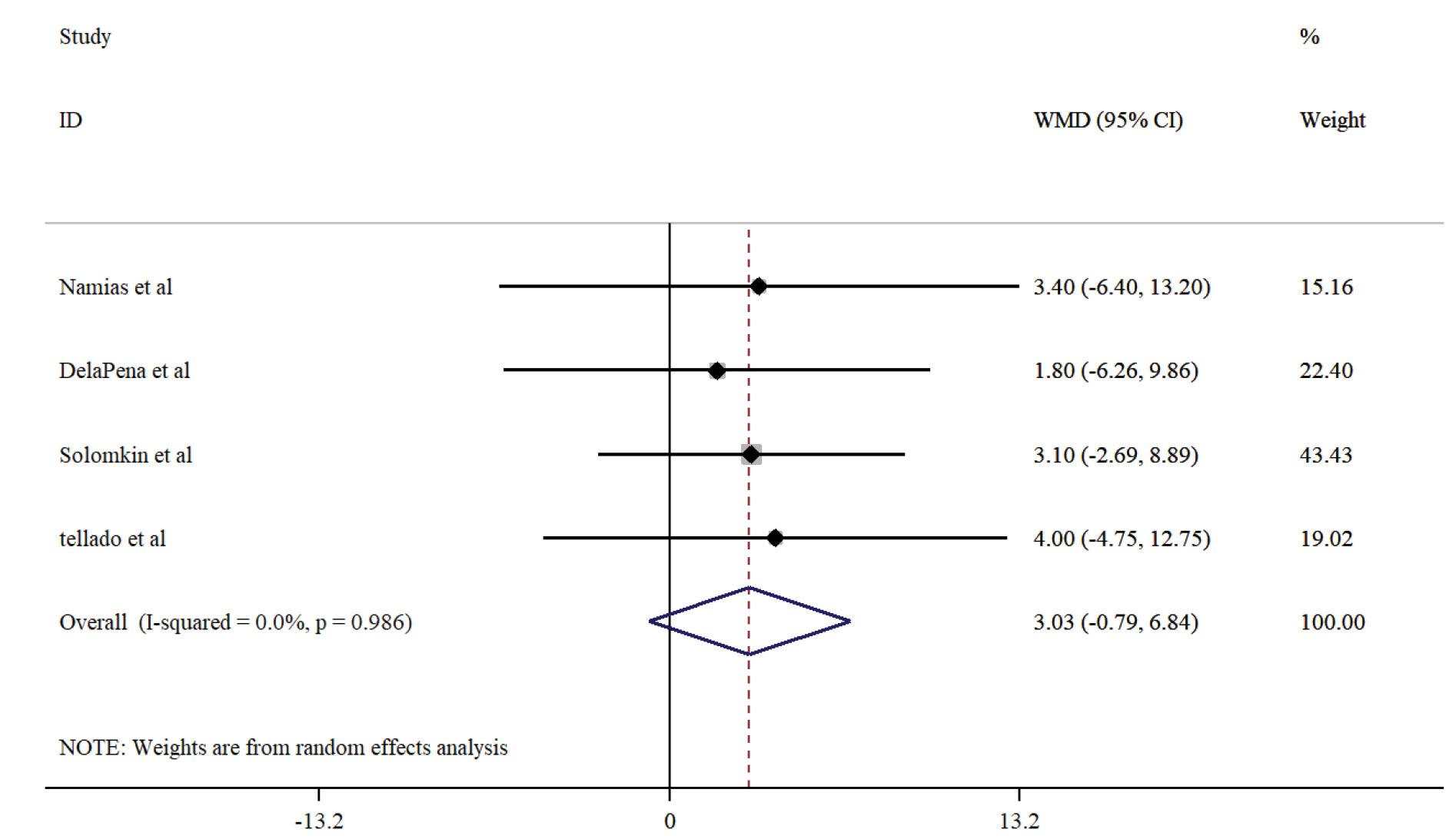
Figure 2.
Analysis of 4 Studies Including 767 Patients.
.
Analysis of 4 Studies Including 767 Patients.
Publication Bias
Based on the funnel plot, there is no visually detectable bias in studies. In addition, Egger’s test (P = 0.86) and Begg’s test provided no evidence for publication bias (Figure 3).
Sensitivity Analysis
The sensitivity analysis showed that no individual studies significantly influenced the final results (Figure 4).
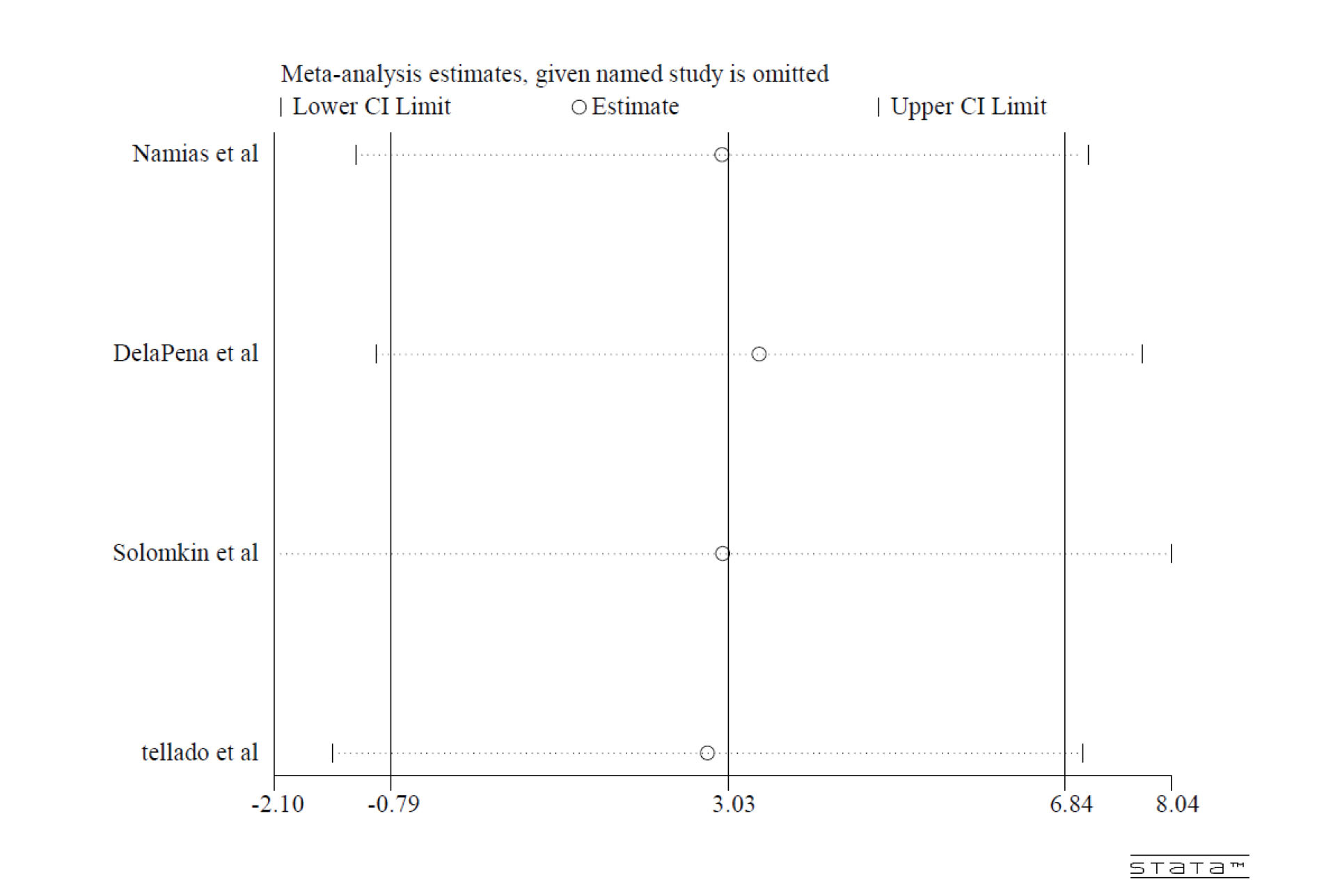
Figure 4.
Sensitivity Analysis.
.
Sensitivity Analysis.
Discussion
A disruption in a normal mucosal barrier and subsequent leakage of normal bowel flora may lead to intra-abdominal infections (20).
The empiric regimen should cover enteric streptococci, non-resistant Enterobacteriaceae, and anaerobes in patients with mild to moderate community-acquired intra-abdominal infections with no risk factors for antibiotic resistance or treatment failure (21).
Ertapenem is a carbapenem antibiotic that can be used for patients with mild to moderate community-acquired intra-abdominal infections. It targets bacterial membrane proteins and consequently bacterial cell wall synthesis which leads to pathogen killing (22).
Unlike older carbapenems, ertapenem can be administered once daily, which makes it a cost-effective option for patients with mild to moderate intra-abdominal infections. Additionally, this antibiotic drug can be used in the antimicrobial stewardship program due to its narrow spectrum and lower risks of bacterial resistance (23).
This systematic review and meta-analysis was performed to compare the clinical effectiveness of ertapenem and piperacillin/tazobactam medications in patients with mild to moderate intra-abdominal infections. Researchers employ different approaches in reporting clinical trials in terms of effectiveness, including clinical, microbiological, and long-term effectiveness. However, clinical effectiveness is more important in clinical practice because it indicates the alleviation of clinical symptoms during or immediately after the treatment course. Nevertheless, only clinical effectiveness was considered in this study.
Based on the included studies in this meta-analysis, ertapenem was more effective compared to piperacillin/tazobactam although this difference was not statistically significant (P = 0.211).
In a study by Tellado et al, the effectiveness of ertapenem and piperacillin/tazobactam in patients with mild to moderate intra-abdominal infections was 86.4% and 82.4%, respectively, and no related side effect was reported in this regard (19).
In a randomized, double-blind multicentral study, Solomkin et al reported 79.3% and 76.2% effectiveness for ertapenem and piperacillin/tazobactam, respectively, while finding no medication-related side effects (18).
Additionally, Dela Pena et al evaluated the efficacy and safety of ertapenem versus piperacillin-tazobactam for the treatment of intra-abdominal infections requiring surgical intervention and concluded that ertapenem and piperacillin/tazobactam were 98.2% and 96.4% efficacious, respectively (17).
In another study, Namias et al assessed the safety and effectiveness of ertapenem and piperacillin/tazobactam in patients with mild to moderate intra-abdominal infections and found that the effectiveness of ertapenem and piperacillin/tazobactam was 89.6% and 86.2%, respectively (16).
This meta-analysis had some limitations. First, the data, which were defined as cure or improvement of signs and symptoms, were used to analyze the clinical treatment success which may not be accurate compared to complete cure. In addition, ertapenem was not administered for severe cases of intra-abdominal infections. Consequently, the affected patients with resistant pathogens were excluded in most randomized control trials, which may interfere with the finding.
It is hoped that the results of this study help health care policymakers regarding deciding on whether to include Ertapenem in Iran’s National Medication Formulary.
Conclusions
In spite of the limitations, Ertapenem was generally well-tolerated and its effectiveness, when administered 1 gram once daily, was not statistically inferior to that of piperacillin/tazobactam. According to some recent studies, the administration of ertapenem is also associated with lower costs and decreased antimicrobial resistance. Further, no related adverse effects were reported in clinical trials except for nausea and vomiting that was statistically negligible. Therefore, ertapenem is probably a better first-choice treatment in individuals with mild to moderate intra-abdominal infections due to its superior pharmacokinetic properties (e.g., longer half-life and daily regimen) compared to piperacillin/tazobactam and its similar clinical effectiveness profile.
Conflict of Interests
None.
Acknowledgements
There is no financial support or conflict of interests in this study.
Ethical Approval
None.
Authors’ Contribution
MP wrote the manuscript and conducted all statistical analyses. All authors reviewed the final manuscript.
Funding/Support
None.
References
- Brook I, Frazier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol 2000; 49(9):827-30. doi: 10.1099/0022-1317-49-9-827 [Crossref] [ Google Scholar]
- Jones RS, Claridge JA. Acute Abdomen. In: Townsend C, Beauchamp RD, Evers BM, Mattox K, eds. Sabiston Textbook of Surgery. Elsevier/Saunders; 2004. p. 219-33.
- Johnson CC, Baldessarre J, Levison ME. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin Infect Dis 1997; 24(6):1035-45. doi: 10.1086/513658 [Crossref] [ Google Scholar]
- Mishra SP, Tiwary SK, Mishra M, Gupta SK. An introduction of tertiary peritonitis. J Emerg Trauma Shock 2014; 7(2):121-3. doi: 10.4103/0974-2700.130883 [Crossref] [ Google Scholar]
- Arguedas MR, Fallon MB. Spontaneous bacterial peritonitis and its complications. In: Carpenter CCJ, Griggs RC, Loscalzo J, eds. Cecil Essential of Medicine. 6th ed. WB Saunders; 2004. p. 414-5.
- Sazhin VP, Avdovenko AL, Iurishchev VA. [Current trends in surgical treatment of peritonitis]. Khirurgiia (Mosk) 2007(11):36-9.
- Henry MM, Thompson JN. Surgical Aspects of Acute abdominal conditions. In: Henry MM, Thompson JN. Clinical Surgery. London: WB Saunders; 2001. p. 315-26.
- Swenson RM, Lorber B, Michaelson TC, Spaulding EH. The bacteriology of intra-abdominal infections. Arch Surg 1974; 109(3):398-9. doi: 10.1001/archsurg.1974.01360030050013 [Crossref] [ Google Scholar]
- Bartlett JG. Intra-abdominal sepsis. Med Clin North Am 1995; 79(3):599-617. doi: 10.1016/s0025-7125(16)30059-1 [Crossref] [ Google Scholar]
- Jalali SA, Jalali SM. Segmental infarction of omentum-a case report. Iran J Public Health 2001; 30(3-4):147-8. [ Google Scholar]
- Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50(2):133-64. doi: 10.1086/649554 [Crossref] [ Google Scholar]
- Baron Barshak M. Antimicrobial approach to intra-abdominal infections in adults. Available from: https://www.uptodate.com/contents/antimicrobial-approach-to-intra-abdominal-infections-in-adults.
- Livermore DM, Carter MW, Bagel S, Wiedemann B, Baquero F, Loza E. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob Agents Chemother 2001; 45(6):1860-7. doi: 10.1128/aac.45.6.1860-1867.2001 [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151(4):264-9. doi: 10.7326/0003-4819-151-4-200908180-00135 [Crossref] [ Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. doi: 10.1136/bmj.d5928 [Crossref] [ Google Scholar]
- Namias N, Solomkin JS, Jensen EH, Tomassini JE, Abramson MA. Randomized, multicenter, double-blind study of efficacy, safety, and tolerability of intravenous ertapenem versus piperacillin/tazobactam in treatment of complicated intra-abdominal infections in hospitalized adults. Surg Infect (Larchmt) 2007; 8(1):15-28. doi: 10.1089/sur.2006.030 [Crossref] [ Google Scholar]
- Dela Pena AS, Asperger W, Köckerling F, Raz R, Kafka R, Warren B. Efficacy and safety of ertapenem versus piperacillin-tazobactam for the treatment of intra-abdominal infections requiring surgical intervention. J Gastrointest Surg 2006; 10(4):567-74. doi: 10.1016/j.gassur.2005.06.015 [Crossref] [ Google Scholar]
- Solomkin JS, Yellin AE, Rotstein OD, Christou NV, Dellinger EP, Tellado JM. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg 2003; 237(2):235-45. doi: 10.1097/01.sla.0000048551.32606.73 [Crossref] [ Google Scholar]
- Tellado J, Woods GL, Gesser R, McCarroll K, Teppler H. Ertapenem versus piperacillin-tazobactam for treatment of mixed anaerobic complicated intra-abdominal, complicated skin and skin structure, and acute pelvic infections. Surg Infect (Larchmt) 2002; 3(4):303-14. doi: 10.1089/109629602762539535 [Crossref] [ Google Scholar]
- Lucasti C, Jasovich A, Umeh O, Jiang J, Kaniga K, Friedland I. Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra-abdominal infection: a phase III, prospective, multicenter, randomized, double-blind, noninferiority study. Clin Ther 2008; 30(5):868-883. doi: 10.1016/j.clinthera.2008.04.019 [Crossref] [ Google Scholar]
- Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis 2013; 57(4):e22-e121. doi: 10.1093/cid/cit278 [Crossref] [ Google Scholar]
- Fuchs PC, Barry AL, Brown SD. In vitro activities of ertapenem (MK-0826) against clinical bacterial isolates from 11 North American medical centers. Antimicrob Agents Chemother 2001; 45(6):1915-8. doi: 10.1128/aac.45.6.1915-1918.2001 [Crossref] [ Google Scholar]
- Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet 2005; 366(9498):1695-703. doi: 10.1016/s0140-6736(05)67694-5 [Crossref] [ Google Scholar]