Avicenna Journal of Clinical Microbiology and Infection. 7(2):50-55.
doi: 10.34172/ajcmi.2020.11
Original Article
Molecular Evidence on Theileria annulata Infection and Ixodid Ticks Infestation in the Cattle of Kurdistan Province, West of Iran
Shadieh Afrasiabian 1  , Mohammad Yakhchali 2, *
, Mohammad Yakhchali 2, * 
Author information:
1Ph.D. Candidate of Parasitology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
2Department of Pathobiology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
*
Corresponding author: Mohammad Yakhchali, Department of Pathobiology, Faculty of Veterinary Medicine, Urmia University, Nazlu Campus, Sero Road, Urmia, Iran; Postal Code: 5756-151818, Tel: + (98) 44 32752844, Fax: + (98) 44 32771926, Email:
m.yakhchali@urmia.ac.ir
Abstract
Background: Bovine theileriosis is an important disease in Iran and throughout the world with economic losses in Iranian cattle husbandry. The aim of the current study was to determine prevalence and geographic distribution of Theileria annulata infection in cattle and ixodid ticks species diversity in Kurdistan Province, West of Iran.
Methods: A total number of 193 blood samples were randomly taken from jugular vein. Ixodid ticks were also collected from body surface of examined cattle in three sub-areas of the region, i.e. north, center and south. The genomic DNA was extracted and PCR was performed to amplify a 721-bp-long fragment of the 30 Kilo Dalton major merozoite surface antigen of T. annulata.
Results: The overall prevalence was 50.2% (97/193) with lymphadenopathy (54.4%) and petechia in mucosal membrane (95%) of cross-breed cattle (24.9%) aged <3 year in north part of the region (82%). Of all cattle infected with T. annulata, 9.3% (18/193) were infested with a total of 147 unfed ixodid ticks. The ixodid ticks indices was 8.17. Eight species of ixodid ticks of two genus, i.e. Hyalomma (52.9%) and Rhipicephalus (23.3%) were identified. The predominant infesting tick in all examined cattle was R. sanguineus (12%, 23/193) in south area of the region.
Conclusions: The results revealed that T. annulata infection was prevalent and ixodid ticks abundance, geographic distribution and the variety of species were wide in this part of Iran.
Keywords: Theileria annulata, Ixodid tick, Cattle
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Theileriosis is a parasitic disease caused by an obligate intracellular protozoan genus Theileria (Apicomplexa, Piroplasmida) and transmitted by ixodid ticks in the ruminants of the tropical and sub-tropical areas of the world and Iran (1). Theileria annulatais the cause of tropical theileriosis in Mediterranean regions, North Africa, South and South-West of Europe, Middle East, China, and Central Asia (2). Other species with moderate pathogenicity in the cattle are T. sergenti, T. buffeli, and T. orientalis from Asia and Europe, as well as T. mutans, T. thaortragi, and T. wulfartia from Africa (3). The causal agent of bovine theileriosis is T. annulata in Iran. Theileria orientalis also exists in the north part of the country (4).
According to Guglielmone et al (5), 702 out of 896 species of ticks belong to ixodid ticks (Acarina, Metastigmata). Approximately 10% of ixodid ticks re fed on domestic animals, particularly cattle, buffaloes, sheep, and goats (6). The hard ticks fauna and the role of some of them in the transmission of T. annulata were first reported in 1949 and 1972 in Iran, respectively (7). In addition, the genusHyalomma was reported as the vector of bovine theileriosis in different parts of Iran (6,8,9). The species of H. anatolicum anatolicum (Koch, 1844), H. anatolicum excavatum (Koch1844), H. asiaticum asiaticum (Schulze and Schlottke, 1930), H. dromedarii(Koch 1844), H. detritum (Schulz, 1919), and H. marginatum are common ixodid ticks in Iran (7).
The accurate diagnosis of theileriosis is essential in epidemiological studies (10). Several laboratory methods (i.e., histopathology, immunology bioassay, and molecular tools) are now available for the detection of T. annulata infection in cattle. Of those, thin blood smear and lymph node examination were the most frequent methods for detecting acute theileriosis in Iranian cattle for many decades (4). However, these methods had low sensitivity due to difficulties in Theileriaspecies discrimination and asymptomatic carrier detection (11). Accordingly, DNA-based techniques such as polymerase chain reaction (PCR), Semi-nested PCR, Nested PCR, and PCR-restriction fragment length polymorphism were developed to detect T. annulata infections in cattle with low levels of parasitemia in the chronic stage and carriers (10,12).
There are suitable climate conditions for ixodid ticks infestation as the main source of the Theileria infection for cattle in different parts of Iran (13). Based on the geographic distribution of ixodid ticks and bovine theileriosis in different parts of Iran, the collection of accurate data in this regard is important for assessing the potential Theileria infection risk for cattle (8). In addition to industrial cattle farming, traditional breeding in the rural areas of Iran is usual. Additionally, global warming may affect the climate conditions and habitats of Iran, and new ixodid tick species may be anticipated to spread into Iran. Thus, the role of ixodid ticks in the epidemiology of important humans and livestock diseases should be examined in detail.
Objectives
This investigation aimed to determine the prevalence of T. annulata and ixodid ticks infestation in the cattle of different parts of Kurdistan province, located in the west of Iran.
Methods
Field Study Area
Kurdistan province is located in the west part of Iran (35◦19’N and 46◦59’E) and is an important livestock production region in the west of Iran. There is a climatic trilogy of cold, temperate, and hot with annual relative humidity of 49.1%, relative rainfall of 375.6 mm, and mean temperature of +14.4ºC due to mountainous areas in the region according to the Iranian Metrological Organization. This part of the country is ecologically considered as a semi-arid area. Economically, cattle breeding plays an important role in this province.An average population of 269 692 cattle is reared in this region (Iranian Veterinary Organization, 2015).
Collection of Blood Samples
Overall, 193 cattle (58.6% female and 41.4% male) were randomly selected and clinically examined during the study in 2018. The blood samples were also taken from the jugular vein and stored at -20°C until DNA extraction. Then, blood smears were prepared, stained with Giemsa, and microscopically examined at 1000×. Data pertaining to each examined animal (i.e., animal location, management system, time of day, tag number, breed, age, and gender) were recorded to determine parasitemia. The cattle were raised and grazed during the day following traditional practices. Further, cattle breeds were Holstein (28%), cross-breed (40%), and indigenous (32%). The place of the study was divided into three sub-areas, namely, north (82 cattle in 13 villages of Saqez and in 13 villages of Divandareh suburb), center (53 cattle in 13 villages of Sanandaj suburb), and south (58 cattle in 13 villages of Kamyaran suburb), the related data are shown in Figure 1 and Table 1. The examined animals were also divided into three age groups based on the eruption of permanent incisor teeth (14), the details of which are presented in Table 1.
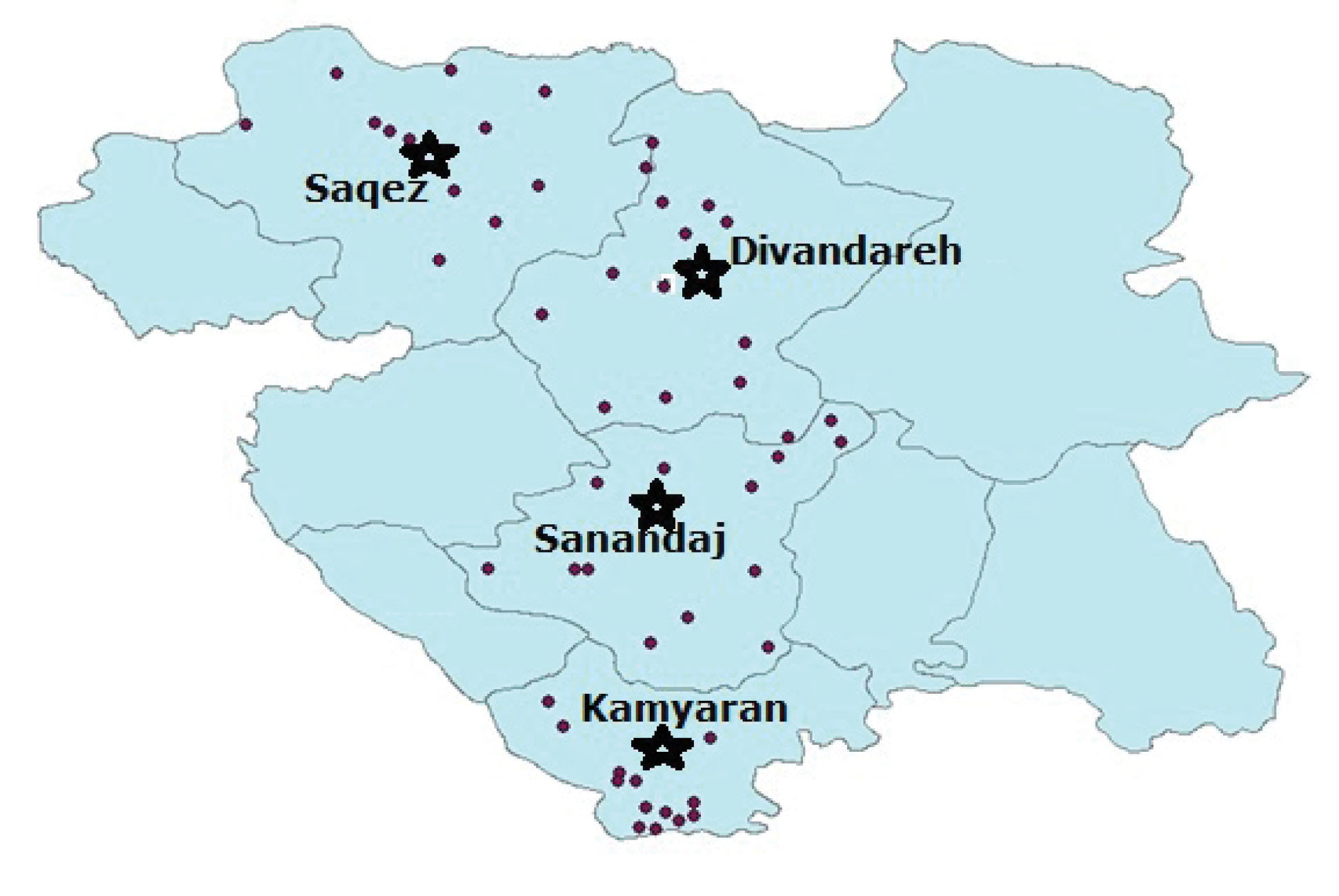
Figure 1.
Map of sampling areas in Kurdistan Province, West of Iran.
.
Map of sampling areas in Kurdistan Province, West of Iran.
Table 1.
The Prevalence of Theileria annulata in Cattle According to Age, Gender, and Breed of the Examined Cattle
|
Geographic Distribution
|
N
|
P (%, n/N)
|
Gender (%)
|
Age (year, %)
|
Breed (%)
|
| North |
82 |
21.2 |
M
6.7 |
F
c
14.5 |
1>
3.6 |
3-1
8.8 |
3<
8.8 |
H
6.2 |
CB
6.2 |
IG
8.8 |
| Center |
53 |
15 |
4.7 |
10.4 |
4.3 |
6.2 |
6.7 |
4.2 |
4.7 |
6.2 |
| South |
58 |
14 |
5.2 |
8.8 |
4.2 |
3.6 |
6.2 |
1 |
14 |
0.5 |
| Total |
193 |
50.2 |
16.6 |
33.7 |
12.1 |
18.6a |
21.7 |
11.4 |
24.9b |
15.5 |
Note. CB: Cross-breed; H: Holstein; IG: Indigenous; N: Number of examined animals; P: Prevalence.
aχ2=0.87, P > 0.05; bχ2=5.29, P > 0.05; cχ2=0.3, P > 0.05; dχ2= 4.19, P > 0.05.
Collection of Ixodid Ticks
Ixodid ticks were collected in early mornings and evenings from the body surface of the examined cattle but never from the ground in order to avoid the accidental occurrence from other livestock. Next, ixodid ticks were directly collected from the body surface of the examined animals (i.e., the head, neck, ear, perinea, mammary glands, testes, tail, and groin by rubbing alcohol pads surrounding the skin) to remove embedded living ticks using forceps and gloves (7). During tick collection, care was taken to ensure that the mouthparts were not left behind during the traction with thumb forceps. The data pertaining to predilection sites, the stages of the collected ixodid ticks (i.e., larva, nymph, and adult), and the recent use of acaricides were recorded as well. The collected ixodid ticks were placed into 70% ethanol (Merck, Germany) in glass vials and labelled with the date and place of collection. Next, the ixodid ticks species were identified using identification keys as described by Soulsby (15) and Walker et al (16).
Molecular Procedures
DNA Extraction
The genomic DNA extraction of T. annulata in whole blood samples was performed by using the genomic DNA purification kit (Thermo Fisher Scientific, USA).
Polymerase Chain Reaction
A pair of primers (Forward: 5’GTAACCTTTAAAAACGT-3’ and Reverse: 5’GTTACGAACATGGGTTT-3’) was used to amplify a fragment length of 721 bp of the large subunit rRNA gene sequence encoding the 30-kDa major merozoite surface antigen of T. annulata(17). Furthermore, PCR was carried out in a 25 μL reaction mixture containing 2 μL (100 ng) of genomic DNA, 1.5 U of Taq DNA polymerase (Fermentas, Germany), 50 mM of each dNTPs (Cinna Gen, Iran), 2 mM of MgCl2, 2.5 μL of PCR buffer (10×), and 0.2 μM of each primer with positive and negative controls. The reaction was performed in the Applied Biosystem thermal cycler. The samples were subjected to an initial denaturation step at 94ºC for 2 minutes, followed by 30 cycles of 1 minute at 94ºC, 1 minute at 48ºC, and 1 minute at 72ºC, and a final extension step at 72 ºC for 5 minutes. A volume of 10 μL of each PCR product was analyzed by electrophoresis on 2% (w/v) agarose gel for 90 minutes at 85 V. Finally, the gels were visualized by staining with ethidium bromide (1 μg/mL).
Statistical Evaluation
The non-parametric Chi-square test was used to evaluate the association between prevalence and all data (i.e., age, gender, and breed) pertaining to the examined cattle and collected ixodid ticks (SPSS 16.0, Chicago, IL, USA). A probability score of P≤0.05 was considered statistically significant.
Results
Clinical Findings
Parasitemia in the infected cattle of all three regions ranged from 11.1% to 48.3%. Of all examined blood samples, 22 (11.4%) cases were positive for piroplasm with lymphadenopathy (54.4%) and petechia in the mucosal membrane (95%) in cross-breed cattle aged <3 years (Tables 1 and 2).
Table 2.
Prevalence of Clinical Signs ofTheileria annulataInfection in Examined Cattle
Geographic
Distribution
|
Parasitemia
(%)
|
Clinical Findings (%)
|
|
|
|
S
|
F
|
C
|
Pe
|
Pa
|
J
|
Ly
|
| North |
12.2 |
70 |
30 |
75 |
95 |
95 |
70 |
53.3 |
| Center |
48.3 |
70 |
50 |
70 |
100 |
92 |
70 |
55 |
| South |
11.1 |
50 |
50 |
60 |
90 |
95 |
60 |
55 |
| Total |
11.4 |
63.3 |
46.7 |
68.3 |
95 |
94 |
66.7 |
54.4 |
Note: C: Cough; F: Fever; J: Jaundice; Ly: Lymphadenopathy; Pa: Pale mucosa; Pe: Petechia; S: Stagger; n: Animals infected with Theileria annulata; N: Total examined animals.
Tick infestation of Examined Cattle
Of 147 ixodid ticks, there were two genera of Hyalomma (52.9%) and Rhipicephalus (23.3%) with eight species including Hyalomma anatolicum anatolicum (3.6%), H. anatolicum excavatum (5.2%), H. asiaticum asiaticum(4.2%), H. marginatum marginatum(3.6%), H. marginatum turanicum (6.2%), H. detritum (4.7%), Rhipicephalus bursa (6.2%), and R. sanguineus(12%). In all areas of the region, Hyalomma and Rhipicephalus were found on 18 (9.3%) and 27 (14%) infected cattle with T. annulata (P > 0.05, χ2= 0.003), respectively (Table 3). The ixodid ticks index (i.e., the number of ticks per infested animal) was 0.76. Moreover, the highest tick infestation belonged to R. sanguineus (12%) in the south area of the region (18.1%, P > 0.05). The most infested body sites in male and female cattle were testis (25.9%) and mammary glands (25.9%), respectively (Table 3).
Table 3.
Identified Ixodid Ticks Species and Body Distribution in the Examined Cattle in Kurdistan Province, Iran
|
Geographic Distribution
|
No. of Unfed
Ticks
|
Tick Genus
(%)
|
Tick Species
(%)
|
Body Site Distribution
(%)
|
|
|
|
H
|
R
|
Rs
|
Rb
|
Hmt
|
Hd
|
Hmm
|
Has
|
Hae
|
Haa
|
E
|
I
|
M
|
Ta
|
Te
|
| North |
53 |
24.9 |
3 |
7.8 |
3.1 |
3.1 |
4 |
1.6 |
1 |
0 |
0.5 |
0 |
26.5 |
26.5 |
20.4 |
26.5 |
| Center |
50 |
23.3 |
3 |
4.2 |
3.1 |
3.1 |
1.6 |
1.6 |
2.6 |
0 |
0 |
10 |
22 |
24 |
20 |
24 |
| South |
44 |
4.7 |
18.1 |
0 |
0 |
0 |
1 |
0.5 |
0.5 |
5.2 |
3.1 |
4.5 |
18.8 |
27.2 |
22 |
27.2 |
| Total |
147 |
52.9 |
23.3 |
12 |
6.2 |
6.2 |
4.7 |
3.6 |
4.2 |
5.2 |
3.6 |
4.8 |
22.4 |
25.9 |
21 |
25.9 |
Note: E: Ear; H: Hyalomma; Haa:Hyalomma anatolicum anatolicum; Hae: Hyalomma anatolicum excavatum; Has: Hyalomma asiaticum asiaticum; Hmm: Hyalomma marginatum marginatum; Hmt:Hyalomma marginatum turanicum; Hd:Hyalomma detritum; I: Inner thighs; M: Mammary glands; R: Rhipicephalus; Rb: Rhipicephalus bursa; Rs: Rhipicephalus sanguineus; Ta: tail; Te: Testis.
Molecular Findings
The PCR-based assay revealed that 97 out of 193 blood samples (50.3%) were infected with T. annulata (Figure 2, Table 1). Furthermore, the highest prevalence of T. annulata infection was found in cattle over three years of age (21.8%, 42/193) in the north part of the region (8.8%, P > 0.05, χ2=0.87), the details of which are provided in Table 1. The examined cross-breed cattle had the highest infection with T. annulata (23.2%, 95% CI = 1.17-13.6%). Based on the results (Table 1), there was no significant difference between prevalence and gender, including 16.6% males and 36.7% females, (P > 0.05, χ2= 0.3). Eventually, the breed of the infected cattle had no association with the prevalence of the T. annulata infection (P> 0.05, χ2= 5.29).
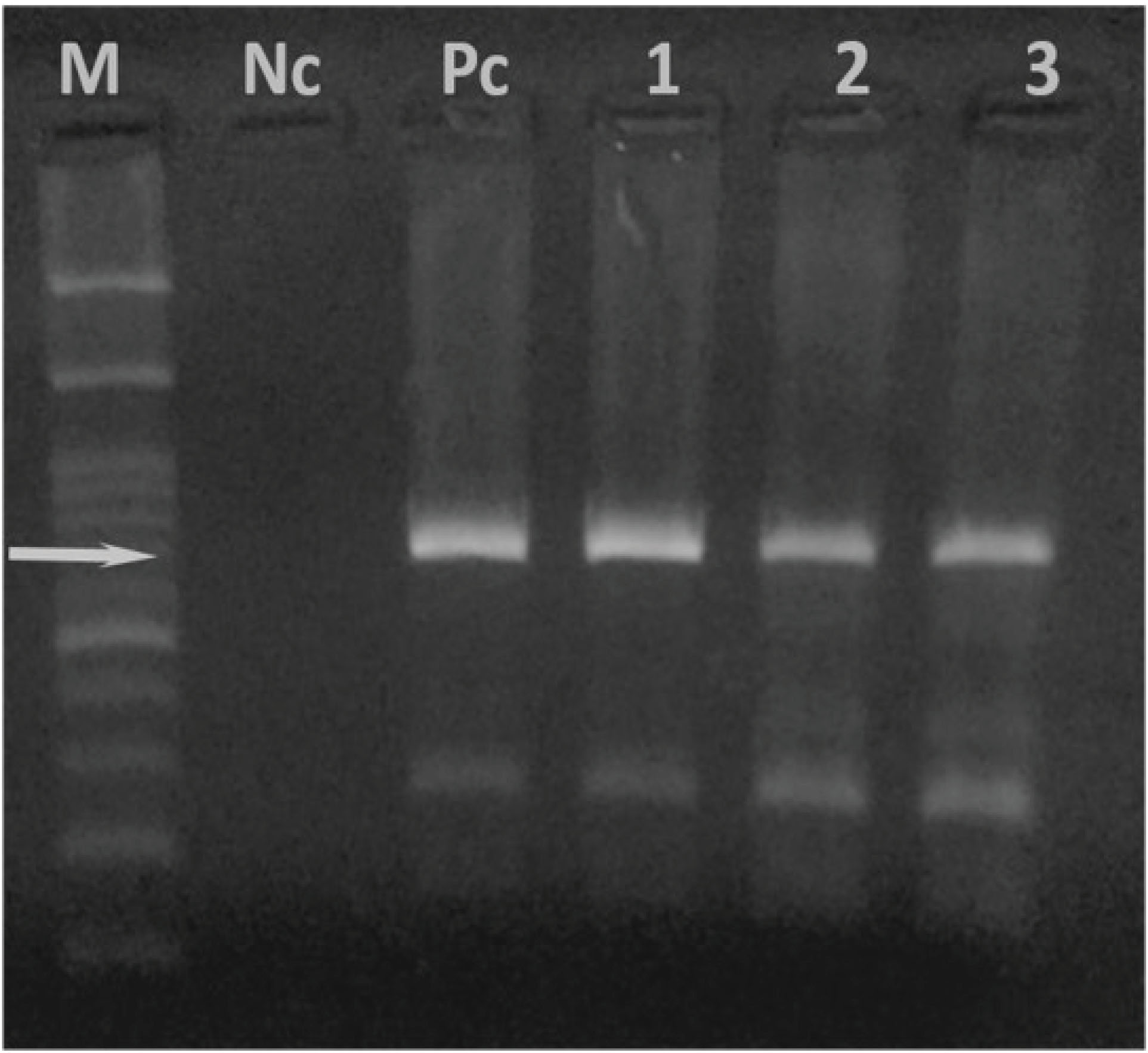
Figure 2.
PCR-amplified fragment length of 721 bp of 30KDa major merozoite surface antigen of Theileria annulata (Lanes 1-3). M: DNA marker 100bp; Nc: negative control; Pc: positive control.
.
PCR-amplified fragment length of 721 bp of 30KDa major merozoite surface antigen of Theileria annulata (Lanes 1-3). M: DNA marker 100bp; Nc: negative control; Pc: positive control.
Discussion
Bovine theileriosis is regarded as an important protozoan disease in cattle. The first case of the Theileria infection in Iranian cattle was recorded in 1935 (4). With regard to the importance of the T. annulatainfection in Iranian cattle, there are various reports on high mortality (5%-90%), morbidity (40-80%), and economic losses such as animal husbandry, productions, and tick control programs (4,7,18,19).
Based on the findings of the present study, the T. annulata infection had the highest prevalence in cross-breed old female cattle in the north area of the region. The prevalence of 50.3% in examined cattle was extremely higher than those reported from the south (31.5%), north-east (20%), north-west (18.65%), north (7.5%), south-east (5.6%), and the west (2.17%) of Iran (6,20-26). The prevalence of the T. annulatainfection (70% and 1.28-39.28%) was also reported in other neighboring countries of Iran like Iraq and Turkey, respectively (4,12,27,28).
In the current study, Hyalomma and Rhipicephalus were found to be prevalent ixodid ticks with eight species. So far, 14 species of family Ixodidae ticks have been reported from different areas of Iran with indices of 1-2, 2.9, and 0.4-4.6 in the north-west, south, and west of Iran, respectively (4,7,29-35).Hyalomma anatolicum excavatum and H. marginatum were reported as important vectors of T. annulata in the semi-arid areas of the Mediterranean region (4,36). Hyalomma species play an important role as the vectors of tropical theileriosis in Iranian cattle (4,33). H. marginatum marginatum was also reported from different areas of the country. However, H. detritumexists on the Caspian Sea coast of northern Iran (6,29,30). In this region like the other areas of the west of Iran, R. sanguineus was the prevalent ixodid tick in the examined cattle (34). In contrast, the predominant tick infestation in cattle was H. anatolicum anatolicum from the east, center, north-east, south, and west of Iran (4,6,7,9,10,29,30,32,35) and H. anatolicum asiaticum from the north-west of Iran (31). In Turkey, H. anatolicum anatolicum was also reported as a prevalent ixodid tick from cattle (37). These differences may be due to various factors like sampling and investigation methods, geographic conditions, temperature, and adaption of ticks species with different climate conditions (10,18,38,30,39). In this study, the R. sanguineus group was recorded in the examined cattle with similar results obtained for the north, south, and east areas of Iran (7,21,40).
In this work, the preferred body sites of identified ixodid ticks were testis and mammary glands in male and female cattle, respectively. In addition, predilection sites for ixodid ticks were reported from the same parts of the body surface of the examined animals in other investigations in the north and north-west of Iran (30,32). It may be due to ixodid ticks preferring warm, moist, and hidden sites with a good vascular supply and thin skin (7,41).
Conclusions
Based on the results of this work, the Hyalommaspecies may play an important role in the transmission of T. annulatain this part of Iran. In addition, the current investigation gives an update on the prevalence of ixodid ticks in the west of Iran. Furthermore, these may provide a valuable basis for designing and launching an all-round control program in this part of the country. Accordingly, it is recommended to conduct further studies in order to determine what economic losses are caused by these parasites in the region.
Conflict of Interests
The authors declare no conflict of interests associated with this study.
Acknowledgments
This study was financially supported by the Faculty of Veterinary Medicine at Urmia University. The authors wish to acknowledge A. Vaziry, Z. Karimikuredestani, A. Badali, and the members of Iranian Veterinary Organization, Kurdistan branch for their technical supports.
Ethical Approval
All ethical standards were respected in this study. The samples were taken from the cattle according to the recommendation of the Faculty of Veterinary Medicine, Urmia University and official rules related to animal ethics and welfares.
References
- Norouzi F, Hashemitabar GH, Razmi GR. Detection of midgut antigens of Hyalomma anatolicum anatolicum tick using SDS-PAGE and Western blot. Iran J Vet Res 2007; 8(2):166-9. [ Google Scholar]
- Hashemi-Fesharki R, Najjar E, Bozorgi S, Esmaeil-Nia K, Habibi GR, Bordbar N. PCR-based detection of Theileria infection and molecular characterization of Tams1 T annulata vaccine strain. Arch Razi Inst 2007; 62(2):83-9. doi: 10.22092/ari.2007.103772 [Crossref] [ Google Scholar]
- Moretti A, Mangili V, Salvatori R, Maresca C, Scoccia E, Torina A. Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: a preliminary study. Vet J 2010; 184(3):346-50. doi: 10.1016/j.tvjl.2009.03.021 [Crossref] [ Google Scholar]
- Sohrabi S, Yakhchali M, Ghashghaei O. PCR-RELP for detecting of Theileria annulata infection in cattle and Hyalomma species in Kermanshah province, Iran. Arch Razi Inst 2015; 70(1):7-12. doi: 10.7508/ari.2015.01.002 [Crossref] [ Google Scholar]
- Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Penañ A, Horak IG. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2010; 2528:1-28. doi: 10.11646/zootaxa.2528.1.1 [Crossref] [ Google Scholar]
- Razmi GR, Ebrahimzadeh E, Aslani MR. A study about tick vectors of bovine theileriosis in an endemic region of Iran. J Vet Med B Infect Dis Vet Public Health 2003; 50(6):309-10. doi: 10.1046/j.1439-0450.2003.00677.x [Crossref] [ Google Scholar]
- Yakhchali M, Bahramnejad K, Almasi O. Ticks (Acari: Ixodida: Ixodidae and Argasidae) abundance and associated risk factors for animals in the natural habitat of Sanandaj suburb, Iran. Int J Acarol 2012; 38(4):353-61. doi: 10.1080/01647954.2011.651155 [Crossref] [ Google Scholar]
- Azizi H, Shiran B, Farzaneh-Dehkordi A, Salehi F, Taghadosi C. Detection of Theileria annulata by PCR and its comparison with smear method in native carrier cows. Biotechnology 2008; 7(3):574-7. doi: 10.3923/biotech.2008.574.577 [Crossref] [ Google Scholar]
- Tavassoli M, Tabatabaei M, Esmaeil Nejad B, Hasani Tabatabaei M, Najafabadi A, Pourseyed SH. Detection of Theileria annulata by the PCR-RFLP in ticks (Acari, Ixodidae) collected from cattle in West and North-West Iran. Acta Parasitol 2011; 56(1):8-13. doi: 10.2478/s11686-011-0001-6 [Crossref] [ Google Scholar]
- Naoman V, Abdi-Goudarzi M, Nabinejad AR, Heidari MR, Khalilifard M. Identification of hard ticks of domestic ruminants in two ecological zones of Isfahan province, Iran. Pajouhesh-va-Sazandegi 2008; 77:88-95. [ Google Scholar]
- Kirvar E, Wilkie G, Katzer F, Brown CG. Theileria lestoquardi--maturation and quantification in Hyalomma anatolicum anatolicum ticks. Parasitology 1998; 117(Pt 3):255-63. doi: 10.1017/s0031182098002960 [Crossref] [ Google Scholar]
- Altay K, Aydin MF, Dumanli N, Aktas M. Molecular detection of Theileria and babesia infections in cattle. Vet Parasitol 2008; 158(4):295-301. doi: 10.1016/j.vetpar.2008.09.025 [Crossref] [ Google Scholar]
- Hamidinejat H, Razi Jalali M, Rasouli A, Nourmohammadi M. Study on some hematologic and biochemical factors in asymptomatic cattle infected with Theileria annulata. Iranian Veterinary Journal 2016; 11(4):26-33. [ Google Scholar]
- Smallwood JE. A Guided Tour of Veterinary Anatomy. Philadelphia, Pa: W.B. Saunders; 1992.
- Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. London: Baillière Tindall; 1982.
- Walker AR, Bouattour A, Camicas JL, Estrada-Peña A, Horak IG, Latif AA, Pegram RG, et al. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. 1st ed. Edinburgh, Scotland: Bioscience Reports; 2003.
- d’Oliveira C, van der Weide M, Habela MA, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol 1995; 33(10):2665-9. [ Google Scholar]
- Hashemi-Fesharki R. Control of Theileria annulata in Iran. Parasitol Today 1988; 4(2):36-40. doi: 10.1016/0169-4758(88)90062-2 [Crossref] [ Google Scholar]
- Ghadrdan-Mashhadi AR, Razmi-Jalali M, Kavand M. To determine serum ALP, AST, GGT AND bilirubin changes in theileriotic cows (Mediterranean coast fever). Journal of the Faculty of Veterinary Medicine, University of Tehran 2006; 61:23-8. [ Google Scholar]
- Mozafari A, Noorollahifard S, Mohammadi V. A survey of bovine theileriosis in Zahedan cattle. Iranian Veterinary Journal 2008; 3:67-70. [ Google Scholar]
- Razmi GR, Barati F, Aslani MR. Prevalence of Theileria annulata in dairy cattle in Mashhad area, Iran. J Vet Parasitol 2009; 23(1):81-3. [ Google Scholar]
- Hoghooghi-Rad N, Ghaemi P, Shayan P, Eckert B, Sadr-Shirazi N. Detection of native carrier cattle infected with Theileria annulata by Semi-nested PCR and smear method in Golestan province of Iran. World Appl Sci J 2011; 12(3):317-23. [ Google Scholar]
- Saleh Zadeh S, Fathi S, Mirzaei Dehaghi M, Norouzi Asl E, Asghary Nezhad H. Survey of Theileria annulata and Anaplasma marginale in cattle in Kerman area, Southeast of Iran. Sci Parasitol 2011; 12(2):61-6. [ Google Scholar]
- Jalali SM, Khaki Z, Kazemi B, Rahbari S, Shayan P, Bandehpour M. Molecular detection and identification of Theileria species by PCR-RFLP method in sheep from Ahvaz, Southern Iran. Iran J Parasitol 2014; 9(1):99-106. [ Google Scholar]
- Khodabandeh S, Razmi GH. Molecular detection of Theileria species and its vectors in cattles in Yazd area by Semi-nested PCR method. J Vet Res 2015; 70(3):249-53. [ Google Scholar]
- Narimani B, Hoghooghi-Rad N, Shayan P, Rahbari S. Molecular and microscopic detection of Theileria spp among cattle and buffaloes in West Azarbaijan, Iran. Arch Razi Inst 2017; 72(3):189-95. doi: 10.22092/ari.2017.111605 [Crossref] [ Google Scholar]
- Aktas M, Altay K, Dumanli N. A molecular survey of bovine Theileria parasites among apparently healthy cattle and with a note on the distribution of ticks in eastern Turkey. Vet Parasitol 2006; 138(3-4):179-85. doi: 10.1016/j.vetpar.2006.01.052 [Crossref] [ Google Scholar]
- Mohammad Al-Saeed AT, Omer LT, Abdo J, Habibi G, Salih DA, Seitzer U. Epidemiological studies on tropical theileriosis (Theileria annulata infection of cattle) in Kurdistan region, Iraq. Parasitol Res 2010; 106(2):403-7. doi: 10.1007/s00436-009-1675-7 [Crossref] [ Google Scholar]
- Mazlum Z. Tick species of Iran, its distribution, host and seasonal activity. Journal of Veterinary Faculty of University Tehran 1972; 19:1-28. [ Google Scholar]
- Rahbari S, Nabian S, Shayan P. Primary report on distribution of tick fauna in Iran. Parasitol Res 2007; 101 Suppl 2:S175-7. doi: 10.1007/s00436-007-0692-7 [Crossref] [ Google Scholar]
- Telmadarraiy Z, Bahrami A, Vatandoost H. A survey on fauna of ticks in West Azerbaijan province, Iran. Iran J Public Health 2004; 33(4):65-9. [ Google Scholar]
- Yakhchali M, Haji Hasanzadehzarza SH. Study on some ecological aspects and prevalence of different species of hard ticks (Acarina: Ixodidae) on cattle, buffalo, and sheep in Oshnavieh suburb. Pajouhesh-va-Sazandegi 2004; 17(2):30-5. [ Google Scholar]
- Yakhchali M, Azizi C. A study on ixodid ticks infestation of cattle, sheep, and goats in Bukan suburb, Iran. Iranian Veterinary Journal 2007; 3:100-4. [ Google Scholar]
- Sohrabi S, Yakhchali M, Ghashghaei O. Hard ticks (Acarina: Ixodidae) diversity in the natural habitat of Iranian domestic ruminants: a provincial study in Kermanshah. J Vet Res 2013; 68(1):39-46. [ Google Scholar]
- Yakhchali M, Rostami A, Esmailzadeh M. Diversity and seasonal distribution of ixodid ticks in the natural habitat of domestic ruminants in north and south of Iran. Rev Med Vet 2011; 162(5):229-35. [ Google Scholar]
- Viseras J, Hueli LE, Adroher FJ, García-Fernández P. Studies on the transmission of Theileria annulata to cattle by the tick Hyalomma lusitanicum. Zentralbl Veterinarmed B 1999; 46(8):505-9. doi: 10.1111/j.1439-0450.1999.tb01242.x [Crossref] [ Google Scholar]
- Aktas M, Dumanli N, Angin M. Cattle infestation by Hyalomma ticks and prevalence of Theileria in Hyalomma species in the east of Turkey. Vet Parasitol 2004; 119(1):1-8. doi: 10.1016/j.vetpar.2003.10.013 [Crossref] [ Google Scholar]
- Estrada-Peña A, Santos-Silva MM. The distribution of ticks (Acari: Ixodidae) of domestic livestock in Portugal. Exp Appl Acarol 2005; 36(3):233-46. doi: 10.1007/s10493-005-5107-9 [Crossref] [ Google Scholar]
- Kabir MHB, Mondal MMH, Eliyas M, Mannan MA, Hashem MA, Debnath NC. An epidemiological survey on investigation of tick infestation in cattle at Chittagong district, Bangladesh. Afr J Microbiol Res 2011; 5(4):346-52. [ Google Scholar]
- Nabian S, Rahbari S, Shayan P, Haddadzadeh HR. Current status of tick fauna in North of Iran. Iran J Parasitol 2007; 2(1):12-7. [ Google Scholar]
- Muchenje V, Dzama K, Chimonyo M, Raats JG, Strydom PE. Tick susceptibility and its effects on growth performance and carcass characteristics of Nguni, Bonsmara and Angus steers raised on natural pasture. Animal 2008; 2(2):298-304. doi: 10.1017/s1751731107001036 [Crossref] [ Google Scholar]