An In Vitro Investigation of the Effects of the Glucocorticoid on Leishmania major Amastigotes
Avicenna J Clin Microbiol Infect, 6(4), 127-132; DOI:10.34172/ajcmi.2019.23
Original Article
An In Vitro Investigation of the Effects of the Glucocorticoid on Leishmania major Amastigotes
Elham Hosseini Renani1, Simindokht Soleimanifard1, Seyyed Hossein Hejazi1, Zahra Ghayour Najafabadi1*
1
Department of Parasitology and Mycology, School of Medicine Isfahan University of Medical Sciences, Isfahan, Iran
*Corresponding author:
Zahra Ghayour Najafabadi,
Hezar Jerib Street, Medical
Parasitology Department of
Parasitology and Mycology,
School of Medicine
Isfahan University of
Medical Sciences, Isfahan,
Iran, 8174673461 Tel:
09133291279
Email: ghayour@med.mui.ac.ir
Abstract
Background: Leishmaniasis is a vector-borne disease caused by Leishmania species. The transcription factor (NF-κB) activates and the innate immune system starts working when this parasite attacks macrophages. In addition, glucocorticoids increase nitric oxide (NO) and INFγ leads to the apoptosis of the cell by inhibiting the NF-κB activity. The aim of this study was the in vitro investigation of the effects of the glucocorticoids on Leishmania major amastigotes.
Methods: Leishmania major was produced in a massive volume. Then, promastigotes penetrated into macrophages and converted to amastigotes by adding promastigotes to the J774 mouse macrophage cell line and providing appropriate conditions. Next, the infected macrophages with L. major parasite were treated with different concentrations of prednisolone and mometasone. After 24 and 48 hours, the effect of the drugs was evaluated based on the average number of amastigotes in the infected macrophages. In addition, the amount of NO and the mean interleukin 12 (IL-12) level secreted by the infected macrophages treated with different concentrations of drugs were measured by Griess reagent and ELISA reader, respectively.
Results: The average number of amastigotes in the infected macrophages treated with different concentrations of the drug was significantly different from the control group. Further, the amount of NO and the mean level of IL-12 secreted by infected macrophages had a direct and significant relationship with different concentrations of drugs, but the results of Tukey post hoc test showed that the reduction in the number of promastigotes was not time-dependent.
Conclusions: In general, prednisolone and mometasone stimulated macrophages increased the IL-12 levels and NO secretion and finally decreased the number of parasites in infected macrophages.
Keywords: Glucocorticoids, Leishmania major, IL 12, Nitric oxide
Background
Leishmaniasis is a common parasitic infection between humans and animals, which is caused by the intracellular parasitic protozoa of the genus Leishmania from the Trypanosomatidae family (1,2). The disease is common in tropical and subtropical regions. Nearly 350 million people are at the risk of leishmaniasis and about two million new cases of leishmaniasis are reported every year (3,4). Cutaneous leishmaniasis and visceral leishmaniasis are the most common forms of the disease. Approximately 90% of all Leishmania infections are localized cutaneous forms, and healing is related to the development of disfiguring scars at the exposed skin areas.
The common treatments of leishmaniasis include pentavalent antimony such as glucantime. Other treatments for leishmaniasis are amphotericin B, paromomycin and miltefosine, metronidazole (5), diamidine (6), pentamidine (7), itraconazole (8), and monomycin (9). Among the mentioned drugs, glucantime is known as the main treatment for this disease.
The side effects of glucantime are increasing liver enzymes and changing the electrocardiogram. These side effects result in specific limitations in the drug dose while parasite drug resistance is increasing in different regions (10-12). In general, the side effects of this drug, the drug resistance of the parasite and the absence of an effective vaccine against leishmaniasis lead to the continuous investigation of leishmaniasis treatments.
Different strategies for the development of leishmaniasis can inhibit several macrophage functions including nitric oxide (NO), phagocytosis, and proliferation, as well as the production of IL-12 (1,13,14). The Th1 lymphocyte, which causes the secretion of IFNγ, IL-2, and TNF, has an important role in the immunity, resistance, and recuperate of the disease while Th2 lymphocytes secreting IL-4, IL-5, IL-10, and IL-13, lead to disease progression and host vulnerability. In addition, IL-12, secreted from macrophages, is an effective substance in the production of the experimental vaccination and treats the L. major infection. Further, it plays a key role in developing the cellular immune by stimulating Th0 cells and differentiating them into Th1 cells, stimulating natural lethal cells, and the generation of IFNγ. Furthermore, NO causes non-oxidative mechanisms inside macrophages, along with intercellular oxidative mechanisms to kill Leishmania major parasites.
In fact, the transcription factor (NF-κB) activates and the innate immune system starts working when this parasite attacks macrophages, which increases the Th2 cytokines and reduces the cytokine IL-2 and NO while causing no apoptosis in the macrophage and parasite surviving (14).
Moreover, glucocorticoids inhibit NF-κβ activity and by preventing destruction (IKβ), lead to the preservation of NF-Kβ in the cytosol (15). This can increase the NO and interferon-gamma and finally leads to the apoptosis of the cell and the death of the parasite.
Therefore, the development of modulating strategies by targeting this transcription factor may provide a new therapeutic tool for treating the leishmaniasis. This study focused on an in vitro investigation of parasite development in macrophages and the effects of the glucocorticoids on L. major amastigotes with prednisolone and mometasone.
Materials and Methods
This experimental study was performed in the Parasitology Laboratory of the Medical School of Isfahan University of Medical Sciences under the ethical code of IR.MUI. REC.1397.3.049.
The first step in this experimental study was the massive production of the L. major parasite. For this purpose, BALB/c mice were inoculated with the Iranian strain of L. major (MRHO/IR/75/ER). After about 4 weeks at the inoculation site, leishmaniasis wound was appeared and used as a source of amastigotes. The isolated samples from the wounds of mice were cultured in a growth medium of the modified Novy-MCNeal-Nicolle with 100 μg/mL streptomycin and 100 IU/mL penicillin G at 24 ± 1˚C. Then, they were transferred to another growth medium of 1640- RPMI enriched with inactive 10% fetal calf serum (FCS) with the mentioned antibiotics and stored at 24 ± 1°C in order to avoid the use of highly passaged parasites and save them until a massive production of amastigotes.
Macrophage Culture and Preparation of the Amastigote- Macrophage In Vitro Model
Subsequently, the J774 mouse macrophage cell line from Pasteur Institute of Iran was used to produce an amastigote-macrophage in vitro model. The cells were isolated from the bottom of the cell culture flask using the cell scraper after massive cell culturing in a RPMI-1640 growth medium enriched with FCS 20%, and penicillin and streptomycin at 37°C and CO2 5%. About 106 cells were transferred to each well with a diameter of 3.5 cm from a 6-well treated cell culture plates containing sterile slides and were incubated at 37°C and CO2 5% for 2 to 3 days after adding 2 mL of RPMI-1640 enriched with FCS 20% complement complex, penicillin, and streptomycin.
The L. major promastigotes were previously counted in the stationary phase and added to each of the wells with a ratio of 7 to 1 (the number of parasites to macrophages). Further, the promastigotes were taken up by macrophages after four hours at 33~34 °C. The extracellular parasites were washed with phosphate-buffered saline and 2 mL of RPMI-1640 enriched growth medium was added to the plates and then the plates were placed at 37 °C for 24 hours to allow them become an amastigote.
Infected Cell Line Treatment With Prednisolone and Mometasone
In the next step, the wells of the cultivating plates were exposed to concentrations of 20, 40, 80, 160, and 320 μg/mL of prednisolone (Sigma p6004) and mometasone (Sigma m4074) as triplicate while three wells without any treatment were considered as the controls. DMSO was used as the solvent for mometasone. More precisely, three wells were exposed to this substance at a concentration of 20 μg/mL to ensure that it was ineffective at the concentration used to dilute the drug. After 24 and 48 hours, the slides were separated from the bottom of the plates and fixed using methanol and stained with Giemsa. In the microscopic investigation step, the number of infected macrophages, as well as the number of intracellular amastigotes was counted, and finally, the average number of amastigotes in each macrophage were calculated by examining 100 macrophages in each drug concentration and compared to the control group. The effect of each treatment on the amastigotes was evaluated using statistical analyses. The supernatant of each well was collected in sterile conditions in small vials and kept for the next steps at -70°C.
Measurement of IL-12 and NO
The amount of IL-12 secreted by the parasite-infected macrophages exposed to different treatments in the supernatant of the culture medium, which was stored at -70°C in the previous step, was measured by the ELISA method using the ELISA kit (Mouse IL-12 Biolegend) according to the kit protocol. Eventually, NO secreted by macrophages in the supernatant of the growth medium when exposed to different concentrations of the drug at 24 and 48 hours was measured by Griess reagent.
Results
The Results of J774 Cell Culturing and Their Infection With Promastigotes
The results showed that 5 to 6 days after culturing J774 cells in 6-well cell culture plates, the cells changed in number, size, and shape (Figure 1a). At this time, promastigotes penetrated into the macrophages and became amastigotes by adding stationary promastigotes to the macrophages and providing other appropriate conditions (Figure 1b).
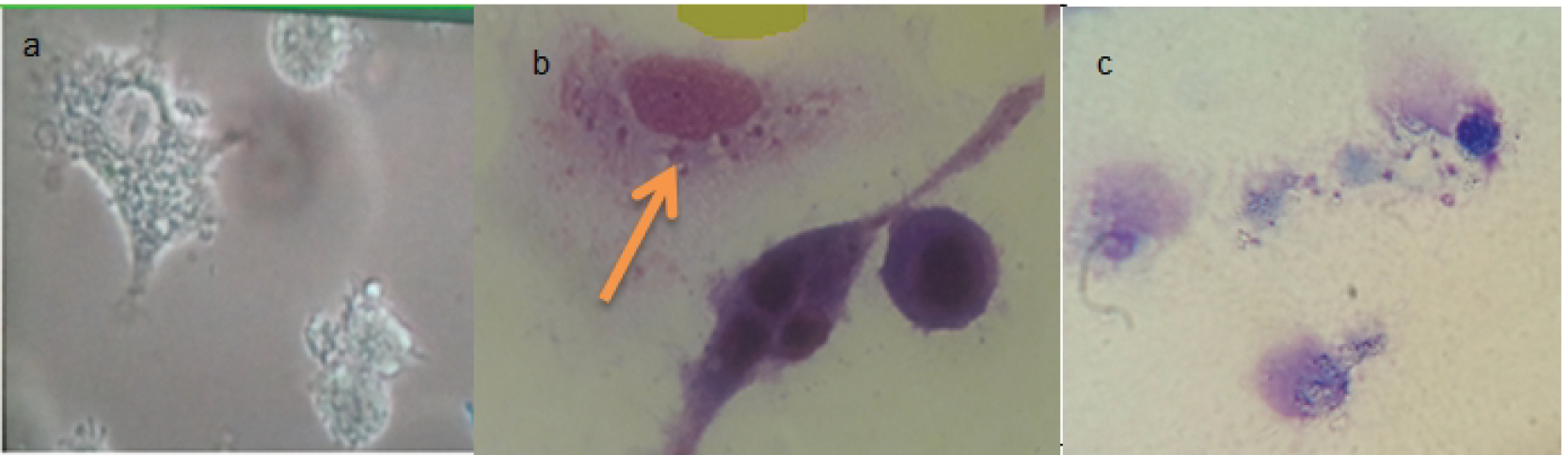
(a) J774 Cell Line Cultured on 100x Magnification Cell Culture Plates After 6 Days of Culture, (b) Macrophages Containing Phagocytic Parasites Transformed From Promastigote to Amastigote, (c) Macrophages Disintegrated due to High Drug Concentration.
The Results of the Average Numbers of Amastigotes in Infected Macrophages Treated With Drugs
Figures 2 and 3 illustrate the results of the anti-leishmaniasis effect of prednisolone and mometasone by considering the average number of amastigotes in each macrophage.
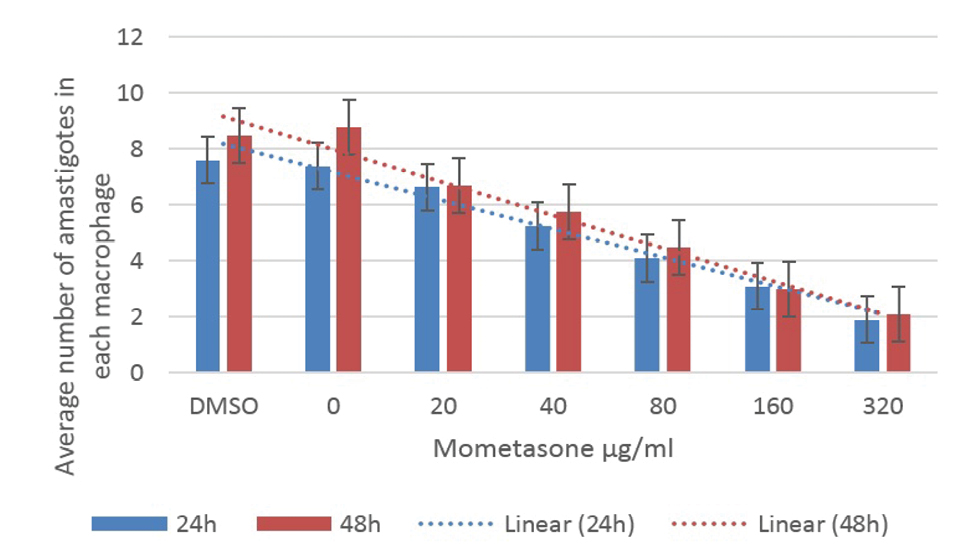
The Average Number of Amastigotes in Each Macrophage Under Different Concentrations of Mometasone.
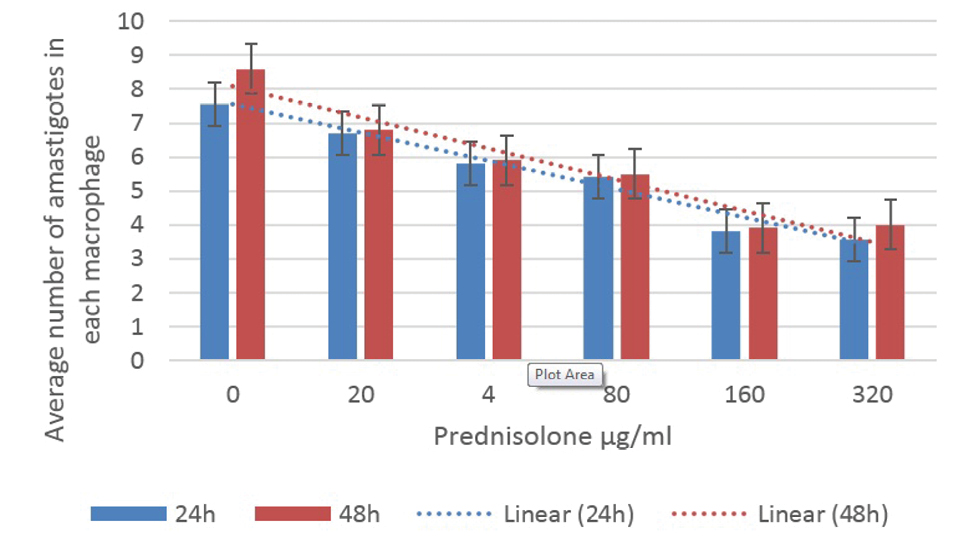
The Average Number of Amastigotes in Each Macrophage Under Different Concentrations of Prednisolone
Using the average of the number of amastigotes in the infected macrophages treated with mometasone and prednisolone was significantly different from the control group (P < 0.05). In other words, these drugs reduced the number of amastigotes within the macrophages while the Tukey’s post hoc test showed that the reduction in the number of promastigotes was not time-dependent. Although the effect of the drugs on the parasites was more at concentrations greater than 160 μg/mL, destructive effects were observed in the macrophages, as most of them were decomposed (Figure 1c), therefore, the best concentration was 160 μg/mL for both prednisolone and mometasone.
The Results of IL-12 and NO Secretion From Treated and Untreated Parasitic Macrophages
After the inoculation of macrophages with Leishmania parasite, they were exposed to different drug concentrations and after 24 and 48 hours, the supernatant was collected and the IL-12 and NO secreted by macrophages were measured by the ELISA and Griess reagent methods, respectively. The results demonstrated that IL-12 and NO releases after 24 hours were directly correlated with an increase in the drug concentration and secretion, so that the amount of secreted IL-12 and NO was not significantly different at concentrations less than 160 μg/mL but this difference was significant at higher concentrations.
Although there was a significant difference in the amount of IL-12 and NO secreted by different drug concentrations at 24 hours, the results of Tukey’s post hoc test showed that this was not time-dependent and at 48 hours after adding the drug, the amount of IL-12 and NO secreted represented no differences compared to the control group.
The results of the mean concentration of IL-12 and NO secreted by macrophages under different concentrations of the drug were shown in Figures 4 and 5.
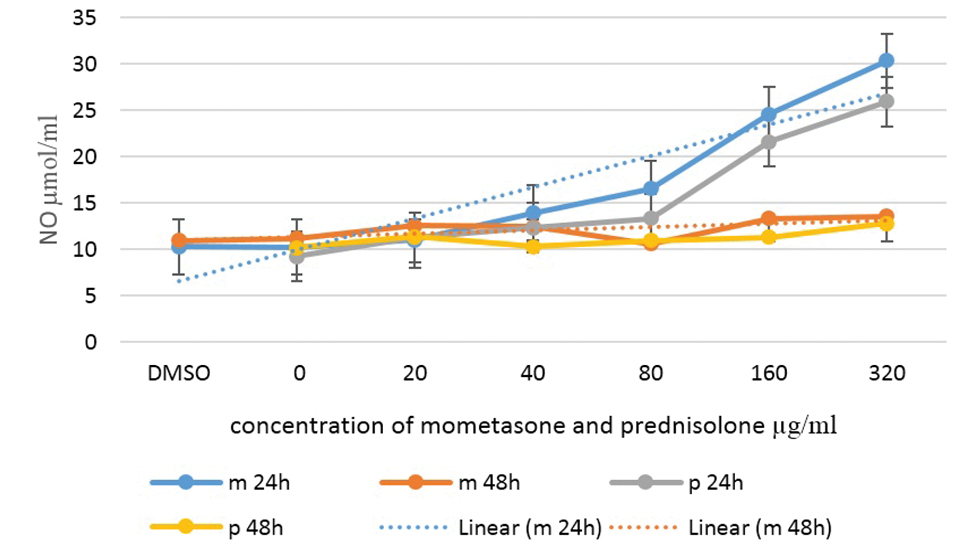
NO Secretion by Macrophages Infected With Leishmania Parasite Under Different Concentrations of Mometasone and Prednisolone.
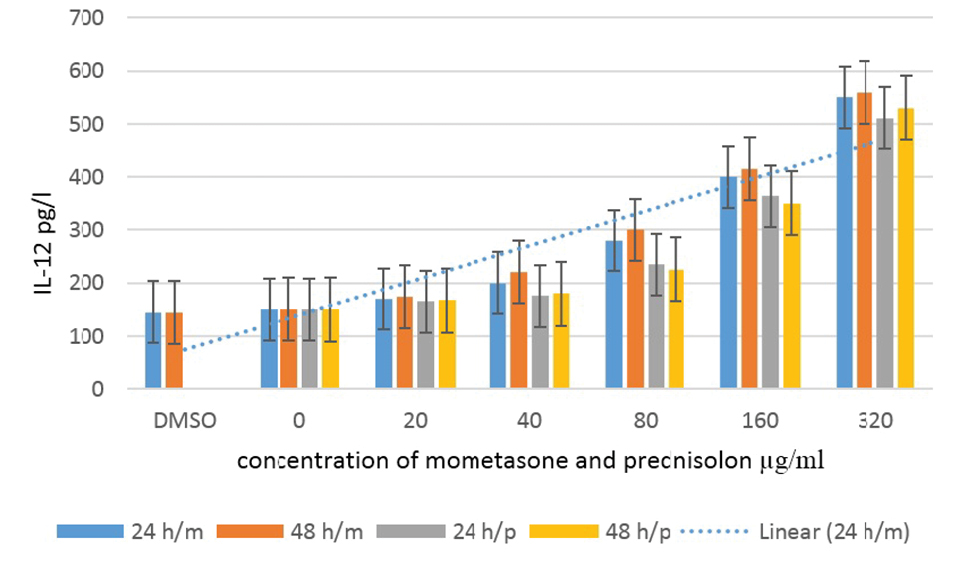
The Secretion of IL-12 by Macrophages Infected With Leishmania Parasite Under Different Concentrations of Mometasone and Prednisolone.
Discussion
The chemical treatment of leishmaniasis heavily relies on the development of the immune response that activates macrophages to produce toxic nitrogen and oxygen mediators to kill amastigotes. This process is suppressed by the parasite itself, which results in modest signaling between the macrophages and the T-cells. During the treatment of macrophages with the glucocorticoid, the genes responsible for inducing NO synthase production increase leading to the production of a large amount of NO (1).
On the other hand, the main mechanisms of the macrophage cells are the destruction of amastigotes, the production of cytokines such as IL-12, NO, and toxic oxidizing metabolites such as superoxide and hydrogen peroxide, which are harmful to all stages of the parasite (14).
In the present study, it was found that the average number of amastigotes in the infected macrophages treated with mometasone and prednisolone was significantly lower than that of the control group and these drugs reduced the number of amastigotes within the macrophages. Moreover, the best concentration of both drugs was 160 μg/mL due to the destructive effects on macrophages and their degradation at high doses. Osero et al studied the effect of glucocorticoids (hydrocortisone and dexamethasone) on macrophages infected with the L. major parasite and found that the parasite in the macrophages of the treated group with glucocorticoids reduced compared to the control group. They further suggested glucocorticoids to control leishmaniasis infection (1). These findings are in line with those of the present study.
The parasites of the genus Leishmania are capable of inhibiting the effector functions of macrophages. These parasites develop the adaptive ability to escape the host defenses. For example, they inactivate the NF- κ B complex and suppress iNOS expression in infected macrophages, which are responsible for the production of the major antileishmanial substance NO, favoring then its replication and successful infection.
According to some studies, the inhibition of the NO production led to the disability of macrophages for inhibiting L. major reproduction in the culture medium (17,18). In this study, NO production was observed by the macrophage stimulated with prednisolone and mometasone. NO is a potent microbial agent that causes the death of intracellular parasites and other pathogens (19-22). Another study reported the need for iNOS for the response of NK cells to L. major (14).
Recently, several studies have shown that NO production in human monocytes or macrophages in vitro is also stimulating (16,23). IFN-γ and INF-α have synergistic effects on the induction of NO and iNOS by macrophages in vitro (24).
The results of this study indicated that the secreted NO had a significant increase by increasing the concentration of the drugs after 24 hours. The results of another study showed similar results, demonstrating that glucocorticoids increased the expression of TNF-α genes, along with NO and IL-1 in macrophages as compared to the control group (1).
IL-12 increases cellular immunity by enhancing the differentiation of TCD4+ cells into the Th1. Additionally, the role of IL-12 in the activation of Nk cells leads to the activation of macrophages because it increases the production of IFN-γ from NK cells, as well as IL-12 and IL-18, has a greater effect on the stimulation of IFN-γ production by Nk cells (25-27).
Finally, cytokines that increase the response of Th1 such as IL-12 are more secreted and IL-12 is an essential stimulant for secreting IFNγ and inducing intrinsic and cellular responses against intracellular parasites including Leishmania and IL-10 acts in a modest way for these reactions (28).
In this study, IL-12 secreted by macrophages was measured and the results represented that the amount of IL-12 secretion was significantly and positively correlated with the increase in drug concentration after 24 hours (P < 0.05).
Based on the results of other studies, human cytokine response to L. major at the initial stage of infection and in patients susceptible to treatment increased IL-12 and IFN-γ expression, but lower levels of Th2 cytokines were secreted as IL-5 and IL-4 (29-30).
The results of the present study showed that the average of L. major parasites in mouse J774 cells decreased with prednisolone and mometasone. In addition, the level of NO and the mean level of IL-12 secreted by infected macrophages treated with both drugs indicated an increase. The decrease in amastigotes appears to be due to an increase in the mean level of NO and IL-12.
Although glucocorticoids in this study and previous studies did not completely eliminate the parasite, in vitro experiments reduced parasites while increasing cytokines in the macrophages (1). On the other hand, the symptoms of the disease, the size of the lesion, and the duration of the disease reduced in in vivo experiments (31). Therefore, the use of glucocorticoid drugs has many benefits. Glucocorticoids are available and considered as cost-effective drugs. Further, their efficiency is approved regarding reducing the symptoms in the case of many inflammatory diseases. Furthermore, their mechanism in the inhibition of the NF-kβ transcription factor, which increases the level of Th1 cytokines that are effective in treating the leishmaniasis lesion. Accordingly, glucocorticoids can probably be useful in combination with anti-leishmaniasis drugs for reducing the parasitic infections and managing immunity toward Th1 and faster treatment with fewer side effects.
Conclusions
In this study NO and mean IL-12 secretion were measured as major macrophage cytokines against L. major in order to evaluate the performance of glucocorticoid drugs. The results of the analysis showed that the amount of NO and mean IL-12 in the supernatant in treated groups with prednisolone and mometasone drugs increased compared to the control groups. Given the addition of drugs without any inducer, glucocorticoid drugs may directly affect the function of macrophages and increase the release of NO and the mean IL-12. Thus, it is recommended to investigate the effect of glucocorticoids on leishmaniasis in vivo in order to obtain more information.
Acknowledgements
This article is a master’s degree program in parasitology under number 397049 in collaboration with Isfahan University of Medical Sciences. We appreciate the collaboration of the Research Deputy and the Department of Parasitology of Isfahan University of Medical Sciences.
References
- Osero BO, Mosigisi A, Ogeto TK, Mugambi R, Ingonga J, Karanja RM, et al. Effects of glucocorticoids in Leishmania major infection. Int J Fauna Biol Stud 2015;2(3):16-22.
- Roberts MT. Current understandings on the immunology of leishmaniasis and recent developments in prevention and treatment. Br Med Bull 2005;75-76:115-30. doi: 10.1093/ bmb/ldl003. [Crossref]
- Ngumbi PM, Irungu LW, Ndegwa PN, Maniania NK. Pathogenicity of Metarhizium anisopliae (Metch) Sorok and Beauveria bassiana (Bals) Vuill to adult Phlebotomus duboscqi (Neveu-Lemaire) in the laboratory. J Vector Borne Dis 2011;48(1):37-40.
- Nilforoushzadeh MA, Shirani-Bidabadi L, Jafari R, Zolfaghari- Baghbaderani A, Ghahraman-Tabrizi M, Moradi S, et al. Topical effectiveness of different concentrations of nanosilver solution on Leishmania major lesions in mice (Balb/c). Journal of Isfahan Medical School 2011;29(158):1575-82. [Persian].
- Pennisi MG, De Majo M, Masucci M, Britti D, Vitale F, Del Maso R. Efficacy of the treatment of dogs with leishmaniosis with a combination of metronidazole and spiramycin. Vet Rec 2005;156(11):346-9. doi: 10.1136/vr.156.11.346. [Crossref]
- Chauhan PM, Iyer RN, Shankhdhar V, Guru PY, Sen AB. Effect of new diamidines against Leishmania donovani infection. Indian J Exp Biol 1993;31(2):196-8.
- Soto J, Buffet P, Grogl M, Berman J. Successful treatment of Colombian cutaneous leishmaniasis with four injections of pentamidine. Am J Trop Med Hyg 1994;50(1):107-11. doi: 10.4269/ajtmh.1994.50.107. [Crossref]
- Consigli J, Danielo C, Gallerano V, Papa M, Guidi A. Cutaneous leishmaniasis: successful treatment with itraconazole. Int J Dermatol 2006;45(1):46-9. doi: 10.1111/j.1365- 4632.2004.02429.x. [Crossref]
- Kellina OI, Inyakhina AV, Yastrebova RI. Treatment of cutaneous leishmaniasis with monomycin. Meditsinskaya Parazitol i Parazit Bolezn 1966;35(3):283-7.
- Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med 2006;3(5):e162. doi: 10.1371/journal.pmed.0030162. [Crossref]
- Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol Microbiol 2005;57(6):1690- 9. doi: 10.1111/j.1365-2958.2005.04782.x. [Crossref]
- Al-Majali O, Routh HB, Abuloham O, Bhowmik KR, Muhsen M, Hebeheba H. A 2-year study of liquid nitrogen therapy in cutaneous leishmaniasis. Int J Dermatol 1997;36(6):460-2. doi: 10.1046/j.1365-4362.1997.00045.x. [Crossref]
- Belkaid Y, Butcher B, Sacks DL. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur J Immunol 1998;28(4):1389- 400. doi: 10.1002/(sici)1521-4141(199804)28:04>1389::aidimmu1389<3.0.co;2-1. [Crossref]
- Diefenbach A, Schindler H, Rollinghoff M, Yokoyama WM, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science 1999;284(5416):951-5. doi: 10.1126/science.284.5416.951. [Crossref]
- Bogdan C. The function of nitric oxide in the immune system. In: Mayer B, ed. Nitric Oxide. Berlin, Heidelberg: Springer; 2000. p. 443-92.
- Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Bécherel P, Debré P, et al. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci U S A 1995;92(17):7804-8. doi: 10.1073/pnas.92.17.7804. [Crossref]
- Assreuy J, Cunha FQ, Epperlein M, Noronha-Dutra A, O’Donnell CA, Liew FY, et al. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol 1994;24(3):672-6. doi: 10.1002/eji.1830240328. [Crossref]
- Green SJ, Meltzer MS, Hibbs JB Jr, Nacy CA. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol 1990;144(1):278-83.
- Bogdan C, Röllinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev 2000;173:17-26. doi: 10.1034/j.1600-065x.2000.917307.x. [Crossref]
- Liew FY, Parkinson C, Millott S, Severn A, Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology 1990;69(4):570-3.
- Liew FY, Wei XQ, Proudfoot L. Cytokines and nitric oxide as effector molecules against parasitic infections. Philos Trans R Soc Lond B Biol Sci 1997;352(1359):1311-5. doi: 10.1098/ rstb.1997.0115. [Crossref]
- Murray HW, Nathan CF. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med 1999;189(4):741-6. doi: 10.1084/jem.189.4.741. [Crossref]
- Zhou A, Chen Z, Rummage JA, Jiang H, Kolosov M, Kolosova I, et al. Exogenous interferon-gamma induces endogenous synthesis of interferon-alpha and -beta by murine macrophages for induction of nitric oxide synthase. J Interferon Cytokine Res 1995;15(10):897-904. doi: 10.1089/jir.1995.15.897. [Crossref]
- Mirzaei F, Khazaei M. Role of nitric oxide in biological systems: a systematic review. Journal of Mazandaran University of Medical Sciences 2017;27(150):192-222. [Persian].
- Nabors GS, Afonso LC, Farrell JP, Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc Natl Acad Sci U S A 1995;92(8):3142-6. doi: 10.1073/pnas.92.8.3142. [Crossref]
- Carrada G, Caneda C, Salaiza N, Delgado J, Ruiz A, Sanchez B, et al. Monocyte cytokine and costimulatory molecule expression in patients infected with Leishmania mexicana. Parasite Immunol 2007;29(3):117-26. doi: 10.1111/j.1365- 3024.2006.00924.x. [Crossref]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001;19:423-74. doi: 10.1146/annurev.immunol.19.1.423. [Crossref]
- Shio MT, Olivier M. Leishmania survival mechanisms: the role of host phosphatases. J Leukoc Biol 2010;88(1):1-3. doi: 10.1189/jlb.0210088. [Crossref]
- Habibi GR, Khamesipour A, McMaster WR, Mahboudi F. Cytokine gene expression in healing and non-healing cases of cutaneous leishmaniasis in response to in vitro stimulation with recombinant gp63 using semi-quantitative RT-PCR. Scand J Immunol 2001;54(4):414-20. doi: 10.1046/j.1365- 3083.2001.00990.x. [Crossref]
- Rogers KA, Titus RG. The human cytokine response to Leishmania major early after exposure to the parasite in vitro. J Parasitol 2004;90(3):557-63. doi: 10.1645/ge-3317. [Crossref]
- Nyamao RM, Lagat ZO, Christopher KW, Jumba BN, Waihenya RW, Karanja R, et al. Efficacy of glucocorticoids in controlling Leishmania major infecting Balb/c mice. J Infect Dis Immun 2014;6(2):10-18. doi: 10.5897/jidi2014.0130. [Crossref]