Avicenna Journal of Clinical Microbiology and Infection. 6(2):44-48.
doi: 10.34172/ajcmi.2019.09
Original Article
Prevalence of Multidrug-Resistant, Extensively Drug-Resistantand Pandrug-Resistant Uropathogens Isolated From UrinaryTract Infection Cases in Dhaka, Bangladesh
Runa Asma 1  , Md. Jahangir Alam 2, *
, Md. Jahangir Alam 2, *  , Md Rahimgir 3, Mohammad Asaduzzaman 4, AKM Mynul Islam 5, Nasir Uddin 6, Md. Saiful Islam Khan 7, Nur-Wa-Bushra Jahan 8, Syeda Subrin Siddika 9, Suvomoy Datta 1
, Md Rahimgir 3, Mohammad Asaduzzaman 4, AKM Mynul Islam 5, Nasir Uddin 6, Md. Saiful Islam Khan 7, Nur-Wa-Bushra Jahan 8, Syeda Subrin Siddika 9, Suvomoy Datta 1
Author information:
1Department of Microbiology, Primeasia University, Banani, Dhaka 1213, Bangladesh
2Department of Microbiology and Biochemistry, North South University, Bhasundhara, Dhaka-1212, Bangladesh
3Department of Microbiology, Armed Forces Medical College, Dhaka Cantonment, Dhaka-1206, Bangladesh
4Department of Biochemistry, Primeasia University, Banani, Dhaka 1213, Bangladesh
5Department of Hematology, National Institute of Cancer Research and Hospital, Mohakhali, Dhaka-1212, Bangladesh
6Department of Biochemistry, National Institute of Cardiovascular Disease and Hospital, Sher-e-Bangla Nagor Thana, Dhaka-1207, Bangladesh
7Department of Biochemistry and Immunology, Ibn Sina Diagnostic and Consultation Center, Badda, Dhaka-1212, Bangladesh
8Department of Gynecology and Obstetrics, Sir Salimullah Medical College and Mitford Hospital, Dhaka-1000, Bangladesh.
9Department of Community Medicine, Mugda Medical College, Mugda, Dhaka-1214, Bangladesh
Abstract
Background: The resistance pattern of uropathogens is increasing very rapidly because of the unsorted, insufficient, and incoherent usage of antibiotics. The aim of this study was to evaluate the prevalence of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) uropathogens which were isolated from the urinary tract infection (UTI) cases in Dhaka, Bangladesh.
Methods: In this cross-sectional study, a total of 21167 urine samples were collected from January 2016 to December 2018, followed by using conventional methods, as well as Kirby-Bauer disc diffusion method for urine culture and susceptibility, respectively. Finally, SPSS software was utilized to analyze the obtained data.
Results: From among 21167 urine samples, 2469 (11.66%) cases were bacteriologically positive. In UTI cases, males proportion were higher compared to females (in ≤ 10 and > 60 to ≤90 years age groups) and females in the age groups between 10 and 60 and >90 years suffered more than males (P<0.05). In addition, 172 (7.0%), 1337 (54.2%), and 845 (34.2%) cases were identified as XDR, single drug-resistance (SDR), and nondrug-resistance (NDR), respectively. Although the number of female XDR cases was higher than males, the percentages of male cases were higher compared to female cases in this study. The most predominant drugresistance cases (18.7%) were found in the age group between 21 and 30 years (P<0.05). Eventually, the isolates of Escherichia coli were the most prevalent cases that carried XDR (5.4%) and MDR (39.7%).
Conclusions: In general, it is extremely alarming to increase XDR and MDR uropathogens. This bacterial resistance can be prevented through control measures that limit the spread of resistant bacteria and the regular monitoring of this resistance phenotype of uropathogens, along with the rational use of antimicrobial therapy.
Keywords: UTI, MDR, XDR, PDR, Uropathogens
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
The resistance of antimicrobial agents in pathogenic bacteria has become a significant public health problem. According to Magiorakos et al,(1) this pattern occasionally extends to multidrug-resistant (MDR) or extensively drug-resistant (XDR) or even pandrug drug-resistant (PDR). Urinary tract infection (UTI) is considered as a different type of infection which is caused by the above-mentioned resistant bacteria. It is known that UTI is the most common infection in the world, especially Bangladesh. This infection is due to the main colonization of normal and opportunistic microflora (2). In the third world country like Bangladesh, the rate of UTI patients is high due to poor hygiene, long time catheterization, uncontrolled sexual intercourse, pregnancy, spermicidal contraception, and the like (3-6).
Many of antibiotics and their super generations are used to prevent the UTIs. Unfortunately, several studies indicate that many uropathogens have become resistant to a wide range of antibiotics due to abuse, overuse, and uncompleted dosages (7-9). Some are also getting resistant to efficacious antibiotics by adopting genetic transformations and different mechanism of mutations (10-13). In addition, this resistant bacterium provides daily challenges for individuals to infectious diseases throughout the world (14), leading to fatalities from simple microbial infections to treatment-mediated complications (15).
Furthermore, worldwide increases in SDR, MDR, and XDR bacteria are certainly well-known. The incidence of the following bacteria has also received special attention, especially in developing countries:
-
Methicillin-resistant Staphylococcus aureus
-
Vancomycin-resistant S. aureus
-
Coagulase-negative staphylococci
-
Glycopeptides intermediate-sensitive S. aureus
-
Vancomycin-resistant Enterococcus species
-
Penicillin-resistant Streptococcus pneumoniae
-
Extended-spectrum β-lactamase-producing bacteria
-
Carbapenem-resistant bacteria (16).
Accordingly, the present study sought to investigate the prevalence of MDR, XDR, and PDR uropathogens in Dhaka which caused UTIs as per standardized international terminology created by the European Centre for Disease Control, along with the Centre for Disease Control and Prevention, Atlanta (1).
Materials and Methods
Study Design and Setting
The study was designed to collect urine samples from 21167 cases with (>70%) or without the symptoms of UTIs. After getting permission from IBN SINA trust Ethical Committee, verbal informed consent was obtained from the subjects, and they completed a written standardized questionnaire which was kept confidential during the research. The whole study was conducted in the Microbiology Laboratory (IBN SINA D. Lab, Badda, Hosen Market, Dhaka-1212, Bangladesh) which was approved by the International Standards Organization and Gulf Accreditation Center.
Sample Collection and Bacteriological Assessment
The early-morning midstream urine samples were aseptically collected from 2 1167 subjects including 5081 males and 16086 females. The MacConkey agar (Oxoid), blood agar (Oxoid), and HiCrome UTI agar (HiMedia) media were applied for the inoculation of all the urine samples, maintaining biosafety level II. To observe the growth of bacteria, all the plates were incubated at 37°C for 48 hours. Finally, the isolates were identified by using biochemical tests like Triple Sugar Iron (TSI-Oxoid), Motility Indole Urea (MIU-Oxoid), and Simmons Citrate (Oxoid) agar, as well as colonial morphology with the naked eye and/or microscopic examination (17).
Antibiotic Susceptibility Assessment
For antibacterial susceptibility, Kirby Bauer disc diffusion method (commercially available antibiotic discs in Bangladesh) was utilized (17). The following commercially available antibiotic discs were employed for susceptibility test: Amikacin (30 μg, Oxoid), amoxyclav (amoxicillin + clavulanic acid, 30 μg, Oxoid), amoxycillin (20 μg, Oxoid), azithromycin (15 μg, Oxoid), aztreonam (30 µg, Oxoid), colistin (10 μg, Oxoid), cefepime (30 μg, Oxoid), ceftriaxone (30 μg, Oxoid), cefixime (5 μg, Oxoid), ceftazidime (30 μg, Oxoid), cefotaxime (30 μg, Oxoid), ciprofloxacin (5 μg, Oxoid), cloxacillin (5 μg, Oxoid), doxycycline (30 µg, Oxoid), fusidic acid (10 μg, Oxoid), gentamicin (10 μg, Oxoid), imipenem (10 μg, Oxoid), linezolid (30 μg, Oxoid), levofloxacin (5 μg, Oxoid), meropenem (10 μg, Oxoid), mecillinam (25 μg, Oxoid), nitrofurantoin (300 μg, Oxoid), nalidixic acid (30 μg, Oxoid), netilmicin (30 μg, Oxoid), piperacillin-tazobactam (110 μg, Oxoid), trimethoprim-sulfamethoxazole (25 μg, Oxoid) and vancomycin (30 μg, Oxoid).
Then, the results were interpreted by measuring the zone of inhibition against each of the organisms. Further, American Type Culture Collection (ATCC) strains were applied as the control strain (8,18). The isolated microorganisms were classified as MDR (non-susceptible to ≥1 agent in ≥3 antimicrobial categories), extensively resistant (non-susceptible to ≥1 agent in all but ≤2 antimicrobial categories), and PDR (non-susceptible to all antimicrobial agents listed.) microorganism as described by Magiorakos et al (1).
Statistical Analysis
SPSS (Statistical Package for Social Science) software, version 18 was used for data analysis. Furthermore, the chi-square test was utilized to compare the groups and P<0.05 was considered as statistically significant.
Results
A number of 2 1167 urine samples were collected in this study, among which 2469 (11.66%) and 18698 (88.34%) were found bacteriologically positive and negative, respectively.
Table 1 shows the distribution of UTI cases by age and sex. Most of the subjects were female and the highest number of subjects were within 21-30 years age group. Furthermore, a higher number of males were found in the age groups of ≤10 and > 60 to ≤90 years and a higher number of females were found within the age groups of 10 to 60 and ≥90 years as compared to males.
Table 1.
Distribution of Urinary Tract Infection Cases by Age and Sex
|
Age Group (y)
|
Male (n=531)
|
Female (n=1938)
|
|
No
|
(%)
|
No
|
(%)
|
| 1-10 |
116 |
(21.8) |
201 |
(10.4) |
| 11-20 |
11 |
(2.1) |
144 |
(7.4) |
| 21-30 |
46 |
(8.7) |
415 |
(21.4) |
| 31-40 |
45 |
(8.5) |
255 |
(13.2) |
| 41-50 |
50 |
(9.4) |
286 |
(14.8) |
| 51-60 |
67 |
(12.5) |
284 |
(14.6) |
| 61-70 |
114 |
(21.5) |
230 |
(11.9) |
| 71-80 |
64 |
(12.1) |
95 |
(4.9) |
| 81-90 |
17 |
(3.2) |
22 |
(1.1) |
| 91-100 |
1 |
(0.2) |
6 |
(0.3) |
|
Total
|
531
|
(100)
|
1938
|
(100)
|
Figure 1 illustrates the distribution of non-drug-resistance (NDR), single drug-resistance (SDR), MDR, XDR, and PDR uropathogens. MDR, XDR, and NDR cases were observed in 54.2%, 7%, and 4.7%, respectively. No PDR case was found in our study.
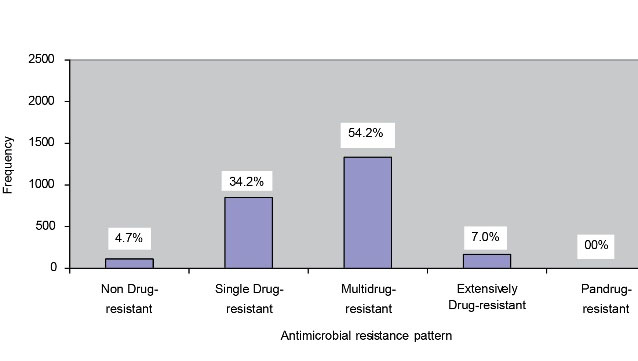
Figure 1.
Distribution of antimicrobial resistance pattern. (n=2469)
.
Distribution of antimicrobial resistance pattern. (n=2469)
Figure 2 displays the distribution of drug-resistance pattern by gender. As shown, females were predominating in all cases of SDR, MDR, and XDR.
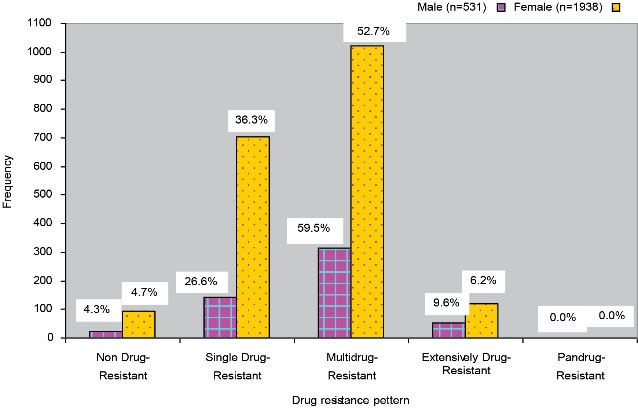
Figure 2.
Distribution of drug resistance pattern by gender
.
Distribution of drug resistance pattern by gender
Table 2 represents the drug-resistance pattern of UTI cases in different age groups. Predominant drug-resistance cases (18.7%) were found in the 21-30-year age group. The numbers of XDR cases (1.3%) were observed in the age group of 61-70 years which was higher than all the other age groups. The results of the study also revealed that the number of MDR cases were higher than that of all other drug-resistance patterns in all age groups.
Table 2.
Distribution of Antimicrobial Agent Resistance Pattern by the Age Group of UTI Cases
|
Age Group(y)
|
Non-drug-Resistance
|
Single Drug-Resistance
|
Multidrug-Resistance
|
Extensively Drug-Resistance
|
Total
|
|
No. (%)
|
No. (%)
|
No. (%)
|
No. (%)
|
No. (%)
|
| 1-10 |
12 (0.5) |
101 (4.1) |
185 (7.5) |
19 (0.8) |
317 (12.8) |
| 11-20 |
12 (0.5) |
58 (2.3) |
77 (3.1) |
8 (0.3) |
155 (6.3) |
| 21-30 |
30 (1.2) |
192 (7.8) |
214 (8.7) |
25 (1.0) |
461 (18.7) |
| 31-40 |
14 (0.6) |
111 (4.5) |
155 (6.3) |
20 (0.8) |
300 (12.2) |
| 41-50 |
12 (0.5) |
112 (4.5) |
189 (7.7) |
23 (0.9) |
336 (13.6) |
| 51-60 |
18 (0.7) |
114 (4.6) |
195 (7.9) |
24 (1.0) |
351 (14.2) |
| 61-70 |
15 (0.6) |
96 (3.9) |
202 (8.2) |
31 (1.3) |
344 (13.9) |
| 71-80 |
2 (0.1) |
46 (1.9) |
96 (3.9) |
15 (0.6) |
159 (6.4) |
| 81-90 |
0 (0.0) |
14 (0.6) |
21 (0.9) |
4 (0.2) |
39 (1.6) |
| 90-100 |
0 (0.0) |
1 (0.1) |
3 (0.1) |
3 (0.1) |
7 (0.3) |
|
Total
|
115 (4.7)
|
845 (34.2)
|
1337 (54.2)
|
172 (7.0)
|
2469 (100)
|
Abbreviation:UTI, Urinary tract infection.
The distribution of the drug-resistance pattern in the isolated pathogens of UTI cases is provided in Table 3. Based on the results, E. coli was the most predominant isolate (72.4%). In the present study, the highest number of XDR cases were E. coli (5.4%), followed by Klebsiella spp. (0.6%), Pseudomonas spp. (0.4%), and Enterococcus spp. (0.3%). The maximum number of MDR cases were also contributed to E. coli (39.7%) and Enterococcus spp. (4.1%).
Table 3.
Distribution of Drug-Resistance Pattern Among the Isolated Pathogens
|
Isolated Uropathogens
|
Non-drug-Resistance
|
Single Drug-Resistance
|
Multidrug-Resistance
|
Extensively Drug-Resistance
|
Total
|
|
No. (%)
|
No. (%)
|
No. (%)
|
No. (%)
|
No. (%)
|
|
E. coli
|
82 (3.3) |
593 (24) |
980 (39.7) |
133 (5.4) |
1788 (72.4) |
|
Klebsiella spp. |
6 (0.2) |
44 (1.8) |
66 (2.7) |
15 (0.6) |
131 (5.3) |
|
Enterobacter spp. |
18 (0.7) |
83 (3.4) |
36 (1.5) |
0 (0.0) |
137 (5.5) |
|
Pseudomonas spp. |
1 (0.0) |
9 (0.4) |
33 (1.3) |
11 (0.4) |
54 (2.2) |
|
Proteus spp. |
1 (0.0) |
9 (0.4) |
20 (0.8) |
2 (0.1) |
32 (1.3) |
|
Acinetobacter spp. |
0 (0.0) |
3 (0.1) |
5 (0.2) |
1 (0.0) |
9 (0.4) |
|
Citrobacter spp. |
1 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (0.0) |
|
Serratia spp. |
0 (0.0) |
2 (0.1) |
0 (0.0) |
0 (0.0) |
2 (0.1) |
|
Staph. aureus
|
1 (0.0) |
34 (1.4) |
42 (1.7) |
2 (0.1) |
79 (3.2) |
|
Staph. saprophyticus
|
2 (0.1) |
26 (1.1) |
41 (1.7) |
0 (0.0) |
69 (2.8) |
|
Enterococcus spp. |
1 (0.0) |
25 (1.0) |
100 (4.1) |
8 (0.3) |
134 (5.4) |
|
Streptococcus group B |
2 (0.1) |
17 (0.7) |
14 (0.6) |
0 (0.0) |
33 (1.3) |
| Total |
115 (4.7) |
845 (34.2) |
1337 (54.2) |
172 (7.0) |
2469 (100) |
Discussion
As previously described, the misuse and abuse of antibiotics are responsible for drug resistance and some bacteria naturally develop resistance by the consequence of their adaptation to the environment. In addition, resistance develops by the exposure of microorganisms to different antibiotics which increases the selective pressure and favors the development of resistance (12). Whether it is natural or man-made, it is a more alarming message regarding increasing the drug resistance. This study aimed to evaluate the prevalence of antibiotic resistance pattern among bacteria which were isolated from patients with UTIs. For this reason, 2 1167 urine samples were tested, of which 2469 (11.66%) of them were bacteriologically positive. Other studies also showed that the bacteriological positivity rate in UTI cases was around 10% in Bangladesh (4,8).
UTIs were frequently found in the age group of 21-30 years. For example, Asaduzzaman et al reported that UTIs were prevalent among the age group of 21-30 years. They further showed that people were sexually active in this age group. Furthermore, they used different types of contraceptives, foams, gels, diaphragms, and spermicides which were at higher risk of developing UTIs. The results of the study revealed that males in the age group ≤10 years further suffered from UTI compared to females because uncircumcised male infants appeared to be an increased risk of UTIs (13). A higher number of females than males were observed in the age group of 10 to 60 and >90 years (P < 0.05).
Figure 1 shows the distribution of the antibiotic resistance pattern of NDR (susceptible to all listed antimicrobial agents), SDR (non-susceptible to ≥1 agent in ≥2 antimicrobial categories), MDR (non-susceptible to ≥1 agent in ≥3 antimicrobial categories), XDR (non-susceptible to ≥1 agent in all but ≤2 antimicrobial categories), and PDR (non-susceptible to all listed antimicrobial agents.) cases (1). Overall, 115 (4.7%) NDR, 845 (34.2%) SDR, 1337 (54.2%) MDR, and 172 (7.0%) XDR cases were detected in our study while not observing any PDR cases. On the other hand, Begum et al. in their study found 70.67% MDR and 14% XDR cases but no PDR cases (2). The results showed the distribution of drug-resistance pattern by gender (Figure 2). The percentages of male cases were higher than those of females regarding MDR (59.5%) and XDR (9.6%) cases. However, no PDR cases were detected in both males and females (P<0.05).
The predominant drug resistance was found in the age group between 21 to 30 years (18.7%), followed by 14.2%, 13.9%, 13.6%, and 12.8% in the respective age group of 51-60, 61-70, 41-50, and 1-10 years, respectively (Table 2). The XDR cases in the age group of 61 to 70 were higher (1.3%) than the other age groups. The results also revealed that the percentage of all MDR cases were higher than the other drug-resistance patterns in all age groups. The age groups of 21-30 years were the most predominant subjects for NDR, SDR, and MDR cases but the most predominant age group was 61-70 years in XDR cases (P<0.05).
Table 3 presented the distribution of the isolated uropathogens by the drug-resistance pattern in UTI cases. Based on the data, E. coli was the most predominant isolate (72.4%) in the present study. Several studies also indicated that E. coli was the most predominant uropathogen (4,8). In our study, the highest number of XDR uropathogens belonged to E. coli (5.4%), followed by Klebsiella spp. (0.6%), Pseudomonas spp. (0.4%), and Enterococcus spp. (0.3%). The prevalence of MDR cases was also predominated by E. coli (39.7%) and then Enterococcus spp. (4.1%). Contrarily, XDR cases were not found in Enterobacter spp., Citrobacter spp., Serratia spp., Staphylococcus saprophyticus, and Streptococcus group B (P<0.05).
Conclusions
The results of the study revealed that the MDR and XDR cases but not PDR cases are increasing steadily. Based on the results, different awareness programs are needed for both the population and health professionals about the adverse effect of resistance and the importance of the correct use of antibiotics. By adopting this initiative, it is expected to control the spread of resistant bacteria which cause UTIs, and finally, reduce morbidity and mortality.
Ethical Approval
The ethical permission was obtained from the Ethical Committee of IBN SINA trust.
Patients’ Consent
The verbally informed consent and filled-up written standardized questionnaire were used before study initiation.
Conflict of Interest Disclosures
The authors declare no conflict of interest.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
I would like to gratefully acknowledge IBN SINA Diagnostic Center, Badda, Dhaka, Bangladesh for their wholehearted support for this study.
References
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3):268-81. doi: 10.1111/j.1469-0691.2011.03570.x [Crossref] [ Google Scholar]
- Begum N, Shamsuzzaman SM. Emergence of multidrug resistant and extensively drug resistant community acquired uropathogens in Dhaka city, Bangladesh. Bangladesh J Med Microbiol 2015; 9(2):7-12. doi: 10.3329/bjmm.v9i2.31414 [Crossref] [ Google Scholar]
- Manges AR, Tabor H, Tellis P, Vincent C, Tellier PP. Endemic and epidemic lineages of Escherichia coli that cause urinary tract infections. Emerg Infect Dis 2008; 14(10):1575-83. doi: 10.3201/eid1410.080102 [Crossref] [ Google Scholar]
- Alam J, Mousumi SJ, Rana R, Islam S, Akter S, Juliana FM. Ceftriaxone resistance patterns of uropathogens isolated from urinary tract infection patients in selected areas of Dhaka city, Bangladesh. IOSR Journal of Nursing and Health Science 2017; 6(5):28-34. [ Google Scholar]
- Hossain N, Das B, Alam J, Juliana FM, Islam MJ, Hossain MN. Susceptibility pattern of nitrofurantoin against uropathogens in selected areas of Dhaka city, Bangladesh. IOSR Journal of Nursing and Health Science 2017; 6(5):60-5. doi: 10.9790/1959-0605086065 [Crossref] [ Google Scholar]
- Alam J, Juliana FM, Rahimgir M, Hossain MN, Fatema B, Asaduzzaman M. Resistance pattern of ciprofloxacin against common uropathogens in selected area of Dhaka city, Bangladesh. IOSR Journal of Nursing and Health Science 2017; 6(5):52-7. doi: 10.9790/1959-0605015257 [Crossref] [ Google Scholar]
- Tamberkar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol 2006; 5(17):1562-5. [ Google Scholar]
- Alam J, Asma R, Chowdury SS, Rahimgir M. Sensitivity pattern of cefotaxime against common uropathogens in vitro in Dhaka, Bangladesh. Drugs Ther Perspect 2019; 35(3):145-9. doi: 10.1007/s40267-019-00603-1 [Crossref] [ Google Scholar]
- Asaduzzaman M, Ullah M, Redwan S, Alam J, Juliana FM, Hossain N. Emergence of meropenem resistance in pathogens recovered from urine cultures in Bangladesh. IOSR Journal of Pharmacy and Biological Sciences 2018; 13(3):41-7. doi: 10.9790/3008-1303044147 [Crossref] [ Google Scholar]
- Asaduzzaman M, Baral K, Islam M, Nayem A, Alam J, Juliana FM. Susceptibility pattern of second line antibiotic colistin against gram-negative bacteria causing urinary tract infection in selected areas Dhaka city, Bangladesh. European J Biomed Pharm Sci 2018; 5(3):874-9. [ Google Scholar]
- Asaduzzaman M, Hasin Z, Kumar S, Akter T, Alam J, Juliana FM. Efficacy of Amikacin antibiotic to uropathogens isolated from patients urine sample causing urinary tract infection in selected areas of Dhaka city, Bangladesh. Int J Curr Innov Adv Res 2018; 1(3):89-99. [ Google Scholar]
- Asaduzzaman M, Hasan Z, Khatun M, Alam J, Hossain N, Das B. Resistance pattern of levofloxacin against uropathogens causing urinary tract infection in selected areas of Dhaka city Bangladesh. J Biol Agric Healthc 2018; 8(4):74-81. [ Google Scholar]
- Asaduzzaman M, Shamim A, Mian S, Alam J, Juliana FM, Hossain N. Resistance pattern of cefixime against uropathogens causing urinary tract infection in selected areas of Dhaka city, Bangladesh. Int J Eng Sci 2018; 7(1):33-9. [ Google Scholar]
- Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant gram-negative bacilli in Europe. Euro Surveill 2008; 13(47).
- Dutta S, Hassan MR, Rahman F, Jilani MSA, Noor R. Study of antimicrobial susceptibility of clinically significant microorganisms isolated from selected areas of Dhaka, Bangladesh. Bangladesh J Med Sci 2013; 12(1):34-42. doi: 10.3329/bjms.v12i1.13351 [Crossref] [ Google Scholar]
- Alam SMS, Kalam MA, Munna MS, Munshi SK, Noor R. Isolation of pathogenic microorganisms from burn patients admitted in Dhaka Medical College and Hospital and demonstration of their drug-resistance traits. Asian Pac J Trop Dis 2014; 4(5):402-7. doi: 10.1016/S2222-1808(14)60596-X [Crossref] [ Google Scholar]
- Clinical and Laboratory Standard Institute (CLSI). Performance standards for antimicrobial susceptibility testing (Twenty-third Informational Supplement. CLSI document M100-S23). Wayne, PA: CLSI; 2013.
- Asaduzzaman M, Miah AA, Bhuiyan KA, Alam J, Juliana FM, Hossain N. Resistant pattern of nalidixic acid against uropathogens in selected areas of Dhaka city, Bangladesh. European J Biomed Pharm Sci 2018; 5(3):90-5. [ Google Scholar]