Avicenna Journal of Clinical Microbiology and Infection. 6(2):49-56.
doi: 10.34172/ajcmi.2019.10
Research Article
Etiologic Profile and Sensitivity Pattern of Germs Responsible for Urinary Tract Infection Among Under-five Children in Douala, Cameroon: A Hospital-Based Study
Dorgelesse Francine Kouemo Motse 1, *  , Guy Pascal Ngaba 1, Danielle Christiane Kedy koum 1, Loick Pradel Kojom Foko 2, Cecile Okalla Ebongue 3, Désiré Dieudonné Adiogo 1, *
, Guy Pascal Ngaba 1, Danielle Christiane Kedy koum 1, Loick Pradel Kojom Foko 2, Cecile Okalla Ebongue 3, Désiré Dieudonné Adiogo 1, *
Author information:
1Faculty of Medicine and Pharmaceutical Science, the University of Douala, P.O. Box 24157, Douala, Cameroon
2Department of Animal Sciences, Faculty of Science, The University of Douala, P.O. Box 24157, Douala, Cameroon
3Douala General Hospital, P.O. Box 4856, Douala, Cameroon
Abstract
Background: Urinary tract infection (UTI) is considered as one of the most common diseases encountered in medical practice. This study aimed at determining the prevalence and antibiotic susceptibility of bacteria responsible for UTI among under five-year-old children.
Methods: A cross-sectional study was conducted at the Bonassama District Hospital of Douala, Cameroon. Sociodemographic and clinical information was documented, followed by collecting urine samples for bacteriological examination and antibiotic sensitivity test.
Results: The prevalence of UTI was 32.25% (129/400) and girls were more infected than boys (57.4% vs. 42.6, P=0.007). In addition, Escherichia coli (41.08%) and Enterobacter cloacae (18.6%) were the main bacteria which were isolated in this study and the resistance rates of E. coli isolates were higher for penicillin and second- and first-generation cephalosporins. This pattern was similar for E. cloacae and Klebsiella pneumoniae as well.
Conclusions: Overall, UTI is still a major public health problem in Cameroon.
Keywords: Uropathogens, Children, Prevalence, Antibiotic resistance
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Urinary tract infection (UTI) is usually defined as the presence of actively multiplying organisms in any part of the urinary tract such as kidneys, bladder, and urethra (1,2). Bacterial agents are mainly implicated as the causative germs of UTIs (3,4) which account for more than 95% of all cases (5). Viruses, parasites, and fungi may also be responsible for this type of infection, especially in immuno-compromised individuals (1,3). Each year, about 150 million urinary infection cases are recorded worldwide costing the world economy over six billion US dollars (5,6). According to some reports, several factors such as age, gender, race, and circumcision status are associated with an increased risk of UTI (1). The bulk of UTI-induced burden is concentrated on children, pregnant women, child-bearing women, and immunocompromised individuals (1,3).
Similarly, evidence-based information regarding the epidemiology of UTIs is increasingly released but disseminated in the African continent. However, the existing studies evaluating the prevalence of UTI causative germs emphasized the significant predominance of Gram-negative bacteria with Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae as the most prevalent germs (7-10).
Young children represent one of the most social groups who are at the risk of UTI (1,3) which is a common and important public health problem since its symptoms in children may be subtle or non-specific making the diagnosis more complicated (11,12). In general, the symptoms in children may include fever, vomiting, diarrhoea, poor appetite, irritability, and the overall feeling of illness (4). When UTI is not early diagnosed, life-threatening complications such as sepsis and renal scarring may occur as a consequence. In addition, renal scarring is the most common cause of hypertension in later childhood and renal failure in adulthood (3,7,12).
In developing countries particularly in resource-constrained ones, the treatment of UTI heavily relies on an empiric or probabilistic approach (2,7,12) which owing to various reasons, may be initiated even before the availability of the final laboratory diagnostic test results (6). Accordingly, this increases the drug pressure which the uropathogens are exposed to and thus leads to the emergence of drug-resistant and multidrug resistant germs. Drug resistance is increasingly growing in many parts of the world (13) and undermining different control endeavours in order to eliminate UTI as a public health problem, especially in children who pay the heaviest burden to these infections (1,3). Therefore, knowing about the patterns of UTI-related germs and their antibiotic sensitivity profile is extremely essential in medical practice to adequately define treatment policies.
Objectives
In Cameroon, there is a paucity of studies addressing these issues, especially about the children. Therefore, the current study sought to identify the prevalence of the causative pathogens of UTI and sensitivity patterns to commonly used antibiotics in children less than five years consulting at the Bonassama District Hospital in Douala, Cameroon.
Methods
Study Area
The study was performed at the Paediatrics Department of Bonassama District Hospital, which is a health facility located in the town of Douala, Cameroon.
Study Design and Sample Size
A descriptive and analytical cross-sectional survey was conducted from May 2013 to March 2014. The sample size was calculated using the Lorentz’s formula as follows.
n = Z2p(1-p)/d2
where n and Z represent the required sample size and the statistic for the desired confidence level (1.96 for 95% confidence level). In addition, p and d denote the assumed prevalence of UTI among under-five children and the accepted margin of error (5%). The prevalence value of UTI (13.99%), found by Tchendjou Takam (14), was fitted in the formula as well. Thus, the effective sample size with this value was set at 185 individuals.
Study Population
The population of the study included children aged 0-59 months. All children attending the paediatric department with their parents and having a prescription for urine examination were included in the current study and consent was obtained from their parents while those children who had none of the aforementioned criteria were excluded from the study.
Data Collection
The strategy of urine sampling varied with regards to the age of the children and their ability to control the micturition. A child either under catheter or with difficulty in controlling their micturition was considered unable to urinate. To collect the urine from such children, suprapubic aspiration was performed for new-borns after cleaning the skin with alcohol antiseptic solution. Mid-stream clean catch urine (MSU) was obtained in all children who were able to provide MSU. Further, the sterile container was placed in children unable to control their micturition for 30 minutes. When no urine was sampled, a new sterile collector sac was placed for 30 minutes and continued until successful collection of urine specimen. Prior to urine sampling, children’s genital organs were carefully cleaned with alcohol antiseptic solution. In catheterized children, urine specimens were collected by pressing the tube of the catheter for 30 minutes and then puncturing the accumulated urine upstream with a sterile syringe. The collected urine specimens were placed onto a tray on the same day of recruitment and immediately transported to the laboratory of the Paediatrics Department for analysis. In addition, information regarding gender, age, residence area, weight, and temperature were documented using an ad-hoc collection form.
Laboratory Procedures
Urinalysis
The urine samples were analysed macroscopically for colour and turbidity. Thereafter, they were centrifuged, the supernatant was discarded, and the deposits were examined under the microscope for the presence of white blood cells (pus cells), red blood cells, crystals, epithelial cells, parasites, and yeasts. The count of the forenamed elements was performed using Malassez cell. Furthermore, pH and the presence of nitrates, haemoglobin, proteins, and glucose were determined using urinary dipstick according to the manufacturer’s instructions.
Isolation, Count and Identification of Bacteria Colonies
Ten microliters (10 µL) of each urine sample were inoculated onto the culture media using the calibrated platinum loop and then incubated at 37°C for 24-48 hours. Several culture media were used such as cysteine lactose electrolyte deficient agar, eosin-methylene blue (EMB), Muller Hinton agar, and Sabouraud dextrose agar (SDA). EMB was applied for identifying Enterobacteriaceae. Moreover, SDA was applied for determining yeasts and performing antifungigram while Muller-Hinton agar was used for performing antibiogram. The media were prepared in accordance with the manufacturer’s recommendations and the results were reported in terms of the number of cells/high power field (HPF). High colony counts with more than one species of microorganisms were considered as contamination and the culture was repeated for contaminated plates. A culture plate was considered positive for UTI if the concentration of a single organism was ≥ 105 CFU/mL. Finally, the isolates were identified by the examination of each pure bacterial colony using the API 20E kit and pyuria and haematuria were defined as the presence of more than five leukocytes or erythrocytes per HPF.
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility of the isolates was tested by the disk diffusion method with respect to a standard method proposed by the National Committee for Clinical Laboratory Standards in 2013. Similarly, individual colonies were suspended to 0.5 McFarland using normal saline, followed by inoculating the suspensions on Muller-Hinton agar and incubating at 37°C for 18-24 hours. Twenty-six antimicrobial agents were tested as follows.
Amoxicillin (30 µg); amoxicillin + clavulanic acid (20/10 µg); penicillin g (2 µg); oxacillin (1 µg);cefazolin (30 µg); cefalotin (30 µg); cefamandole (30 µg); cefoxitin (30 µg); cefuroxime (30 µg); cefotaxime (30 µg); ceftriaxon (30 µg); ceftazidime (30 µg); netilmicin (30 µg); gentamicin (10 µg); tobramycin (10 µg); amikacin (30 µg); ofloxacin (5 µg); levofloxacin (5 µg); ciprofloxacin (5 µg); nalidixic acid (30 µg); erythromycin (2 µg); clarithromycin (15 µg); lincomycin (2 µg); vancomycin (30 µg); fusidic acid (10 µg); colistin sulfate (25 µg).
Statistical Analysis
Data were keyed and coded in an Excel sheet and the statistical analysis was performed using StatView software, version 5.0 (SAS Institute Inc., USA). Descriptive statistics were employed where appropriate. Eventually, the association between dependent and independent variables was tested using the independence chi-square test. Statistical significance was set at P< 0.05.
Results
Baseline Data
In total, 400 children were included in the study. As shown in Table 1, boys were more represented (52.7%) when compared to their girl counterparts (47.3%) with a sex ratio (M/F) of 1.14. The mean age was 17 ± 14 months with 1 and 59 as the extreme values. The 6-12-month age group was the most prevalent (29%) and less than one child out of five (15%) had a previous history of antibiotics intake. As regards the signs and symptoms observed at clinical examination, 200 (50.0%) cases presented one clinical sign while only seven (1.8%) of them demonstrated no clinical symptom (Table 1). Additionally, fever (313 cases), cough (95 cases), vomiting (54 cases), and diarrhoea (42 cases) were the most frequent clinical signs and symptoms in children (Figure 1).
Table 1.
Baseline Characteristics of the Children
|
Variables
|
Categories
|
Frequency/Value
|
%
|
| Gender |
Girls |
189 |
47.3 |
| Boys |
211 |
52.7 |
| Age groups (mon) |
(0-6) |
66 |
16.4 |
| (6-12) |
116 |
29.0 |
| (12-24) |
105 |
26.3 |
| (24-60) |
113 |
28.3 |
| Mean age ± SD (month) |
|
17 ± 14 |
|
| Mean temperature ± SD (°C) |
|
37.8 ± 0.9 |
|
| Mean weight ± SD (kg) |
|
8.8 ± 3.1 |
|
| Antibiotic intake history |
No |
344 |
86.0 |
| Yes |
56 |
14.0 |
| Urine collection method |
Pot |
298 |
74.5 |
| Collector Sac |
102 |
25.5 |
Number of signs and symptoms
presented |
None |
7 |
1.8 |
| One |
200 |
50.0 |
| Two |
139 |
34.8 |
| Three |
52 |
13.0 |
| Four |
2 |
0 |
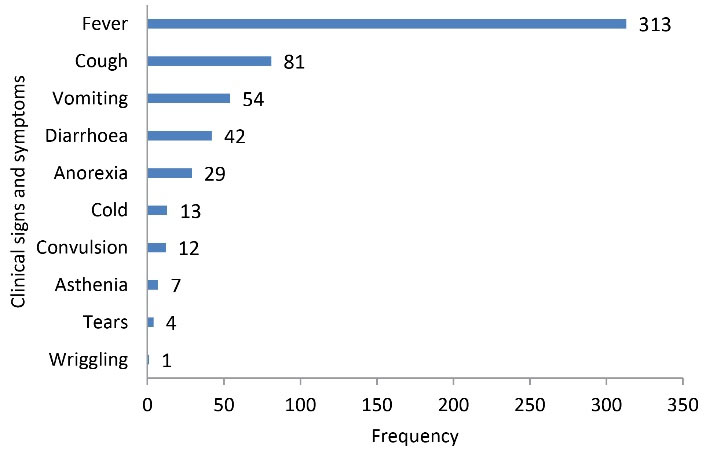
Figure 1.
The Frequency of Clinical Signs and Symptoms
.
The Frequency of Clinical Signs and Symptoms
Urinalysis
Based on the macroscopic analysis, 68.3% and 94.5% of urine specimens were yellowish and bright, respectively (Table 2). Interestingly, the microscope-based analysis revealed the presence of epithelial cells, yeast, crystals in 8.5%, 1.4%, and 0.4% of urine specimens, respectively. In addition, urinary dipstick-based testing highlighted some cases of glycosuria, proteinuria, and haematuria (Table 2).
Table 2.
Results from Urinalysis
|
Variables
|
Category
|
Frequency/Value
|
Percent/Min-Max Values
|
| Colour of urine |
Yellowish |
273 |
68.3 |
| Citrine yellow |
117 |
29.3 |
| Amber-coloured yellow |
5 |
1.2 |
| Pale yellow |
5 |
1.2 |
| Turbidity |
Bright |
378 |
94.5 |
| Cloudy |
22 |
5.5 |
| Presence of epithelial cells |
No |
366 |
91.5 |
| Yes |
34 |
8.5 |
| Mean epithelial cells count ± SD (/mm3) |
|
4 ± 6 |
0 - 80 |
| Presence of yeasts |
No |
395 |
98.6 |
| Yes |
5 |
1.4 |
| Mean yeasts count ± SD (/mm3) |
|
1 ± 1 |
0 - 8 |
| Presence of crystals |
No |
399 |
99.6 |
| Yes |
1 |
0.4 |
| Presence of cylinders, RBC or parasites |
No |
400 |
100 |
| Yes |
0 |
0 |
| Presence of leucocytes |
No |
345 |
86.3 |
| Yes |
35 |
13.7 |
| Mean leucocytes count ± SD (/mL) |
|
4231 ± 6504 |
0 - 30500 |
| Presence of nitrates |
No |
292 |
73.0 |
| Yes |
108 |
27.0 |
| Presence of cetonic bodies |
No |
398 |
99.5 |
| Yes |
2 |
0.5 |
| Glycosuria |
No |
397 |
99.3 |
| Yes |
3 |
0.7 |
| Proteinuria |
No |
395 |
98.8 |
| Yes |
5 |
1.2 |
| Haematuria |
No |
398 |
99.5 |
| Yes |
2 |
0.5 |
| Mean pH ± SD |
|
6 ± 0.4 |
0 - 8.5 |
Prevalence of Urinary Tract Infection and Causative Microbial Agents
The overall prevalence of UTI was 32.25%. Of the positive samples for UTI, Escherichia coli was the most isolated germs with 41.1% of all cases of infection, followed by Enterobacter cloacae (18.6%), Klebsiella pneumoniae (8.5%), and Staphylococcus aureus (6.9%). Three samples (2.2%) were positive for Candida albicans and the Gram-negative bacteria accounted for 90.9% of all the cases of UTI (Table 3).
Table 3.
The Prevalence of the Isolated Germs With Respect to Gram-Staining and Biological Group
|
Groups
|
Isolated germs
|
Frequency
|
Percent
|
Gram-negative bacteria (n = 117, 90.9%)
|
Escherichia coli
|
53 |
41.1 |
|
Enterobacter cloacae
|
24 |
18.6 |
|
Klebsiella pneumoniae
|
11 |
8.5 |
|
Raoultella ornithinolytica
|
4 |
3.1 |
|
Acinetobacter baumanii
|
3 |
2.2 |
|
Serratia liquefaciens
|
3 |
2.2 |
|
Erwina spp
|
2 |
1.6 |
|
Klebsiella oxytoca
|
2 |
1.6 |
|
Kluyvera spp
|
2 |
1.6 |
|
Proteus mirabilis
|
2 |
1.6 |
|
Providencia stuartii
|
2 |
1.6 |
|
Salmonella spp
|
2 |
1.6 |
|
Serratia odorifera
|
2 |
1.6 |
|
Citrobacter koseri
|
1 |
0.8 |
|
Enterobacter sakazakii
|
1 |
0.8 |
|
klebsiella ozaenae
|
1 |
0.8 |
|
Providencia rettgeri
|
1 |
0.8 |
|
Steno maltophilia
|
1 |
0.8 |
| Gram-positive bacteria (n = 9, 6.9%) |
Staphylococcus aureus
|
9 |
6.9 |
| Yeast (n = 3, 2.2%) |
Candida albicans
|
3 |
2.2 |
| |
Total
|
129
|
100
|
Characteristics of Children Diagnosed With Urinary Tract Infection
As presented in Table 4, children diagnosed with UTIs were mainly girls (57.4%) and aged 6-12 months (36.4%) compared to those UTI-negative cases. Further, positive children were significantly younger than their negative counterparts (14.0 ± 13.1 years vs. 17.6 ± 14.2 years (P= 0.014). Besides, the proportion of children having a history of antibiotics intake (P= 0.525) and presenting at least one sign/symptom at admission (P= 0.087) were similar between positive and negative children (Table 4).
Table 4.
Characteristics of Children Diagnosed With Urinary Tract Infection
|
Variables
|
Categories
|
UTI-Negative
|
UTI-Positive
|
P
Value
|
|
No.
|
%
|
No.
|
%
|
| Gender |
Girls |
117 |
43.2 |
74 |
57.4 |
0.007* |
| Boys |
154 |
56.8 |
55 |
42.6 |
| Age (months) |
(0-6) |
41 |
15.1 |
25 |
19.4 |
0.018* |
| (6-12) |
69 |
25.5 |
47 |
36.4 |
| (12-24) |
73 |
26.9 |
32 |
24.8 |
| (24-60) |
88 |
32.5 |
25 |
19.4 |
| Mean age (months) |
|
17.6 ± 14.2 |
14.0 ± 13.1 |
0.014* |
| Mean temperature (°C) |
|
37.7 ± 0.9 |
38.1 ± 0.9 |
0.000* |
| History of ATB treatment |
No |
231 |
85.2 |
113 |
87.6 |
0.525 |
| Yes |
40 |
14.8 |
16 |
12.4 |
| Number of signs/symptoms |
None |
6 |
2.2 |
1 |
0.8 |
0.087
|
| One |
128 |
47.2 |
72 |
55.7 |
| Two |
101 |
37.3 |
38 |
29.5 |
| Three |
36 |
13.3 |
16 |
12.4 |
| Four |
0 |
0.0 |
2 |
1.6 |
UTI: Unitary tract infection; Data are presented as frequency (percentage) and mean ± standard deviation; The Chi-square test and one-way analysis of variance were used to compare proportions and means, respectively; * P value less than 0.05 (statistically significant).
Antibiotic Sensitivity Testing
Overall, the resistance of bacteria found in this study was more emphasized (i.e., more than 50%) for four antibiotic classes (i.e., penicillins, along with the first, second, and third generation cephalosporins) as presented in Table 5. Indeed, E. coli, E. cloacae, and K. pneumoniae isolates were mainly resistant to amoxicillin with the resistance rates of 96.2%, 100%, and 100%, respectively. Similar rates regarding resistance to amoxicillin + clavulanic acid were also found for these three germs (i.e., 90.6%, 100%, and 90.9%, respectively). Likewise, the germs were resistant although less susceptible compared to penicillin class, to second and third generation cephalosporins. Furthermore, the highest rates of resistance were recorded against cefalotin and cefamandole which were 75.5% and 64.2% for E. coli, 87.5% and 75% for E. cloacae, along with 90.9% and 63.6% for K. pneumoniae. However, the lowest resistance rates were recorded for aminosids and quinolones families. On the other hand, S. aureus was most resistant to second and third generation cephalosporins with the overall rate of 77.8% for each antibiotic except for cefazolin (66.7%). Likewise, S. aureus was less resistant against aminosids and quinolones families (Table 5).
Table 5.
The Results of Antimicrobial Sensitivity Testing
|
Antibiotic classes
|
Nature of Antibiotics
|
E. coli
(n = 53)
|
E. cloacae
(n = 24)
|
K. pneumoniae
(n = 11)
|
S. aureus
(n= 9)
|
| Penicillin |
Amoxicillin |
51 (96.2) |
24 (100) |
11 (100) |
6 (66.7) |
| Amoxicillin + clavulanic acid |
48 (90.6) |
24 (100) |
10 (90.9) |
6 (66.7) |
| Oxacillin |
- |
- |
- |
3 (33.3) |
| Penicillin G |
- |
- |
- |
3 (33.3) |
| First generation cephalosporin |
Cefalotin |
40 (75.5) |
21 (87.5) |
10 (90.9) |
7 (77.8) |
| Cefazolin |
38 (71.7) |
20 (86.9) |
7 (63.6) |
6 (66.7) |
| Second generation cephalosporin |
Cefamandole |
34 (64.2) |
18 (75) |
7 (63.6) |
7 (77.8) |
| Cefoxitin |
30 (56.6) |
18 (75) |
6 (54.5) |
7 (77.8) |
| Cefuroxime |
27 (50.9) |
12 (50) |
9 (81.8) |
7 (77.8) |
| Third generation cephalosporin |
Cefotaxime |
23 (43.4) |
11 (45.8) |
6 (54.5) |
7 (77.8) |
| Ceftriaxone |
18 (33.9) |
8 (33.3) |
6 (54.5) |
7 (77.8) |
| Ceftazidime |
16 (30.2) |
9 (37.5) |
6 (54.5) |
7 (77.8) |
| Aminosids |
Netilmicin |
6 (11.3) |
2 (8.30) |
0 (0) |
2 (22.2) |
| Gentamicin |
10 (18.9) |
0 (0) |
0 (0) |
2 (22.2) |
| Tobramycin |
9 (16.9) |
2 (8.30) |
2 (18.2) |
4 (44.4) |
| Amikacin |
6 (11.3) |
0 (0) |
1 (9.1) |
3 (33.3) |
| Quinolones |
Ofloxacin |
9 (16.9) |
4 (16.6) |
1 (9.09) |
0 (0) |
| Levofloxacin |
8 (15.1) |
3 (12.5) |
1 (9.1) |
3 (33.3) |
| Ciprofloxacin |
9 (16.9) |
2 (8.30) |
1 (9.1) |
0 (0) |
| Nalixidic acid |
15 (28.3) |
11 (45.8) |
2 (18.2) |
1 (11.1) |
| Macrolids |
Erythromycin |
- |
- |
- |
7 (77.8) |
| Clarithromycin |
- |
- |
- |
8 (88,88) |
| Peptides |
Lincomycin |
- |
- |
- |
3 (33.3) |
| Vancomycin |
- |
- |
- |
4 (44.4) |
| Fusidic acid |
- |
- |
- |
2 (22.2) |
| Other class |
Colistin sulfate |
15 (28.3) |
9 (37.5) |
3 (27.3) |
5 (55.6) |
Discussion
UTI is a serious public health problem, especially in Cameroon. This study was designed to determine the prevalence and sensitivity pattern of the causative uropathogens germs of UTI in children less than five years consulting at the Bonassama District Hospital of Douala.
The incidence of UTI was 32.5% in this study. This was higher as compared to the reports by Banapurmath and Jayamony (15), Rai et al (12), Farajnia et al (16), Msaki et al (17), Samuel et al. (10), and Khatoon et al. (2) in which it was estimated at 8%, 13.2%, 28.6%, 20.3%, 26%, and 17.8%, respectively. On the other hand, the prevalence of UTI was lower than that of the other studies (3,5,7-9,11). For instance, Festo et al (7) and Saeed et al (11) found 39.7% and 35% incidence rates, respectively. The differences in the study design, as well as the study population and sample size may explain these discrepancies. Moreover, this may be related to the geographical difference of the risk cofactors of UTI in population from this area such as nutritional status or poor life hygiene. Interestingly, this fact may probably explain the dynamic nature of UTI epidemiology over time and space as previously outlined by the other authors (16,18).
Similarly, girls were more affected by UTI than their boy counterparts (37.03% vs. 27.96, respectively) and the association was statistically significant (P=0.0236). This is in line with the results of many previous reports which emphasized that girls exhibited a higher risk of UTI (5,7-11,16,19,20). The shorter length of the urethra and its proximity to the anus in girls enhanced the scope for the pathogens to invade the bladder which resulted in lower UTI. Additionally, Saeed et al pinpointed that the infrequent micturition in girls may increase the risk of bacterial colonisation, and consequently, the infection of the bladder (11). Conversely, Ousseini reported a higher risk of UTI in boys (21). This inversion of the causal relationship may be due to the fact that the authors are interested in UTI in malnourished children.
Twenty germ species were isolated in this study and the gram-negative bacteria were mostly implicated in UTI (90.9%). This is consistent with the findings of several previous reports which outlined them as the main causative germs of UTI (2,5,7-9,17). Similarly, the Gram-positive cocci represented only by S. aureus was regarded as the second causal bacterial group. The ability of Gram-negative germs to produce diverse virulence factors such as adhesins or enzymes may be related to their predominance as germs responsible for UTI (13).
Escherichia coli, E. cloacae, K. pneumoniae, and S. aureus were the most common germs involved in UTI, which demonstrates that UTI possesses a major implication of these germs in this study. This conclusion obviously varies when analysing the results on the same topic and other studies (5,13,22,23). For instance, Ben Abdallah et al reported E. coli (76%), Klebsiella sp. (10.5%), and P. mirabilis (4%) as the main causative uropathogens (13). This inter-studies variability reinforces the aforementioned assumption about the heterogeneity of UTI over time and space. However, the findings of these studies corroborate with the fact that E. coli is the most prevalent germ responsible for UTI (18,24,25).
Our results about the antimicrobial sensitivity highlight the universal problem of resistance. Four major germs species were found in this study, namely, E. coli, E. cloacae, K. pneumoniae, and S. aureus. Overall, the resistance of these germs species was more accentuated (i.e., more than 50%) for the antibiotic classes of penicillins, along with first, second, and third generation cephalosporins. This could be a consequence of high selective pressure due to abusive prescription and the intake of antibiotic drugs (26) was often related to probabilistic treatment and self-medication, respectively (12).
The resistance was the highest against penicillins (amoxicillin 96.2% and clavulanic acid 90.6%) in E. coli isolates. This is in conformity with the findings of previous studies (6,19,27). These isolates were more sensible to aminosids and quinolones antibiotic classes since less than 20% of the isolates were resistant except for nalidixic acid (28.3%).
Regarding S. aureus isolates, the resistance pattern was similar to that of the E. cloacae and K. pneumoniae for penicillins, as well as the first and second generation cephalosporins. Surprisingly, as a proper characteristic, these isolates were resistant to third- and second-generation cephalosporins (77.8% for cefotaxime, ceftriaxone, and ceftazidime) and macrolides (erythromycin 77.8% and clarithromycin 88.8%). These resistance levels are troublesome regarding this pathogen. These bacteria are gaining significance as the causative agents of UTI due to their complex genetic makeup which is responsible for their pathogenicity, toxicity, and the acquisition of resistance trait (28). This genome-based life trait might justify the high rate of resistance recorded in this study. On the other hand, we should be comfortable with our assumption owing to the minor sample size of the S. aureus isolates (9 cases) in this study. Further studies with larger sample size are needed to confirm or invalidate our observation.
More importantly, R. ornithinolytica and Salmonella spp. were found to be involved in some UTI cases in this study. These bacteria are uncommon human pathogens and as a result, the infection cases are rare (29,30). The former is commonly acquired in humans through the ingestion of improperly preserved fish. According to previous research, it is involved in community-acquired cystitis Japanese woman (29). This bacterium can be a pathogen through its ability to express histidine decarboxylase which allows it to elicit histamine toxicity, which is also known as scombroidsyndrome (31). In addition, R. ornithinolytica is thought to be an emergent threat since it naturally expresses beta-lactamases which render it resistant to commonly used antibiotics (29). As regards Salmonella spp., this group of bacteria is generally responsible for typhoid fever. Many reports outlined their implication in UTIs, especially nontyphoidal strains (30,32). Similarly, this pathogen constitutes another health concern as Salmonella UTI which may complicate pyelonephritis, kidney failure, renal lithiasis, and chronic bacteriuria. Moreover, Salmonella UTI can worsen an enteric Salmonella infection (30).
This study has a few limitations. Our results were obtained from one hospital and as a result, cannot be extrapolated to all health facilities. In addition, the susceptibility profile of the isolated bacteria is partial since some antibiotics such as carbapenems were not evaluated in this study.
Conclusions
In general, a high prevalence of UTI was found among under-five children in this study. Further, the gram-negative bacteria group was the main causative agent of UTI with E. coli as the commonest isolated germs. The results of this study revealed worrying resistance rates in isolates uropathogens, especially against penicillins in addition to the first, second, and third generation cephalosporins. Since the epidemiology of UTI varies over time and due to geographical areas and the growing risk of multidrug resistance, it would be crucial to periodically monitor the sensitivity pattern of the uropathogens in order to mitigate the wastage of antibiotic drugs related to probabilistictreatment in health facilities.
Ethical Approval
The study was approved by the Regional Delegation of Public Health for Littoral Region, Douala. The ethical clearance was provided by the Bonassama District Hospital (N° HDB/140/15/2013/T). Before applying the inclusion procedure, the objectives and protocol of the study were explained to children’s parents. All children were included in the study upon parental approval following signing informed consent forms. The parents or guardians and their children were informed that their participation in the study was voluntary, and they could withdraw any time without any explanation and repercussion.
Conflict of Interest Disclosures
None.
Acknowledgements
The authors are grateful to the children who participated in the study, as well as their parents/guardians. Special thank also goes to the officials of Bonassama District Hospital for providing authorizations and facilitating data collection process. Eventually, we appreciate Mr. WEPNJE Godlove BUNDA for English editing.
References
- Laila K, Roy E, Rahman H, Roy RR. Urinary tract infection in children: An update. Bangladesh J Child Health 2012; 36(2):90-7. doi: 10.3329/bjch.v36i2.13085 [Crossref] [ Google Scholar]
- Khatoon A, Rizvi M, Sultan A, Khan F, Shukla I, Khan HM. Prevalence, microbial profile and antimicrobial sensitivity pattern of uropathogens isolated from paediatric patients. Int J Curr Microbiol Appl Sci 2015(1):10-8.
- Manjula J, Boloor R, Prabhu S. Prevalence of Urinary Tract Infection in Febrile Children below 5 Years of Age, Admitted in Tertiary Care Hospital in Dakshina Kannada District, Karnataka, India. Int J Sci Study 2014; 2(5):6-10. [ Google Scholar]
- Vasudevan R. Urinary tract infection: an overview of the infection and the associated risk factors. J Microbiol Exp 2014; 1(2):42-54. doi: 10.15406/jmen.2014.01.00008 [Crossref] [ Google Scholar]
- Akoachere JF, Yvonne S, Akum NH, Seraphine EN. Etiologic profile and antimicrobial susceptibility of community-acquired urinary tract infection in two Cameroonian towns. BMC Res Notes 2012; 5:219. doi: 10.1186/1756-0500-5-219 [Crossref] [ Google Scholar]
- Dibua UM, Onyemerela IS, Nweze EI. Frequency, urinalysis and susceptibility profile of pathogens causing urinary tract infections in Enugu State, southeast Nigeria. Rev Inst Med Trop Sao Paulo 2014; 56(1):55-9. doi: 10.1590/s0036-46652014000100008 [Crossref] [ Google Scholar]
- Festo E, Kidenya BR, Hokororo A, Mshana ME. Predictors of Urinary tract infection among febrile children attending at Bugando Medical Centre Northwestern, Tanzania. Arch Clin Microbiol 2011; 5(2):1-7. [ Google Scholar]
- Moses A, Michael E, Chukwudi A, Nwofoke OE. Asymptomatic urinary tract infection among school children in rural area of Ebonyi State. Ann Biol Res 2012; 3(5):2353-6. [ Google Scholar]
- Anigilaje EA, Bitto TT. Prevalence and predictors of urinary tract infections among children with cerebral palsy in Makurdi, Nigeria. Int J Nephrol 2013; 2013:937268. doi: 10.1155/2013/937268 [Crossref] [ Google Scholar]
- Samuel SO, Salami TAT, Adewuyi GM, Babatope E, Ekozien MI. Prevalence of Urinary Tract Infections among a cohort of HIV Positive Patients accessing care in a rural health centre in Nigeria. J Microbiol Biotechnol Res 2012; 2(4):507-10. [ Google Scholar]
- Saeed CH, AL-Otraqchi KIB, Mansoor IY. Prevalence of urinary tract infections and antibiotics susceptibility pattern among infants and young children in Erbil city. Zanco J Med Sci 2015; 19(1):915-22. doi: 10.15218/zjms.2015.0012 [Crossref] [ Google Scholar]
- Rai GK, Upreti HC, Rai SK, Shah KP, Shrestha RM. Causative agents of urinary tract infections in children and their antibiotic sensitivity pattern: a hospital based study. Nepal Med Coll J 2008; 10(2):86-90. [ Google Scholar]
- Ben Abdallah H, Sahmoun O, Ben Romdhane F. Profil de sensibilité aux antibiotiques des entérobactéries uropathogènes isolées dans la province de Monastir. Rev Tun Infect 2008; 2(2):5-8. [ Google Scholar]
- Tchendjou Takam PY. L’infection urinaire du nouveau-né et de l’enfant à l’hôpital général de Yaoundé : Aspects cliniques, biologiques, thérapeutiques et évolutifs [Thesis]. Yaoundé: Université de Yaoundé I; 2002. [French].
- Banapurmath CR, Jayamony S. Prevalence of urinary tract infection in severely malnourished preschool children. Indian Pediatr 1994; 31(6):679-82. [ Google Scholar]
- Farajnia S, Alikhani MY, Ghotaslou R, Naghili B, Nakhlband A. Causative agents and antimicrobial susceptibilities of urinary tract infections in the northwest of Iran. Int J Infect Dis 2009; 13(2):140-4. doi: 10.1016/j.ijid.2008.04.014 [Crossref] [ Google Scholar]
- Msaki BP, Mshana SE, Hokororo A, Mazigo HD, Morona D. Prevalence and predictors of urinary tract infection and severe malaria among febrile children attending Makongoro health centre in Mwanza city, North-Western Tanzania. Arch Public Health 2012; 70(1):4. doi: 10.1186/0778-7367-70-4 [Crossref] [ Google Scholar]
- Hanna-Wakim RH, Ghanem ST, El Helou MW, Khafaja SA, Shaker RA, Hassan SA. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol 2015; 5:45. doi: 10.3389/fcimb.2015.00045 [Crossref] [ Google Scholar]
- Kaur R, Walia G, Mehta M. Prevalence of Urinary tract infections in children and their sensitivity to various antibiotics. J Acad Indus Res 2012; 1(4):161-3. [ Google Scholar]
- Saravanan S. Prevalence and antimicrobial sensitivity pattern of urinary tract infection in febrile children aged 1 month to 5 years. J Dent Med Sci 2013; 10(5):15-8. [ Google Scholar]
- Ousseini KF. Etude de l’infection urinaire chez l’enfant malnutri dans le service de pédiatrie à de l’hôpital national de Niamey au Niger [Thesis]. Niamey, Niger: Abdou Moumouni University; 2011.
- Isa MA, Ismail HY, Allamin IA, Shettima A, Mustapha A. Prevalence of urinary tract infection among primary school children in Maiduguri, Borno State, Nigeria. Int J Environ 2013; 2(1):9-15. doi: 10.3126/ije.v2i1.9203 [Crossref] [ Google Scholar]
- Renda R. Diagnosis and antibiotic resistance distribution in children with urinary tract infection: a single center experience. Int J Pediatr 2018; 6(1):6815-22. doi: 10.22038/ijp.2017.28352.2462 [Crossref] [ Google Scholar]
- Aghazadeh M, Sari S, Nahaie M, Hashemi SSR, Mehri S. Prevalence and antibiotic susceptibility pattern of E coli isolated from urinary tract infection in patients with renal failure disease and renal transplant recipients. Trop J Pharm Res 2015; 14(4):649-53. doi: 10.4314/tjpr.v14i4.13 [Crossref] [ Google Scholar]
- Jitendranath A, Radhika R, Bhargavi L, Bhai G, Beevi R. Microbiological profile of urinary tract infection in pediatric population from a tertiary care hospital in South Kerala. J Bacteriol Mycol Open Access 2015; 1(1):4-7. doi: 10.15406/jbmoa.2015.01.00002 [Crossref] [ Google Scholar]
- Amine IL, Chegri M, L’Kassmi H. Épidémiologie et résistance aux antibiotiques des entérobactéries isolées d’infections urinaires à l’hôpital militaire Moulay-Ismail de Meknès [Epidemiology and antibiotic resistance of Enterobacteriaceae isolated in urinary tract infections at the Moulay Ismail Military Hospital of Meknes]. Antibiotiques 2009; 11(2):90-6. doi: 10.1016/j.antib.2008.10.004.[French] [Crossref] [ Google Scholar]
- Sharifian M, Karimi A, Tabatabaei SR, Anvaripour N. Microbial sensitivity pattern in urinary tract infections in children: a single center experience of 1,177 urine cultures. Jpn J Infect Dis 2006; 59(6):380-2. [ Google Scholar]
- Kanamori H, Parobek CM, Juliano JJ, van Duin D, Cairns BA, Weber DJ. A prolonged outbreak of KPC-3-producing Enterobacter cloacae and Klebsiella pneumoniae driven by multiple mechanisms of resistance transmission at a large academic burn center. Antimicrob Agents Chemother 2017; 61(2). doi: 10.1128/aac.01516-16 [Crossref]
- Nakasone ES, Kaneshiro R, Min K, Tokeshi J. Emergence of Raoultella ornithinolytica on O’ahu: a case of community-acquired R ornithinolytica urinary tract infection. Hawaii J Med Public Health 2015; 74(5):174-5. [ Google Scholar]
- Klosterman SA. Salmonella-related urinary tract infection in an elderly patient. BMJ Case Rep 2014; 2014. doi: 10.1136/bcr-2014-204552 [Crossref]
- Kanki M, Yoda T, Tsukamoto T, Shibata T. Klebsiella pneumoniae produces no histamine: Raoultella planticola and Raoultella ornithinolytica strains are histamine producers. Appl Environ Microbiol 2002; 68(7):3462-6. doi: 10.1128/aem.68.7.3462-3466.2002 [Crossref] [ Google Scholar]
- Allerberger FJ, Dierich MP, Ebner A, Keating MR, Steckelberg JM, Yu PK. Urinary tract infection caused by nontyphoidal Salmonella: report of 30 cases. Urol Int 1992; 48(4):395-400. doi: 10.1159/000282362 [Crossref] [ Google Scholar]