Avicenna Journal of Clinical Microbiology and Infection. 11(2):55-61.
doi: 10.34172/ajcmi.3539
Original Article
Thymol Enhances Antibiotic Sensitivity and Inhibits Biofilm Formation in Urinary Tract Infection Isolates of Escherichia coli From Gilan Province, Iran
Fatemeh Dizani  , Leila Asadpour *
, Leila Asadpour * 
Author information:
1Department of Biology, Rasht Branch, Islamic Azad University, Rasht, Iran
Abstract
Background: The present study aimed to investigate the effect of thymol on growth inhibition, biofilm inhibition, and antibiotic sensitivity of Escherichia coli isolated from urinary tract infection in Gilan province, Iran.
Methods: The disc diffusion and broth microdilution methods were used to investigate the antimicrobial effect of thymol on 30 urinary tract infection isolates of E. coli. Additionally, the time-kill assay was used to investigate the effectiveness of thymol at different time intervals over a period of 24 hours. The anti-biofilm effect of thymol was investigated using the microplate method. Moreover, the effect of thymol on fimH gene expression was investigated. To investigate the effect of thymol on the sensitivity of E. coli isolates to cefotaxime and ciprofloxacin, the checkerboard assay was used.
Results: ThymolFF exhibits inhibitory effects on the growth of E. coli isolates and the diameter of the zone of growth inhibition caused by 5 mg of thymol in the studied isolates varied between 15 and 30 mm and minimum inhibitory concentration (MIC) of thymol against MDR E. coli isolates ranged from 1 to 4 mg/mL. Depending on the concentration and the exposure time, bacterial killing was promoted by thymol treatment. The use of thymol decreased the biofilm formation of the isolates by more than 50% compared to the control and also bacterial treatment with thymol led to down-regulation of the fimH gene. Furthermore, enhancing the antibacterial effect of cefotaxime and ciprofloxacin by thymol was demonstrated.
Conclusion: The present study found that thymol, a terpenoid of plant origin, exhibited strong killing efficiency, inhibiting the biofilm formation, and enhanced antibiotic sensitivity of E. coli isolated from urinary tract infection.
Keywords: Escherichia coli, Thymol, Biofilm, Antimicrobial
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Dizani F, Asadpour L. Thymol enhances antibiotic sensitivity and inhibits biofilm formation in urinary tract infection isolates of Escherichia coli From Gilan province, Iran. Avicenna J Clin Microbiol Infect. 2024; 11(2):55-61. doi:10.34172/ajcmi.3539
Introduction
Urinary tract infections (UTIs) are the most common cause of hospital infections and the second most common infection among humans. More than 150 million cases of UTIs are reported per year worldwide, affecting all age groups. Most UTIs are caused by bacteria that are part of the normal flora, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species. The treatment of UTIsis costly and is commonly treated with antibiotics, which leads to rapid adaptation of microbiota of the gut and vagina and the development of antibiotic-resistant bacteria (1-3). About 80%-90% of non-hospital UTIs are caused by E. coli (1). This bacterium is a member of the Enterobacteriaceae family which is usually resistant to antibiotics. This resistance is due to multiple intrinsic and acquired mechanisms present in the bacterium. Normally, beta-lactam antibiotics are prescribed to treat E. coli infections, but the emergence and variety of beta-lactamase-producing species have limited the effectiveness of these strains (2). In recent years, because of the resistance of pathogens to common antibiotics and increasing interest in using natural antimicrobial substances, research on the effectiveness of antimicrobial plant substances has increased (3). Plants produce a wide range of secondary metabolites with many desirable biological functions which can protect plants against plant pathogens and against abiotic stresses (4,5). Interactions between active plant substances and antibiotics can lead to antagonistic, additive, or synergistic effects. Usually, the use of several antimicrobial substances together can disrupt several different biochemical processes which destroy microbial agents. Thymol (2-isopropyl-5-methyl phenol) is the most important phenolic monoterpene extracted from plants of the Lamiaceae family, including thyme, oregano, basil, and spearmint. Various studies have shown that thymol has strong antiseptic, anti-inflammatory, and antioxidant properties. According to the Food and Drug Administration (FDA), this substance is considered safe and can be used as a food additive (3,6,7). Moreover, the association between thymol and commercial antibiotics has been studied in several works and the results are promising. Thymol enhanced the antibacterial effect of neomycin against Staphylococcus aureus and showed synergistic effects on the activity of ceftriaxone against Enterococcus faecalis (8). Besides, thymol in combination with streptomycin and kanamycin showed synergic effects against K. pneumoniae biofilms (9). In another study, thymol has been shown to be synergistic with streptomycin and gentamicin against S. aureus and with streptomycin against Streptococcus agalactiae. In addition, thymol showed synergistic activity with chloramphenicol against Acinetobacter baumannii (10). Thymol increases the sensitivity of colistin-resistant gram-negative bacteria to colistin and may be a promising treatment option against infections caused by these bacteria (11). However, there is a lack of studies on the synergistic activity of thymol combined with antibiotics which are commonly used in the treatment of UTIs caused by drug-resistant E. coli isolates. The purpose of this study was to investigate the effect of thymol on growth inhibition, biofilm inhibition, and antibiotic sensitivity of E. coli isolated from UTI using time-kill assay, microtiter plate method, and checkerboard assay in Gilan province, Iran.
Materials and Methods
Test Bacteria
In this study, a total of 30 E. coli were isolated from UTIs in Gilan province, Iran, by transferring clean-voided midstream urine samples to blood agar and MacConkey (MC) agar media using a 0.01-ml platinum loop. After incubation at 37 °C for 24 hours, UTI was diagnosed as described previously (2) and isolates were identified by biochemical tests which include catalase, oxidase, motility, indole production, urease production, methyl red, citrate utilization, growth on triple sugar iron agar (TSI) and lysine iron agar (LIA), and oxidative/fermentative degradation of glucose (3).
The resistance pattern of E. coli to selected antibiotics was established using an antibiogram test (disk diffusion) following the CLSI 2021 guidelines. Antibiotic discs used in this study included gentamicin (10 µg), ceftazidime (30 µg), cefotaxime (30 µg), ampicillin/sulbactam (30 µg), meropenem (10 µg), piperacillin (100 µg), imipenem (10 µg), piperacillin/tazobactam (36 µg), and ciprofloxacin (5 µg), which were purchased from Himedia (India). E. coli ATCC 25922 was included for quality control, and breakpoints were in accordance with CLSI guidelines. Then, the number of strains with multiple drug resistance (MDR) was determined based on CDC definition (12).
Biofilm Forming Ability
Phenotypic assessment of the biofilm-forming ability of test isolates has been done by microplate method. Briefly, standard overnight cultures (1.5 × 108 CFU/mL) of test bacteria have been diluted 100 folds in Tryptic soy broth containing 1% glucose. Subsequently, 200 µL of each dilution was transferred to individual wells of a 96-well plate and incubated for 48 hours at 37 °C. After incubation, the wells were washed with PBS (three times) to remove planktonic bacteria and then fixed with methanol for 20 minutes. Thereafter, each well was stained with 200 μL of 0.02% crystal violet and washed with distilled water for 5 minutes. After drying the plates and adding 200 μL of 33% glacial acetic acid to each well, biofilm was quantitatively analyzed by reading the OD of the wells at 570 nm with an ELISA reader. The level of biofilm formation has been determined according to OD values (OD ≤ ODc = no biofilm producer; ODc < OD ≤ 2 × ODc = weak biofilm producer; 2 × ODc < OD ≤ 4 × ODc = moderate biofilm producer; 4 × ODc < OD = strong biofilm producer) (13).
Investigation of the Inhibitory Effect of Thymol on Escherichia coli Isolates
The disc diffusion method was used to investigate the antimicrobial effect of thymol on clinical isolates of E. coli. For this purpose, 100 µL of standard overnight bacterial suspension (1.5 × 108 CFU/mL) was cultured on Mueller Hinton Agar. Afterwards, the standard discs were coated with 1, 2.5, 5, and 10 mg of thymol and transferred to the culture. Finally, the plates were incubated for 24 hours at 37 °C and the diameter of the zone of inhibition was measured. This experiment was performed in triplicate.
Determination of Minimum Inhibitory Concentration of Thymol
The minimum inhibitory concentration (MIC) of thymol against 10 selected MDR E. coli isolates was obtained by broth microdilution method. To perform this experiment, serial dilution of thymol including 0.5-32 mg/mL was prepared in 100 μL of Mueller Hinton broth medium. Then, 100 µL of standard overnight bacterial suspension (1.5 × 108 CFU/mL) was added to each well. Then, the plate was incubated for 24 hours at 37°C. This experiment was done in duplicate (10).
Investigation of the Anti-biofilm Effect of Thymol
The anti-biofilm effect of thymol was investigated using the microplate method. The procedure was the same as the phenotypic evaluation of biofilm-forming ability, with the difference that the trypticase broth medium contained a sub-MIC of thymol for each isolate (ranging from 0.5 to 2 mg/mL). All tests have been performed in triplicate and the percentage of inhibition was calculated as described previously (14).
Time-kill Assay for Thymol
The effectiveness of thymol against clinical isolate of MDR E. coli has been determined by time-kill assay at different time intervals over a period of 24 hours. In these assays, overnight bacterial suspension (5 × 105 CFU/mL) was incubated in a 96-well plate containing adjusted Mueller Hinton broth supplemented with thymol in separate wells. For the determination of cell viability, aliquots were removed from each tube after 0, 2, 4, 8, 12, and 24 hours of incubation and subsequently were diluted serially (1:10) with PBS. Each dilution was cultured on Muller-Hinton agar plates. The time-kill experiment was performed in triplicate (5).
Investigation of the Effect of Thymol on Antibiotic Sensitivity of Escherichia coli Isolates
The checkerboard assay was used to investigate the effect of thymol on the sensitivity of E. coli isolates to cefotaxime and ciprofloxacin. In brief, in a 96-well microplate, thymol and selected antibiotic were diluted in MHB and 8 gradient concentrations of each, including 1/32 × MIC- 4 × MIC, were prepared. Each horizontal row of wells contained a similar level of antibiotic concentration and each longitudinal column had an equal amount of thymol. Each well had a total volume of 200 μL, including 100 μL of bacterial suspension (5 × 105 CFU/mL), 50 μL of thymol, and 50 μL of cefotaxime/ciprofloxacin. Moreover, several control wells, including drug-free, bacteria-free, and single antibacterial agent, were used. E. coli ATCC 25922 was used as sensitivity control strain. After 24 hours of incubation at 37 °C, the MIC values were read. Each test was done three times. Synergy was determined by the calculation of the fractional inhibitory concentrations index (FICI) as described earlier (15).
Investigation of the Effect of Thymol on fimH Gene Expression
Total RNA was extracted from E. coli isolate treated with a sub-MIC of thymol (1 mg/mL) using the Cinagen kit based on the manufacturer’s protocols and subsequently subjected to DNase treatment. Bacterial culture without the presence of antimicrobial substances was used as a control. Qualitative and quantitative analysis of extracted RNA was done using agarose gel electrophoresis and a NanoDrop spectrophotometer, respectively. Then, 0.2 µg of the extracted RNA was immediately used to synthesize cDNA using the Cinagen RT kit and thermal treatment was performed at 85 °C for 5 seconds, followed by incubation at 42 °C for 60 minutes. Then, the synthesized cDNA was used as a template in real-time PCR. In this study, the gapA gene was used as an internal control. Primer sequences for amplification of fimH gene were the same as described previously (16). The polymerase chain reaction was done using the Genet bio kit (CAT.NO: Q9210, South Korea). The reaction was performed under the following conditions: initial annealing steps at 95 °C for 1 minute, 35 thermal cycles of 95 °C for 30 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds. Gene expression change was calculated using 2-ΔΔCT method (16). A no-template sample was included as a negative control in each run for each gene. This reaction was repeated 3 times.
Statistical Analysis
In this study, t test was used to analyze the difference between the control and treatment groups in SPSS. A P value of < 0.05 was considered statistically significant.
Results
Test Bacteria
Among tested isolates, 25 isolates showed MDR phenotype and all of them showed biofilm-forming ability in phenotypic assay. Among them, 10 isolates which were MDR, strong biofilm former, and resistant to both antibiotics (cefotaxime and ciprofloxacin) were selected for further investigations.
Antibacterial Effects of Thymol against Escherichia coli Isolates
The effect of thymol on inhibiting the growth of E. coli isolates was investigated by diffusion method and MIC determination. The diameter of the zone of growth inhibition caused by 5 mg of thymol in the studied isolates varied between 15 and 30 mm (Figure 1). The MIC of thymol against MDR E. coli isolates ranged from 1 to 4 mg/mL.
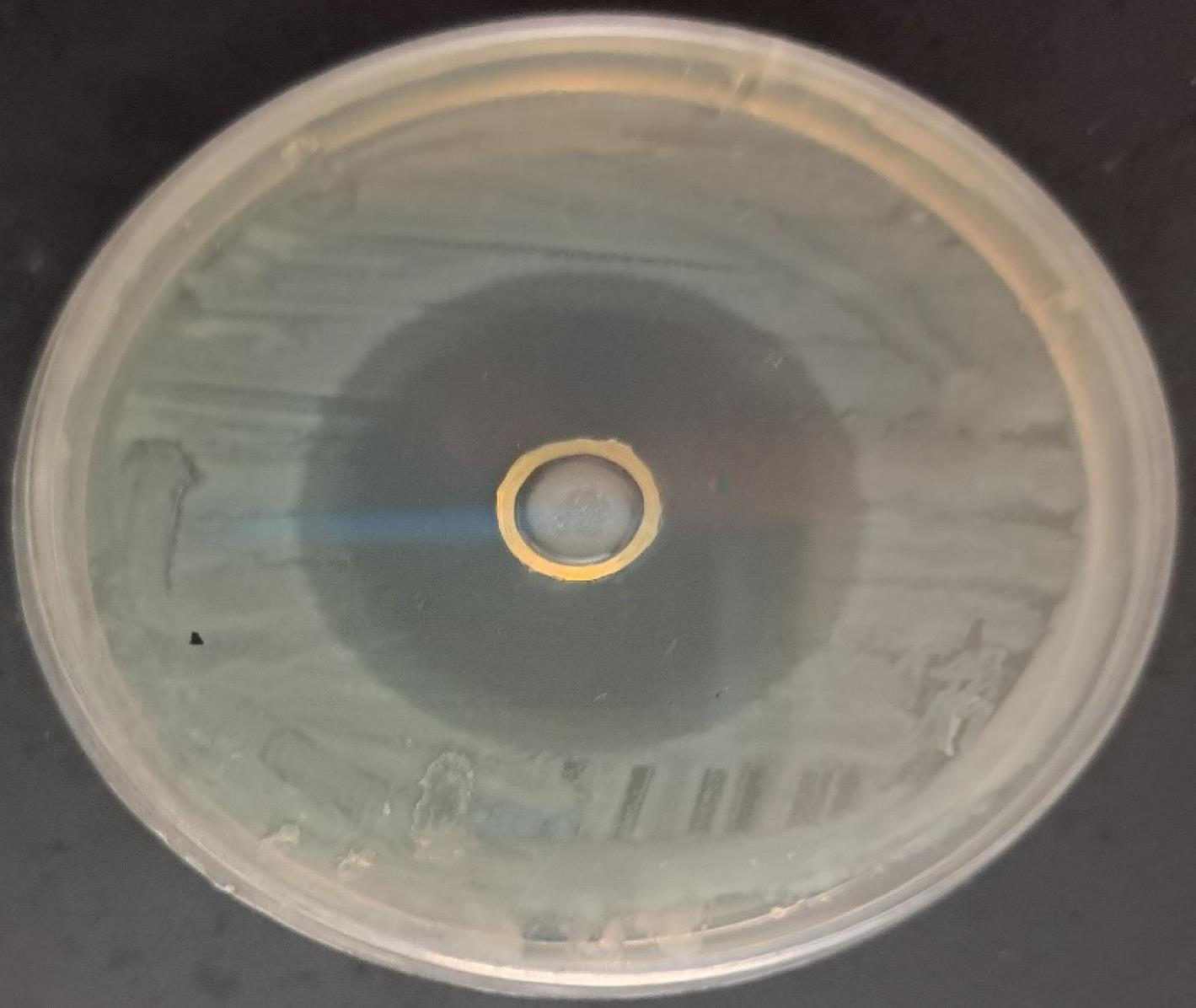
Figure 1.
Growth Inhibition of Escherichia coli Isolate Caused by 5 mg of Thymol
.
Growth Inhibition of Escherichia coli Isolate Caused by 5 mg of Thymol
The Effect of Thymol on Antibiotic Sensitivity of Escherichia coli Isolates
The measurement of the FIC index of thymol and cefotaxime in 10 MDR E. coli isolates (Table 1) showed that thymol and cefotaxime had additive and synergism effects in 8 and 2 isolates, respectively. The use of thymol in combination with cefotaxime reduced the MIC values of this antibiotic 2 to 8 times, but in most cases, no decrease was observed in the MIC of thymol. Thymol and ciprofloxacin had an additive effect in all the tested isolates (Table 2), and the use of thymol in combination with this antibiotic caused a 2-64-fold decrease in the MIC of ciprofloxacin. Enhancing the antibacterial effect of cefotaxime and ciprofloxacin by thymol (obtained from the disc diffusion method) is presented in Figures 2 and 3.
Table 1.
The Effect of the Combination of Cefotaxime and Thymol on MDR Escherichia coli Isolates
|
Bacterial test
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
| MIC of cefotaxime |
64 |
32 |
64 |
64 |
1024 |
512 |
512 |
128 |
32 |
128 |
| MIC of thymol |
4 |
1 |
2 |
2 |
2 |
1 |
4 |
2 |
1 |
2 |
| MIC of cefotaxime in combination with thymol |
4 |
16 |
4 |
8 |
256 |
256 |
64 |
64 |
16 |
16 |
| MIC of thymol in combination with cefotaxime |
4 |
1 |
2 |
2 |
1 |
1 |
2 |
2 |
1 |
2 |
| FIC index |
1.12 |
1.5 |
1.06 |
1.25 |
0.75 |
1.5 |
0. 5 |
1.5 |
1.5 |
1.12 |
MIC: Minumim inhibitory concentration, FIC: Fractional inhibitory concentration.
Table 2.
The Effect of the Combination of Ciprofloxacin and Thymol on Multidrug-Resistant Escherichia coli Isolates
|
Bacterial Test
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
| MIC of ciprofloxacin |
128 |
64 |
16 |
1024 |
1024 |
16 |
512 |
64 |
64 |
128 |
| MIC of thymol |
4 |
2 |
4 |
2 |
2 |
1 |
4 |
2 |
1 |
2 |
| MIC of ciprofloxacin in combination with thymol |
16 |
1 |
1 |
256 |
32 |
8 |
16 |
32 |
4 |
8 |
| MIC of thymol in combination with ciprofloxacin |
4 |
2 |
4 |
2 |
2 |
1 |
4 |
2 |
1 |
2 |
| FIC index |
1.12 |
1.02 |
1.06 |
1.25 |
1.03 |
1.5 |
1. 03 |
1.5 |
1.06 |
1.06 |
MIC: Minumim inhibitory concentration, FIC: Fractional inhibitory concentration.
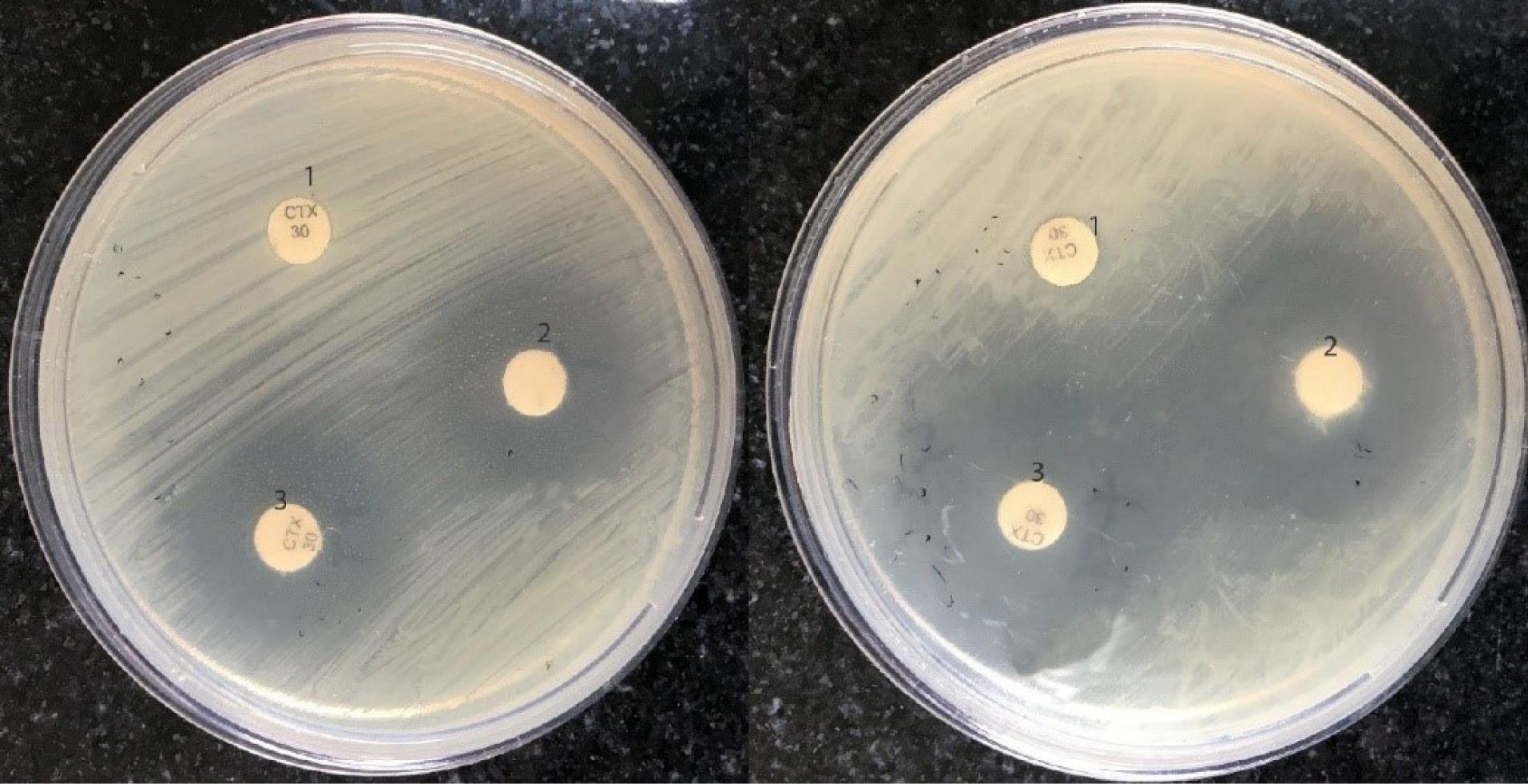
Figure 2.
Growth Inhibition of Escherichia coli Isolates by Cefotaxime (1), Thymol (2), and the Combination of Thymol and Cefotaxime (3) Using the Disc Diffusion Method
.
Growth Inhibition of Escherichia coli Isolates by Cefotaxime (1), Thymol (2), and the Combination of Thymol and Cefotaxime (3) Using the Disc Diffusion Method
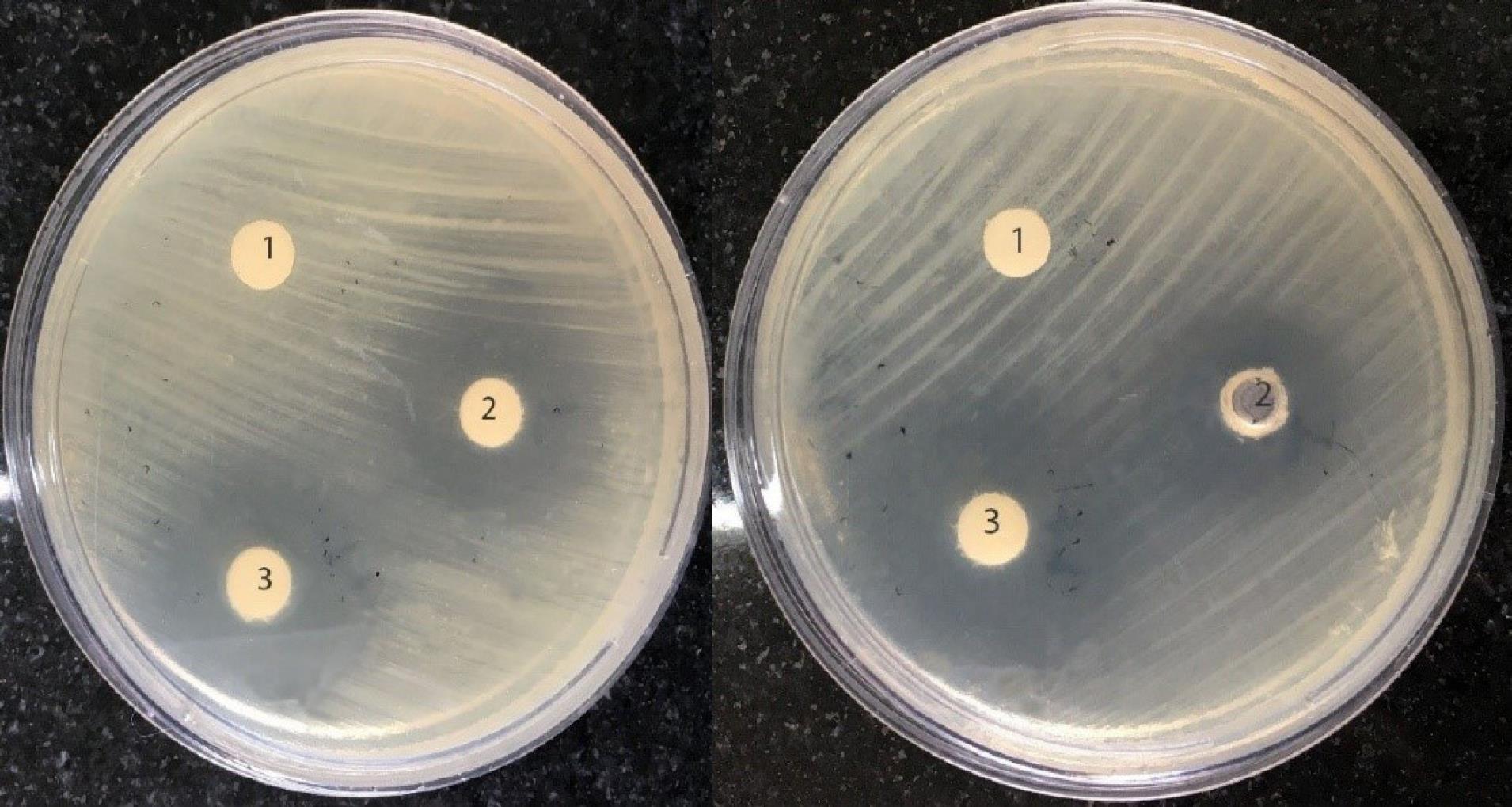
Figure 3.
Growth Inhibition of Escherichia coli Isolates by Ciprofloxacin (1), Thymol (2), and the Combination of Thymol and Ciprofloxacin (3) Using the Disc Diffusion Method
.
Growth Inhibition of Escherichia coli Isolates by Ciprofloxacin (1), Thymol (2), and the Combination of Thymol and Ciprofloxacin (3) Using the Disc Diffusion Method
Investigation of the Anti-biofilm Effect of Thymol
Thymol inhibited the biofilm of E. coli in all studied isolates compared to the control (P˂0.05). The use of thymol decreased the biofilm formation of the isolates by more than 50% compared to the control. The inhibitory effect of thymol on the biofilm formation of each test isolate is shown in Figure 4.
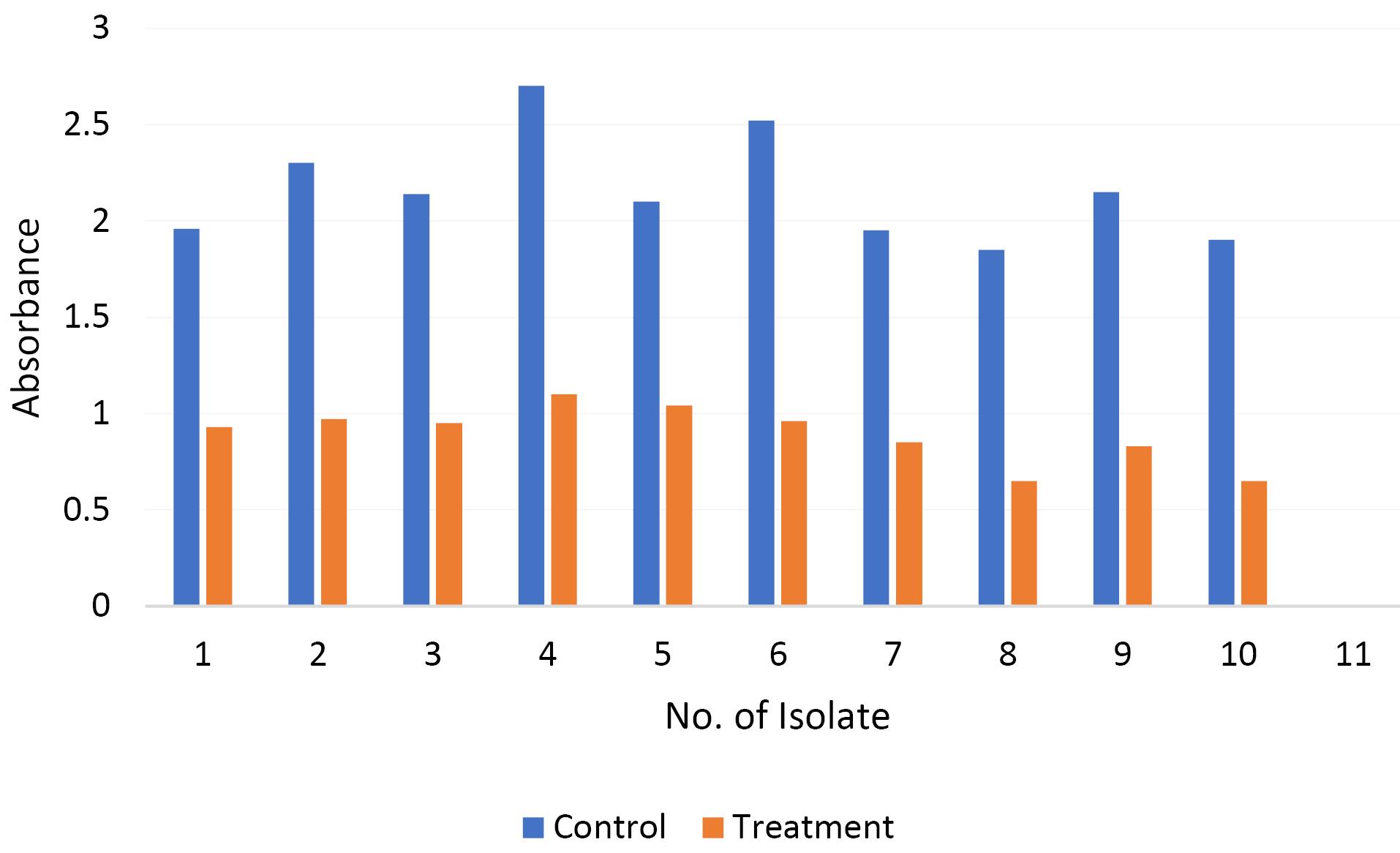
Figure 4.
The Anti-biofilm Effect of Thymol on Multidrug-Resistant Escherichia coli Strains. The diagram shows the absorbance of E. coli isolates in the absence (control) and presence of sub-MIC of thymol (treatment). Thymol decreased the biofilm formation of the isolates by more than 50% compared to the control
.
The Anti-biofilm Effect of Thymol on Multidrug-Resistant Escherichia coli Strains. The diagram shows the absorbance of E. coli isolates in the absence (control) and presence of sub-MIC of thymol (treatment). Thymol decreased the biofilm formation of the isolates by more than 50% compared to the control
Time-kill Assay for Thymol
The bactericidal activity of thymol against the selected E. coli isolates with regard to the changes in the log10 CFU/mL of viable cells is shown in Figure 5. In the time-kill curve assay (Figure 1), after 24 hours of incubation at 37 °C, the bacterial population reached approximately 12 log10 CFU/mL in the control group. When test bacteria were treated with thymol at a concentration of 0.5 × MIC, a gradual decrease in bacterial counts was observed during incubation, and the bacterial population showed a reduction of 2.8-3.4 log10 after 24 hours of exposure. At MIC of thymol, the viability of test bacteria was sharply decreased and no viable cells were detected after 8-12 hours in tested isolates. A sharp drop in the number of viable E. coli cells was detected in treatment with thymol at 2 × MIC and no viable cells were observed within 2-4 hours of incubation. The concentration of thymol and the duration of exposure were also considered to be important factors for promoting bacterial reduction following treatment with thymol.
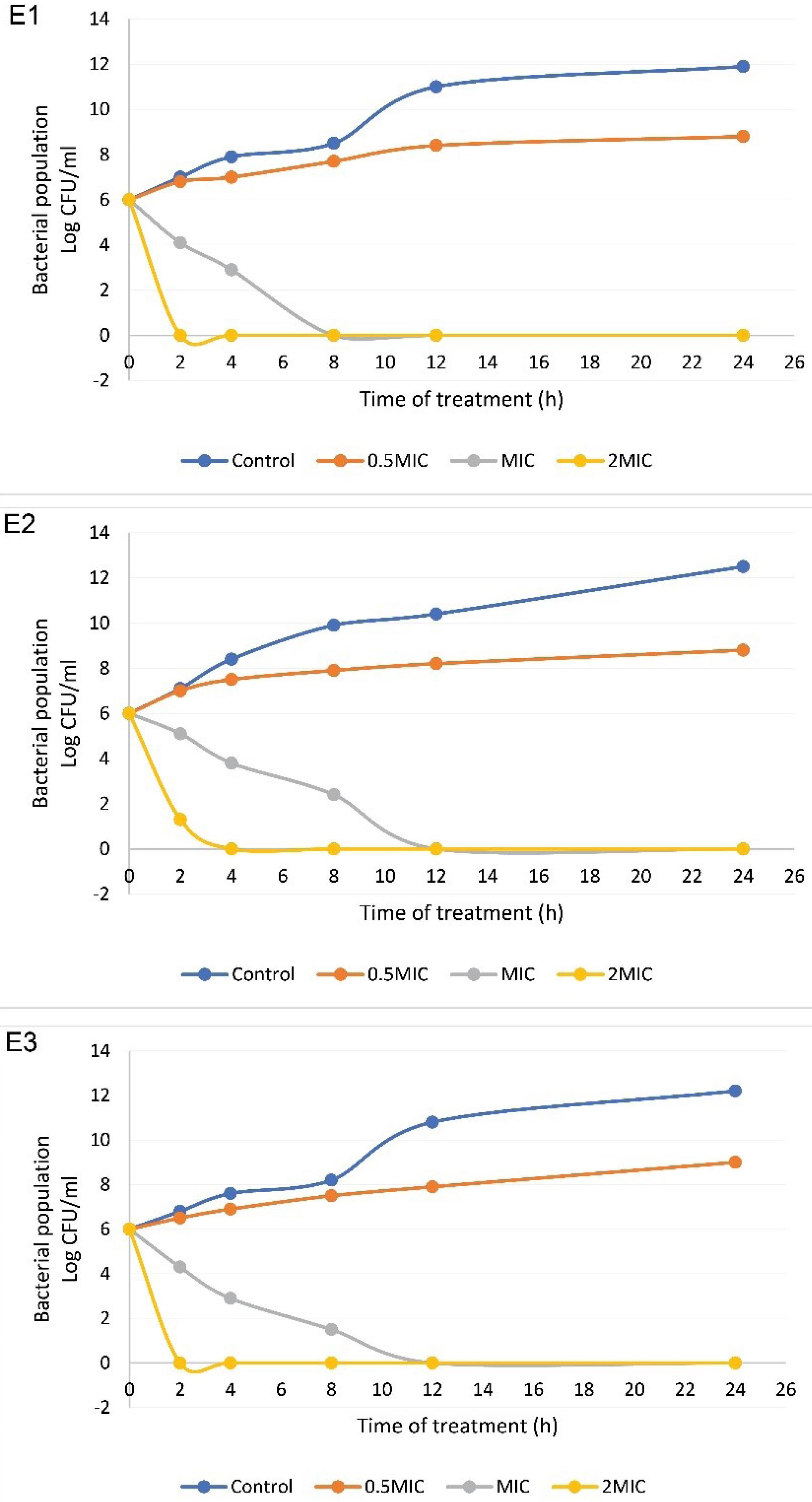
Figure 5.
Bacterial Killing Kinetics of Thymol at 0.5 × MIC, MIC, and 2 × MIC Compared with Control against Selected E. coli Isolates. Treatment with thymol at 0.5 × MIC led to a reduction of 2.8-3.4 log10 CFU/mL after 24 hours of exposure. No viable cells were detected after 8–12 hours in tested isolates by thymol at MIC and E. coli cells decreased sharply by thymol at 2 × MIC and no viable cells were observed within 2-4 hours of treatment
.
Bacterial Killing Kinetics of Thymol at 0.5 × MIC, MIC, and 2 × MIC Compared with Control against Selected E. coli Isolates. Treatment with thymol at 0.5 × MIC led to a reduction of 2.8-3.4 log10 CFU/mL after 24 hours of exposure. No viable cells were detected after 8–12 hours in tested isolates by thymol at MIC and E. coli cells decreased sharply by thymol at 2 × MIC and no viable cells were observed within 2-4 hours of treatment
The Effect of Thymol on the Expression Level of the fimA Gene
The presence of the fimH gene in the studied isolates of E. coli was detected by the PCR. Examining the expression of fimH in E. coli isolates treated with a sub-MIC of thymol showed that the difference between the treatment and control groups was significant at the probability level of 1% and thymol significantly caused a reduction in the expression of this gene compared to the control group (Figure 6).
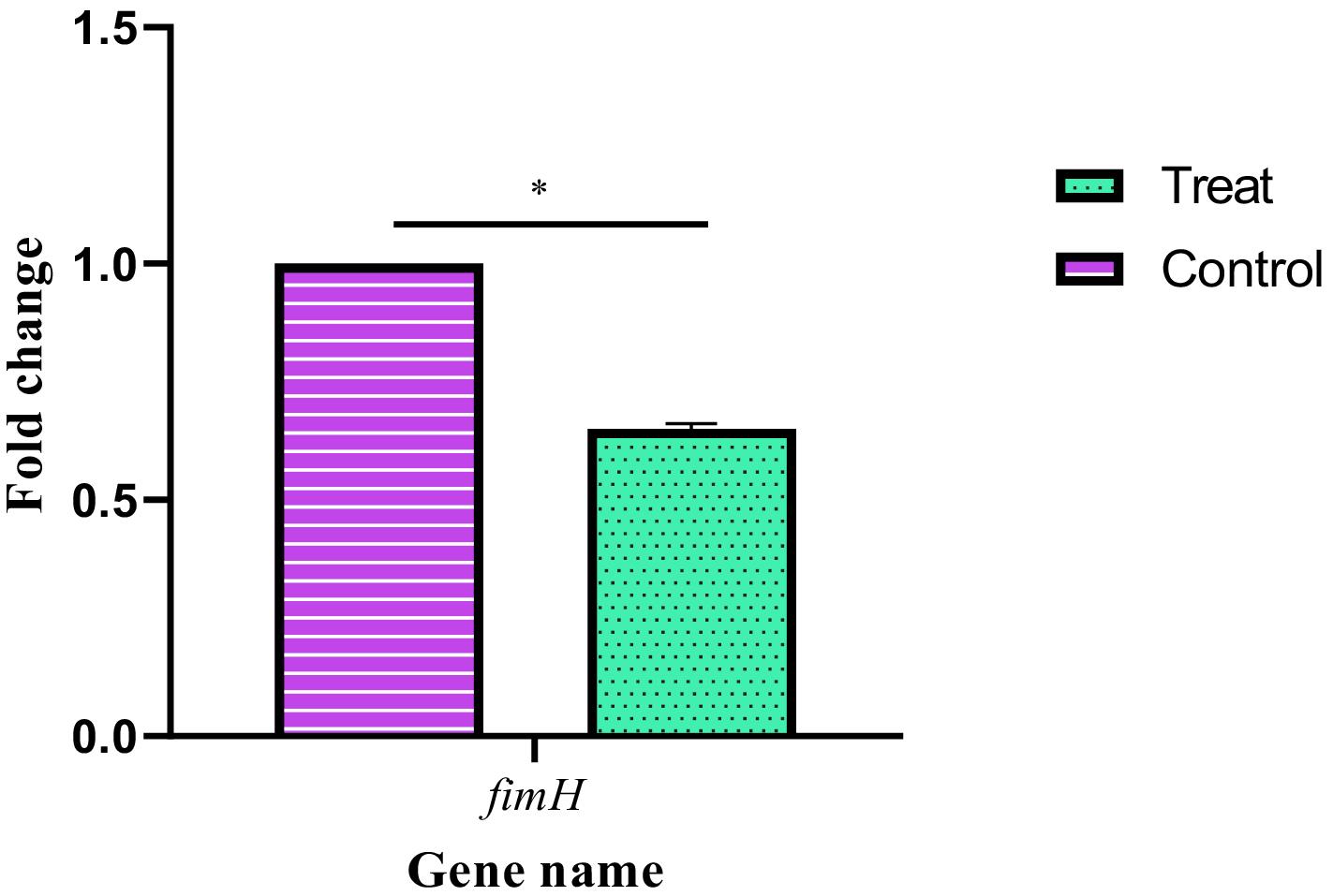
Figure 6.
Changes in Expression Level of fimH Gene in E. coli Isolates Treated with Thymol
.
Changes in Expression Level of fimH Gene in E. coli Isolates Treated with Thymol
Discussion
Urinary tract infections are the most common cause of hospital infections and the second most common infection in humans. About 80%-90% of non-hospital UTIs are caused by E. coli. Since the resistance to antibiotics in the treatment of infections has increased in recent years, the need for new resources and solutions to deal with infections and pathogenic bacteria is felt (1,4,17). The secondary metabolism of plants produces many types of molecules with potential antimicrobial activity. Most of these molecules have weaker antibacterial activity compared to antibiotics but they can act as synergism with antimicrobial drugs to enhance their effect. Thymol is the main active ingredient in thyme oil and extract, and their antimicrobial, antifungal, and antibacterial properties have been proven (5). In the present study, the antimicrobial effect of thymol on urinary isolates of E. coli and its synergistic effect with cefotaxime and ciprofloxacin, which are widely used drugs in the treatment of UTIs, were studied. Thymol has an inhibitory effect on all MDR isolates studied. The lowest growth inhibitory concentration (MIC) of thymol in the examined isolates ranged from 1 to 4 mg/mL. In the agar diffusion method, using the combination of thymol with cefotaxime and ciprofloxacin caused an increase in the diameter of the zone of growth inhibition of the studied bacteria. In addition, thymol in combination with cefotaxime had additive (83.33%) and synergistic (16.66%) effects and in combination with ciprofloxacin in all cases (100%), there were additive effects on tested E. coli isolates. The use of thymol in combination with these antibiotics significantly reduced the MIC of the antibiotic. The use of thymol in combination with cefotaxime caused a 2- to 8-fold decrease in the MIC of cefotaxime, but in most cases, a decrease in the MIC of thymol was not observed. Besides, the use of thymol in combination with ciprofloxacin caused a 2- to 64-fold decrease in the MIC of ciprofloxacin.
The use of herbal antimicrobial compounds in combination with antibiotics can reduce the MIC of antibiotics and prevent the increase in drug resistance (18,19). Since essential oils alone do not have a specific target against bacteria, the risk of resistance is negligible (20) and they can act as antibiotic adjuvants, promising new therapeutic applications (21). Antibiotics may have a different and stronger mechanism of action in the presence of essential oils and thus prevent bacterial resistance (22). Thymol significantly decreased the MIC values of cefotaxime and ciprofloxacin against test bacteria. It even caused the recovery of the sensitivity of the studied bacteria to cefotaxime and ciprofloxacin (according to the MIC). During the last ten years, the effectiveness of herbal compounds in combination with antibiotics, and the use of their combination in reducing the effective concentration of antibiotics, reducing side effects, and preventing the spread of drug resistance have been reported (23,24). Although it is not clearly known how plant compounds can decrease bacterial resistance to antibiotics, several mechanisms are proposed in this context. Due to the hydrophobic nature of plant essential oils and their components, they interact with lipid-containing bacterial membranes and increase their permeability (25,26). The change in the permeability of the target membranes can increase the absorption of antibiotics by bacterial cells. Another plausible explanation is that the plant molecules inhibit the efflux pump activity in bacterial cells that are responsible for reducing the concentration of antibiotics in bacteria (27). Additionally, active compounds such as plant extracts are known as important sources of destroying biofilms. Herbal medicines and their derivatives not only play an effective role in the treatment of biofilm-based diseases but also have fewer side effects compared to chemical medicines (28). The presence of compounds such as flavonoids including quercetin, kaempferol, naringenin, and apigenin, which have the ability to suppress the chromium sensing system for cell-to-cell communication, can explain the inhibitory effect of thymol on biofilm formation. These compounds suppress the expression of genes involved in bacterial biofilm formation and biofilm formation regulators (29-31). It is noteworthy that our results confirmed that bacterial treatment with thymol leads to down-regulation of fimH which is a biofilm-associated gene.
Moreover, to determine the bactericidal power of various concentrations of thymol, a time-kill assay was performed against E. coli isolates. In this assay, thymol showed killing activities against tested isolates in a concentration and time-dependent manner. The main reason for the killing activity of thymol is its interaction with microbial plasma membranes which can cause them to collapse (32). Additionally, a previous related study revealed the advanced killing potential of thymol againsta time and temperature-optimized attached Listeria monocytogenes population in lettuce (33). Moreover, the killing potential of thymol against C. albicans, S. mutans, and Enterobacter spp. has been elucidated (34,35).
The present study has some limitations. It only investigated the effect of thymol on a limited number of E. coli isolates; thus, larger samples should be investigated in further studies to confirm the findings. In addition, further research is needed to investigate the effect of thymol and its combination with antibiotics on other bacterial species and in vivo conditions to support the use of thymol in clinical therapy.
Conclusion
The present study found that thymol, a terpenoid of plant origin, exhibited strong killing efficiency, inhibiting the biofilm formation, and enhanced antibiotic sensitivity of E. coli isolated from UTI. These findings indicated that thymol can be used as a novel antibacterial agent against MDR and biofilm-associated infections. The results showed that it can be used in combination with antibiotics to enhance their antibacterial potential.
Acknowledgments
The authors would like to thank the Islamic Azad University, Rasht Branch, Rasht, Iran, for their support.
Authors’ Contribution
Conceptualization: Leila Asadpour.
Data curation: Fatemeh Dizani.
Formal analysis: Fatemeh Dizani, Leila Asadpour.
Funding acquisition: Fatemeh Dizani, Leila Asadpour.
Investigation: Fatemeh Dizani.
Methodology: Fatemeh Dizani, Leila Asadpour.
Project administration: Leila Asadpour.
Resources: Fatemeh Dizani, Leila Asadpour.
Supervision: Leila Asadpour.
Validation: Fatemeh Dizani, Leila Asadpour.
Visualization: Leila Asadpour.
Writing–original draft: Fatemeh Dizani.
Writing–review & editing: Leila Asadpour.
Competing Interests
The authors declare that there is no conflict of interests.
Ethical Approval
Not applicable.
Funding
The authors did not receive any financial support.
References
- Jalalpoor S. Antibiotic resistant pattern in ESBLs producer Klebsiella pneumoniae strains isolated of hospitalized and out patients acquired urinary tract infection. J Isfahan Med Sch 2011;29(142):695-706. [Persian].
- Sadeghi H, Gheibi N, Karimi Dermani F, Gholamzadeh Khoei S. A retrospective cross-sectional survey on urinary tract infections in a non-hospital medical laboratory. Iran J Med Microbiol 2022; 16(5):465-71. doi: 10.30699/ijmm.16.5.465.[Persian] [Crossref] [ Google Scholar]
- Shivaee A, Mirshekar M, Sadeghi Kalani B, Bordbar D, Ohadi E, Masjedian Jazi F. Evaluation of the effects of curcumin nanoparticles on the expression of genes involved in biofilm formation in UPEC. Infection Epidemiology and Microbiology 2018; 4(4):115-21. [ Google Scholar]
- Heidari-Soureshjani R, Heidari M, Doosti A. Epidemiology of urinary tract infection and antibiotic resistance pattern of E. coli in patients referred to Imam Ali hospital in Farokhshahr, Chaharmahal va Bakhtiari, Iran. J Shahrekord Univ Med Sci 2013;15(2):9-15. [Persian].
- Fahimirad S, Abtahi H, Razavi SH, Alizadeh H, Ghorbanpour M. Production of recombinant antimicrobial polymeric protein beta casein-E 50-52 and its antimicrobial synergistic effects assessment with thymol. Molecules 2017; 22(6):822. doi: 10.3390/molecules22060822 [Crossref] [ Google Scholar]
- Ghorbanpour M, Fahimirad S. Plant nanobionics a novel approach to overcome the environmental challenges. In: Ghorbanpour M, Varma A, eds. Medicinal Plants and Environmental Challenges. Cham: Springer; 2017. p. 247-57. 10.1007/978-3-319-68717-9_14.
- Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022; 27(1):313. doi: 10.3390/molecules27010313 [Crossref] [ Google Scholar]
- Veras HN, Rodrigues FF, Botelho MA, Menezes IR, Coutinho HD, Costa JG. Enhancement of aminoglycosides and β-lactams antibiotic activity by essential oil of Lippiasidoides Cham and the thymol. Arab J Chem 2017; 10:S2790-5. doi: 10.1016/j.arabjc.2013.10.030 [Crossref] [ Google Scholar]
- Bisso Ndezo B, Tokam Kuaté CR, Dzoyem JP. Synergistic antibiofilm efficacy of thymol and piperine in combination with three aminoglycoside antibiotics against Klebsiella pneumoniae biofilms. Can J Infect Dis Med Microbiol 2021; 2021:7029944. doi: 10.1155/2021/7029944 [Crossref] [ Google Scholar]
- Gan C, Langa E, Valenzuela A, Ballestero D, Pino-Otín MR. Synergistic activity of thymol with commercial antibiotics against critical and high WHO priority pathogenic bacteria. Plants (Basel) 2023; 12(9):1868. doi: 10.3390/plants12091868 [Crossref] [ Google Scholar]
- Yao Z, Feng L, Zhao Y, Zhang X, Chen L, Wang L. Thymol increases sensitivity of clinical Col-R gram-negative bacteria to colistin. Microbiol Spectr 2022; 10(4):e0018422. doi: 10.1128/spectrum.00184-22 [Crossref] [ Google Scholar]
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3):268-81. doi: 10.1111/j.1469-0691.2011.03570.x [Crossref] [ Google Scholar]
- Mukane L, Racenis K, Rezevska D, Petersons A, Kroica J. Anti-biofilm effect of bacteriophages and antibiotics against uropathogenic Escherichia coli. Antibiotics (Basel) 2022; 11(12):1706. doi: 10.3390/antibiotics11121706 [Crossref] [ Google Scholar]
- Martínez A, Manrique-Moreno M, Klaiss-Luna MC, Stashenko E, Zafra G, Ortiz C. Effect of essential oils on growth inhibition, biofilm formation and membrane integrity of Escherichia coli and Staphylococcus aureus. Antibiotics (Basel) 2021; 10(12):1474. doi: 10.3390/antibiotics10121474 [Crossref] [ Google Scholar]
- Cai W, Fu Y, Zhang W, Chen X, Zhao J, Song W. Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol 2016; 16(1):181. doi: 10.1186/s12866-016-0797-1 [Crossref] [ Google Scholar]
- Shakerimoghaddam A, Ghaemi EA, jamalli A. Effects of Zno nanoparticles on initial adhesion and FimH gene expression level of uropathogenic Escherichia coli. J Clin Basic Res 2017; 1(3):25-8. doi: 10.18869/acadpub.jcbr.1.3.25 [Crossref] [ Google Scholar]
- Mobasherizadeh M, Bidoki SK, Mobasherizadeh S. Prevalence of CTX-M genes in Escherichia coli strains in outpatient and inpatient cases with urinary tract infections in Isfahan, Iran. J Isfahan Med Sch 2015;33(360):2019-25. [Persian].
- Silva DM, Costa PA, Ribon AO, Purgato GA, Gaspar DM, Diaz MA. Plant extracts display synergism with different classes of antibiotics. An Acad Bras Cienc 2019; 91(2):e20180117. doi: 10.1590/0001-3765201920180117 [Crossref] [ Google Scholar]
- Yang L, Wang K, Li H, Denstedt JD, Cadieux PA. The influence of urinary pH on antibiotic efficacy against bacterial uropathogens. Urology 2014;84(3):731.e1-731.e7. 10.1016/j.urology.2014.04.048.
- Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 2014; 40(1):76-94. doi: 10.3109/1040841x.2013.763219 [Crossref] [ Google Scholar]
- Kissels W, Wu X, Santos RR. Short communication: Interaction of the isomers carvacrol and thymol with the antibiotics doxycycline and tilmicosin: in vitro effects against pathogenic bacteria commonly found in the respiratory tract of calves. J Dairy Sci 2017; 100(2):970-4. doi: 10.3168/jds.2016-11536 [Crossref] [ Google Scholar]
- Yap PS, Yiap BC, Ping HC, Lim SH. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J 2014; 8:6-14. doi: 10.2174/1874285801408010006 [Crossref] [ Google Scholar]
- Mahmoudi H, Zare Fahim N, Alikhani MY, Shokoohizadeh L. Investigation of antimicrobial effect of berberine on ciprofloxacin and imipenem resistance Acinetobacter baumannii isolated from Hamadan hospitals. Iran J Med Microbiol 2020; 14(1):44-54. doi: 10.30699/ijmm.14.1.44.[Persian] [Crossref] [ Google Scholar]
- Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms 2021; 9(10):2041. doi: 10.3390/microorganisms9102041 [Crossref] [ Google Scholar]
- Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 2002; 46(6):1914-20. doi: 10.1128/aac.46.6.1914-1920.2002 [Crossref] [ Google Scholar]
- Ultee A, Bennik MH, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 2002; 68(4):1561-8. doi: 10.1128/aem.68.4.1561-1568.2002 [Crossref] [ Google Scholar]
- Johny AK, Hoagland T, Venkitanarayanan K. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 to antibiotics. Foodborne Pathog Dis 2010; 7(10):1165-70. doi: 10.1089/fpd.2009.0527 [Crossref] [ Google Scholar]
- Shafiei M, Abdi Ali A, Shahcheraghi F, Saboora A, Akbari Noghabi K. Eradication of Pseudomonas aeruginosa biofilms using the combination of n-butanolic Cyclamen coum extract and ciprofloxacin. Jundishapur J Microbiol 2014; 7(2):e14358. doi: 10.5812/jjm.14358 [Crossref] [ Google Scholar]
- Liu Y, Wu L, Han J, Dong P, Luo X, Zhang Y. Inhibition of biofilm formation and related gene expression of Listeria monocytogenes in response to four natural antimicrobial compounds and sodium hypochlorite. Front Microbiol 2020; 11:617473. doi: 10.3389/fmicb.2020.617473 [Crossref] [ Google Scholar]
- Mohammadzadeh A, Sharifi A. Bacterial biofilm and its inhibition mechanisms relying on anti-biofilm properties of plant compounds. Exp Anim Biol 2016;5(1):71-81. [Persian].
- Yuan W, Yuk HG. Effects of sublethal thymol, carvacrol, and trans-cinnamaldehyde adaptation on virulence properties of Escherichia coli O157:H7. Appl Environ Microbiol 2019; 85(14):e00271-19. doi: 10.1128/aem.00271-19 [Crossref] [ Google Scholar]
- Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules 2020; 25(18):4125. doi: 10.3390/molecules25184125 [Crossref] [ Google Scholar]
- Kostoglou D, Tsaklidou P, Iliadis I, Garoufallidou N, Skarmoutsou G, Koulouris I. Advanced killing potential of thymol against a time and temperature optimized attached Listeria monocytogenes population in lettuce broth. Biomolecules 2021; 11(3):397. doi: 10.3390/biom11030397 [Crossref] [ Google Scholar]
- Khanialiakbari S, Yousefimashouf R, Taheri M. Evaluation of thymol effect on antimicrobial susceptibility and biofilm production in clinical Enterobacter spp producing ESBLs and KPC enzymes. J Essent Oil Res 2023; 35(6):570-8. doi: 10.1080/10412905.2023.2276102 [Crossref] [ Google Scholar]
- Priya A, Selvaraj A, Divya D, Karthik Raja R, Pandian SK. In vitro and in vivo anti-infective potential of thymol against early childhood caries causing dual species Candida albicans and Streptococcus mutans. Front Pharmacol 2021; 12:760768. doi: 10.3389/fphar.2021.760768 [Crossref] [ Google Scholar]