Avicenna Journal of Clinical Microbiology and Infection. 11(1):9-16.
doi: 10.34172/ajcmi.3517
Original Article
Sero-prevalence of Rickettsial Infection in the Coastal Area of Bangladesh
Md. Jahangir Alam 1, *  , Al Amin 2, Neamul Hasan Tomal 1, Tareq Mahmud Rakib 1, Md. Abbas Ali 1, Ireen Sultana Shanta 3, Ziaul Islam 1, Munirul Islam 1
, Al Amin 2, Neamul Hasan Tomal 1, Tareq Mahmud Rakib 1, Md. Abbas Ali 1, Ireen Sultana Shanta 3, Ziaul Islam 1, Munirul Islam 1
Author information:
1Nutrition Research Division, International Centre for Diarrhoeal Disease Research, Bangladesh
2Health Care Department, South Eastern Finland University of Applied Science, XAMK, Kotka, Finland
3Infectious Diseases Division, International Centre for Diarrhoeal Disease Research, Bangladesh
Abstract
Background: Rickettsial infection is one of the most frequently occurring neglected diseases, which can be life-threatening if left untreated. Hence, it is important to know the burden of the disease for taking appropriate preventive and control measures. This paper focused on the seroprevalence of rickettsial infection among hospitalized patients residing in the coastal area of Teknaf, Cox’s Bazar, Bangladesh.
Methods: We retrospectively analyzed the hospital records of Weil-Felix test-positive patients from January to December 2022 at Respiratory Disease Hospital, Teknaf. A rapid slide agglutination assay, colorimetric method, KOVA cell counting, flow cytometry method, and SPSS were used for data analysis. The necessary ethical approval was obtained from the Institutional Reviewer Board for using the hospital records.
Results: A total of 91 (16.9%) Rickettsia-positive cases were found out of 538 suspected cases, of which half were male (49.5%). The most predominant age group was 5 to under 18 years of age (41.7%), followed by 18‒30 years of age (23.1%). Fever was the most prominent clinical symptom (97.7%), followed by cough (42.9%), muscle aches (29.7%), and headaches (27.5%). The blood count of the patients showed leukocytosis (53.2%), followed by neutrophilia (23.4%) and thrombocytosis (15.6%). Serum creatinine and C-reactive protein were elevated in 13% and 40% of cases, respectively. Urine analysis detected the presence of high pus cells (83.9%), followed by proteinuria (45.2%) and ketonuria (13.0%).
Conclusion: In-depth confirmatory exploration and preventive measures are necessary to manage and mitigate the spread of infections at Teknaf.
Keywords: Seroprevalence, Rickettsioses, Neglected diseases, Public health
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Alam J, Amin A, Tomal NH, Rakib TM, Ali A, Shanta IS, Islam Z, Islam M. Sero-prevalence of rickettsial infection in the coastal area of Bangladesh. Avicenna J Clin Microbiol Infect. 2024; 11(1):9-16. doi:10.34172/ajcmi.3517
Introduction
Rickettsial infections are a significant global concern as they are infectious diseases that emerge and re-emerge, caused by numerous strains of bacteria categorized under the Rickettsia genus (1). The previously mentioned rickettsial infections are zoonotic diseases that are transmitted by infected ticks, lice, fleas, and mites and are caused by different types of bacteria that can only survive inside host cells (2). Rickettsioses can be contracted through the bites of infected arthropod vectors on humans, or by coming into contact with Rickettsia-infected arthropod excrement on skin openings or mucosal surfaces (3). There are multiple factors that lead to varying clinical outcomes, such as age, early detection and antibiotic therapy, the presence of other medical conditions, and the type of rickettsial infection causing the illness. Individuals afflicted with Rickettsioses usually manifest general clinical indications such as fatigue, rashes, fever, migraines, and emesis that lack specificity (4).
Certain individuals may experience the formation of singular or multiple black spots at the location where a tick has bitten them. This signifies the development of a skin lesion caused by the death of tissue cells in tandem with the initial introduction of the Rickettsia bacterium and subsequent intensive growth of this microorganism. Patients with rickettsial infections commonly manifest maculopapular eruptions due to the Rickettsia-induced harm and inflammation of blood vessels, which results in the leakage of fluid into surrounding tissues (5). During the final stages of rickettsial infections, patients display neurological symptoms and may appear confused or disoriented (4). In severe instances of rickettsioses, there may be a rare occurrence of substantial necrosis and gangrene in the limbs, which may necessitate surgical procedures (4,6). If left untreated, infections caused by highly dangerous Rickettsia species can result in severe complications that endanger one’s life, such as sepsis, acute respiratory distress syndrome, vasculitis, encephalitis, interstitial pneumonia, and organ failure (4,7,8).
If rickettsial fever is not treated, 30%–35% of people suffering from it may die (9). For Rocky Mountain spotted fever, between 10 and 25% of people die, even with treatment, about 5% of people die (10). In addition, it has been reported that untreated patients with scrub typhus have a mortality rate of 10%–50% (11). This implies that people sometimes die more often because they wait too long to visit a doctor or do not get the right medicine (9). Factors that increase the chances of getting very sick from mounted spotted fever are being old, having a weak immune system, drinking a lot of alcohol, having a certain genetic condition called G6PDH deficiency, having diabetes, taking the wrong medicine before, or not getting treatment on time (12).
The diagnosis of Rickettsioses poses a significant challenge, and untreated patients have a startlingly high fatality rate, ranging from 9% to 70%. These debilitating diseases are a major public health concern that affects people worldwide (13-15). In 2017, a study demonstrated that the occurrence of scrub typhus, spotted fever, Q fever, and murine typhus in Bhutan was 22.6%, 15.7%, 6.9%, and 3.5%, respectively (16). A study conducted in Northeast India revealed that the occurrence rate of scrub typhus, spotted fever, and murine typhus was 30.8%, 13.8%, and 4.2%, respectively (17). In South India, it was discovered that 20.4%, 10.4%, and 5.4% of the population had scrub typhus, spotted fever, and murine typhus, respectively (18). A significant incidence of scrub typhus was identified in Nilgiris and Mizoram during the colder and wetter months, as indicated by seroprevalence evidence (19). Scrub typhus prevails widely in flatlands, while spotted fever is primarily found in mountainous areas (18,20).
Although there has been relatively little exploration into rickettsial infections in Bangladesh, the existing research indicates that these illnesses are widespread within the nation. In Bangladesh, rickettsial diseases have been traced back to various Rickettsia species, including Rickettsia conorii, Rickettsia typhi, and Orientia tsutsugamushi. Rickettsial infections such as scrub typhus, murine typhus, and spotted fever are frequently reported in Bangladesh (21). Having a thorough comprehension of how widespread and distributed rickettsial infections are is essential in implementing efficient interventions and strategies to control and manage the disease from a public health perspective. The primary objective of this study is to examine the prevalence of rickettsial infections in the coastal area of Teknaf, Bangladesh.
Materials and Methods
Study Design and Setting
Aretrospective analysis was performed using hospital records of Rickettsia-positive patients admitted to the Respiratory Disease Hospital, icddr,b at Teknaf, Bangladesh, from January 1, 2022, to December 31, 2022. All the data and samples were collected from the study participants with verbal consent, and this process was documented and witnessed by the Institutional Review Board (IRB) of icddr,b. The necessary ethical approval was obtained from the IRB (PR-23064) for accessing and using the above-mentioned patient de-identified records. All the authors can access any information that could potentially reveal the identity of the individuals participating in the study, whether it is during the data collection process or after.
Sample Collection
Overall, 4 mL of venous whole blood samples in a commercially available plain tube (Red cap, BD tube) without anti-coagulant (heparin, ethylenediaminetetraacetic (EDTA) acid, etc.) and 3 mL of them in a tube containing ethylenediaminetetraacetic acid anti-coagulant (purple cap, BD tube) were collected for serological, biochemical, and hematological analyses by venipuncture aseptically. The red cap tube was decanted for 30 minutes for coagulation, followed by the centrifugation of the blood (REMI, China) at 3000 rpm to sample the supernatant serum, and the purple cap tube was kept in the machine, mixing roller (Digilab system, China) at 60 rpm. In addition, 30 mL of spot urine samples were collected and kept in fresh, dry, leak-free urine containers for routine urinalysis.
Serological Analysis
The commercial suspension (Ref: PROX-209) of Proteus OX2, OX19, and OXK was employed to identify the presence of anti-Rickettsia antibodies found in serum samples from humans. This was performed through a fast slide agglutination approach that offered both qualitative and semi-quantitative assessment. In the glycine buffer with a pH rate of 8.2, the concentrated-stained suspension of reagents Proteus OX2, OX19, and OXK can be clumped together when anti-Rickettsia is present in the serum of the patient. The clumping can be observed with the naked eye. The reagent’s sensitivity has been fine-tuned to accurately detect the internal control anti-Proteus (O) titer provided by the commercial company. For qualitative detection, 10 µL of serum and control were used compared to 1 drop (50 µL) of reagent separately. They were then mixed and rotated at 80–100 rpm for 1 minute. The slide could be inspected with the naked eye for the presence or absence of clumps within 60 seconds of taking it off the rotator, and the outcomes of the test sample were contrasted with those of the control serum. If no clumping occurs, it indicates a lack of Rickettsia antibodies. If there is clumping, it implies that the serum is positive for antibodies against Rickettsia (at a concentration of 1:160); thus, it was tested using a serial dilution of 5 µL, 2.5 µL, and 1.25 µL of undiluted serum against 50 µL of the reagent in each test. The results demonstrated titers of 1:320, 1:640, and 1:1280. Rickettsial infection can be indicated by titers greater than 1:80, according to a report (22).
Biochemical Analysis
Serum creatinine was measured with a multi-concept mini-lab, COMBI, clinical chemistry/coagulation/immunochemistry analyzer system (Italy; it can perform end-point, end-point differential, fixed time, and kinetic methods). The COMBI clinical chemistry analyzer system automatically recognizes reagents when added and prepares them as necessary. Prior to testing, the method was calibrated using the manufacturer’s recommended 3-point calibration procedure using a calibrator Cat. No. DC16 (USA). The serum samples were stored in the sample tray and sequentially programmed for serum creatinine in pre-prepared mode after calibration. The COMBI clinical chemistry analyzer system automatically added 25 µL of the serum to the freshly prepared reaction cell and added 125 µL of reagents 1 and 2. The system automatically calculated and printed the results. Bio-Rad chemistry control levels 1 and 2 quality control materials were used for quality control (23).
Hematological Analysis
The samples from the purple tube were immediately mixed to avoid clot formation after collection. These samples were run in a Sysmex XN500 CBC automated analyzer (Sysmex, Kobe, Japan), which automatically aspirated samples and sent samples to the respective sections for analysis. Total white blood cell, differential count of white blood cells, and platelet analysis samples were injected into the center of the sheath line in the flow cell using a syringe. Forward-scattered light and side-scattered light from a fixed volume of the sample were measured by flow cytometry using a semiconductor laser, and the amount was determined by automatic separation. For hemoglobin analysis, the light absorption value of light passing through the diluent was measured each time. The analysis was performed, and this value was subtracted from the absorbance value of light passing through the sample to analyze hemoglobin and obtain its value (the colorimetric method). The analytical method was the SLS-Hb method. The machine had been calibrated by the calibrator and had passed quality control (XN-Check levels 1, 2, and 3). After mechanical analysis, the results were transferred to the laboratory information system (24).
Urinalysis
In general, 30 mL of the spotting urine was collected and immediately (within 30 minutes) sent to the laboratory for routine testing (within 2 hours). Ketonuria and proteinuria were defined as ketone bodies > 0.5 mmol/L and proteinuria > 1 + (30 mg/dL), respectively, by the dipstick test. In accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines, KOVA cell checking plates were used for manual microscopy. Overall, 10 mL of mixed urine was centrifuged at 3000 rpm for 10 minutes, and 9.8 mL of the supernatant was expelled into another dry falcon tube after centrifugation, and the remainder (0.2 mL) was mixed thoroughly. In addition, 20 μL of the mixed residue was expelled with a 1 mL pipette, trickled onto the KOVA cell checking plate, and cleared out to stand for 5 minutes after total expansion. After observing the plate to a power of 10 × 10, each cell component was numbered in the 10 extended arrays to a power of 10 × 40, and the results were recorded, where p/μL = n/(N*50) *90 (n = the numbers of particles constituting the cell, N = all subplots, 50 = times), and the concentration was 10 at 0.2 mL, 90 = 1/(0.0111*1). Microscopic observations were accomplished for the fourth time by four separately qualified, skilled senior medical technologists under a double-blind strategy. Red blood cells, pus cells, and epithelial cells were considered positive indicators when they found their number > 3/HPF, > 5/HPF, and > 5/HPF, respectively. The association between the microscopic test and the KOVA cell checking plate was p/HPF = 1.6∗p/μL (25,26).
Statistical Analysis
Collected data were classified and cleaned with necessary corrections, and missing values were subtracted from the final data set. Categorical and continuous variables were analyzed, presenting descriptive statistics according to means ± standard deviations (SD) and percentages by the Statistical Package for Social Science (IBM SPSS Statistics, version 18).
Results
A total of 91 (16.9%) Rickettsia-positive cases were identified out of a total of 538 suspected cases (Figure 1).
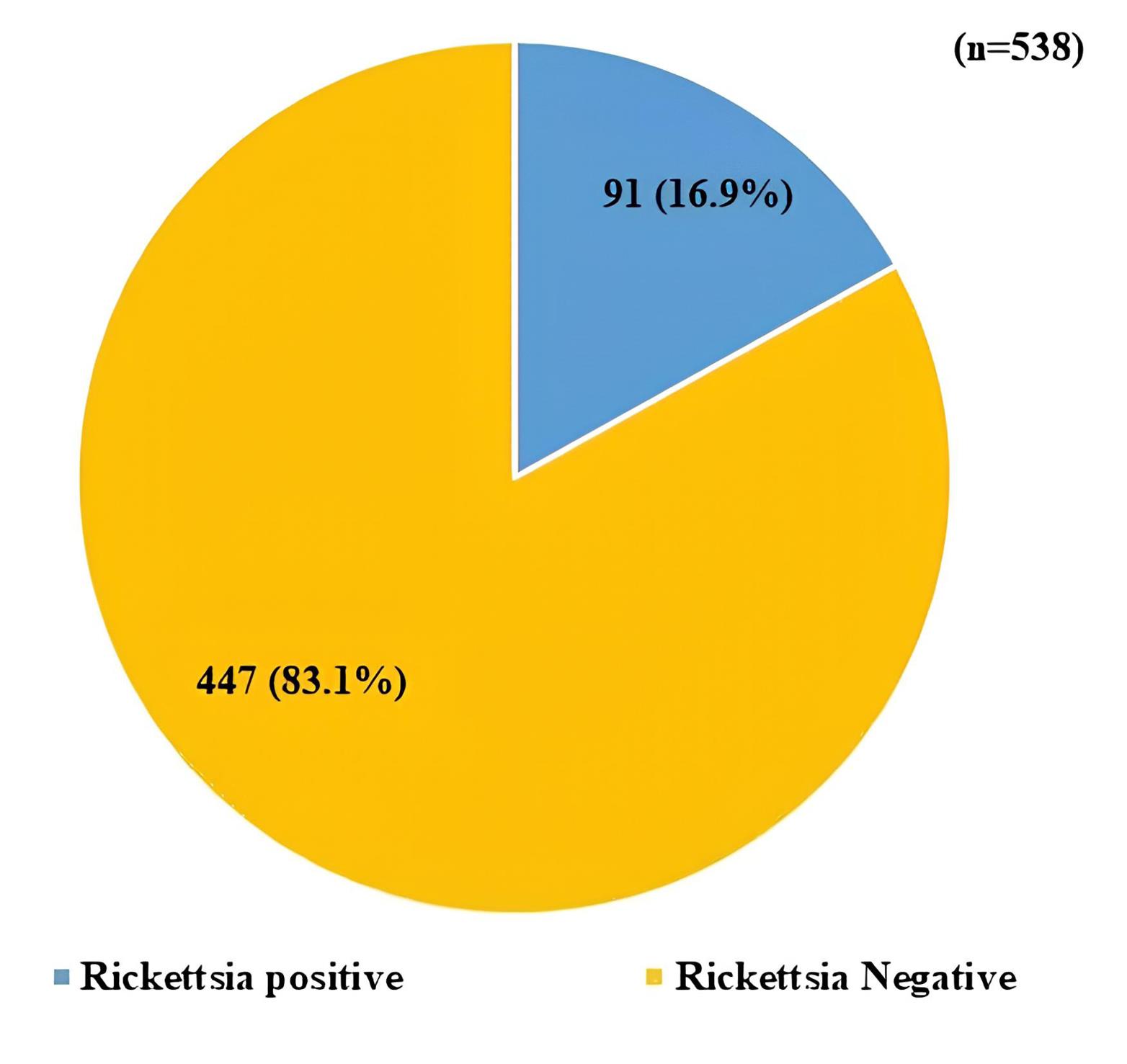
Figure 1.
Seroprevalence of Rickettsial Infection Among the Study Population
.
Seroprevalence of Rickettsial Infection Among the Study Population
In Table 1, both genders were equally affected by Rickettsia. The age group > 5 to < 18 years (41.7%) was the most predominant one, followed by > 18 to < 30 years (23.1%) and under 5 years (13.2%). Among the Rickettsia patients, 44.3% had completed their primary education, followed by 13.5% with secondary-level education. Approximately 42.3% of the students were found to be more prone to rickettsial infection compared to job-holders (21.2%) and housewives (15.4%). The means ± standard deviation (SDs) of the monthly income of the study population were 16315 ± 18626.
Table 1.
Sociodemographic Characteristics of the Study Population
|
Variables
|
No. (%)
|
| Age (n = 91) |
|
| < 5 years |
12 (13.2) |
| > 5 to < 18 years |
38 (41.7) |
| > 18 to < 30 years |
21 (23.1) |
| > 30 to < 45 years |
10 (11.0) |
| > 45 years |
10 (11.0) |
| Gender (n = 91) |
|
| Male |
45 (49.5) |
| Female |
46 (50.5) |
| Education (n = 52) |
|
| Primary school level |
23 (44.3) |
| Secondary school level |
7 (13.5) |
| College level |
6 (11.5) |
| University level |
6 (11.5) |
| Uneducated |
10 (19.2) |
| Occupation (n = 52) |
|
| Student |
22 (42.3) |
| Job-holder |
11 (21.2) |
| Housewives |
8 (15.4) |
| Unemployed |
6 (11.5) |
| Business |
3 (5.8) |
| Day labor/farmer |
2 (3.8) |
| Family income (n = 91) |
Mean±SD
|
| Monthly (BDT) |
16315 ± 18626 |
Note. SD: Standard deviation.
Table 2 represents the clinical features of the study subjects, where fever (97.7%) was the most predominant one, followed by cough (42.9%), muscle aches (29.7%), headache (27.5%), and nausea (24.2%). All vitals demonstrated an increase.
Table 2.
Clinical Manifestations of the Study Population
|
Variables
|
Total (n=91)
|
| Symptoms, No. (%) |
|
| Fever |
88 (97.7) |
| Cough |
39 (42.9) |
| Muscle aches |
27 (29.7) |
| Headache |
25 (27.5) |
| Nausea |
22 (24.2) |
| Runny nose congestion |
19 (20.9) |
| Shortness of breath |
11 (12.1) |
| Sore throat |
4 (4.4) |
| Abdominal pain |
4 (4.4) |
| Diarrhea |
3 (3.3) |
| Anorexia |
2 (2.2) |
| Vitals, Mean ± SD |
|
| Body temperature (°C) |
37.3 ± 4.1 |
| Heart rate (/minute) |
113 ± 27 |
| Respiratory rate (/minute) |
27 ± 08 |
| Systolic blood pressure (mm Hg) |
112 ± 12 |
| Diastolic blood pressure (mm Hg) |
97 ± 11 |
Note. SD: Standard deviation.
The laboratory findings of the study population are provided in Table 3. The seroprevalence of Proteus OX2 (70.3%) was the most predominant finding, followed by Proteus OX19 (24.2%). Leukocytosis was found in 53.2% of specimens tested positive, followed by neutrophilia (23.4%) and lymphocytosis (13.0%). Nearly 16% had thrombocytosis, and 7.8% showed thrombocytopenia. Serum creatinine and C-reactive protein were elevated by 13% and 40.0%, respectively. Above 83% of cases had higher pus cells in their urine samples, followed by proteinuria (45.2%) and ketonuria (13.0%).
Table 3.
Laboratory Findings of the Study Population
|
Laboratory Findings
|
No. (%)
|
| Weil-Felix test (n = 91) |
|
| OX2 |
64 (70.3) |
| OX19 |
22 (24.2) |
| OXK |
5 (5.5) |
| Total count of WBC (n = 77) |
|
| Leukopenia |
8 (10.4) |
| Normal |
28 (36.4) |
| Leukocytosis |
41 (53.2) |
| Differential count of WBC (n = 77) |
|
| Neutropenia |
5 (6.5) |
| Normal |
54 (70.1) |
| Neutrophilia |
18 (23.4) |
| Lymphocytopenia |
23 (29.9) |
| Normal |
44 (57.1) |
| Lymphocytosis |
10 (13.0) |
| Total count of platelet (n = 77) |
|
| Thrombocytopenia Normal |
6 (7.8) |
| 59 (76.6) |
| Thrombocytosis |
12 (15.6) |
| Serum creatinine (n = 54) |
|
| Normal |
47 (87.0) |
| High |
7 (13.0) |
| C-reactive protein (n = 25) |
|
| Normal |
15 (60.0) |
| High |
10 (40.0) |
| Urine Analysis (n = 62) |
|
| Protein (albumin) present ( ≥ 0.3 gm/L) |
28 (45.2) |
| Ketone bodies present ( ≥ 0.5 mmol/L) |
8 (13.0) |
| Pus cell high (0-5/HPF) |
52 (83.9) |
| Epithelial cell high (1-5/HPF) |
43 (69.4) |
| RBC (0-2/HPF) |
20 (32.3) |
Note. CRP: C-reactive protein; Weil Felix test positive: Titer > 1:80; Leukopenia: < 4000 cumm of WBC; Leukocytosis of WBC: > 11000; Neutropenia: < 40% of Neutrophil; Neutrophilia: > 75% of Neutrophil; Lymphocytopenia: < 20% of Lymphocyte; Lymphocytosis: > 45% of Lymphocyte; Thrombocytopenia: < 150000 cumm of Platelet; Thrombocytosis: > 450000 cumm of Platelet. WBC: White blood cell; RBC: Red blood cell. Serum creatinine normal: ≤ 1.3 mg/dL; Serum creatinine high: > 1.3 mg/dL; CRP normal: ≤ 6 mg/dL; CRP high: > 6 mg/dL.
Discussion
The rickettsial infection has a pervasive presence across Bangladesh. Patients who had signs of a rickettsial infection had a higher chance of dying compared to those who had a fever and were diagnosed with other types of infections. This investigation discovered that only 16.9% of the cases tested positive for Rickettsia, which is lower than the percentages (40.0% and 54.1%) found in previous studies conducted in Mymensingh and Bogura (27,28). In addition, 37% of rickettsial infections were identified in the study by Faruque et al (29). In India, 27.3% of cases were diagnosed with rickettsial infection (30). During research conducted in Chittagong between 2014 and 2015, it was found that 16.8% of individuals with fever were affected by scrub typhus, while 5.8% of them had murine typhus, and 4.0% of the cases resulted in fatalities, with a case-fatality rate of 4.0% for both scrub typhus and murine typhus. Approximately a quarter of the patients (23.1%) exhibited signs of curable rickettsial diseases. With its characteristics, high humidity, and intense precipitation, Bangladesh boasts a tropical monsoon climate and is home to a significant population density. These conditions in the environment create ideal living spaces for different types of arthropod vectors, which carry and spread rickettsial infections. Furthermore, the existence of domesticated animals, wildlife, and livestock also aids in the preservation and dissemination of these illnesses within the area (21).
The findings of this study revealed that nearly half of the male participants were impacted by Rickettsia, whereas the corresponding values in the separate studies performed by Maude et al and Yasmin et al were 54.8% and 27.0%, respectively (28,31). In Bangladesh, the prevalence of rickettsial infections was reported to be 68% in males, while in India, 40% of male patients were diagnosed with this type of infection (29,30). Rickettsial infections do not demonstrate a clear gender bias in terms of susceptibility. Both males and females can be equally affected by rickettsial infections. The risk of infection primarily depends on factors such as exposure to infected vectors (e.g., ticks, mites, or fleas), geographical location, and individual activities that may increase the chances of contact with these vectors (32). It is important for individuals of all genders to take appropriate precautions to prevent rickettsial infections, such as wearing protective clothing, using insect repellents, and avoiding tick-infested areas.
In this study, 13.2% of rickettsial infection cases were observed in the under-5-year age group, and the most predominant age group was 5 to under 18 years (41.7%), whereas another study found 24.0% of cases in under-5 year’s children, and 54.0% were 5 to under 18 years of age (29). The most prominent age group reported by Yasmin et al was 21–30 years (29.7%), and 13.5% of cases were under 10 years (28). In addition, the level of education of the people of Teknaf is relatively low. In this study, 44.3% of cases only completed primary education level, followed by secondary school (13.5%). The Inter Sector Coordination Group reported that 15.0% of the population completed secondary school, followed by primary school (13.0%) in Teknaf (33). Around 42% of students affected by Rickettsia did not complete primary school and helped their families earn a livelihood. The average monthly income and standard deviation were 16 315 and 18 626 BDT/month, respectively, indicating a poor economic condition. According to the United Nations World Food Programme in 2017, about 75.0% of the population are very poor or poor in Teknaf (34). This is why the population of Teknaf did not have knowledge of the virulence and prevention of Rickettsia, eventually leading to an increase in the infection rate. Furthermore, Mansoor et al found that people living in rural areas and those with low income are more likely to get rickettsial infections. This is because they often work in agriculture and plantations and are more likely to come into contact with rodents and other animals in these areas (35).
Fever (97.7%) was the most predominant clinical symptom, followed by cough (42.9%), muscle aches (29.7%), headache (27.5%), and nausea (24.2%) found in the present study, while other researchers also found almost similar findings in China (36), Bangladesh (29), and Sri Lanka (37). The most common signs were fever (93–98%), muscle pain (64–75%), headache (48–65%), and tiredness (27%) observed in the study by Crespo et al (38). Moreover, Madeddu et al demonstrated bumpy and spotty skin rash in 85–94% of the patients and black spots in 58–64% of the patients. In addition, about 97.7% and 65.5% of the patients showed the common symptoms of fever and headache, respectively (39).
The seroprevalence of Rickettsia was 16.9% by using the Weil-Felix agglutination assay out of 538 cases, where positive results demonstrated a titer of > 1:80. The low sensitivity and low specificity of the Weil-Felix test can be explained by the low prevalence. About 9% and 10.0% Weil-Felix test positive showing a titer of > 1:80 were revealed by the studies conducted by Mahajan et al and Mathai et al, respectively (40,41). The seroprevalence of agglutination related to the suspension of Proteus OX2 (70.3%) was the most predominant finding, followed by Proteus OX19 (24.2%) and Proteus OXK (5.5%) in this study. The Proteus OX19 antigen reacts strongly with the blood of patients who have typhus or spotted fever. Further, Proteus OX2 and Proteus OXK react strongly with the blood of people who have spotted fever and scrub typhus, respectively (42). The infected people found in this study may have spotted fever mostly, which warrants further investigation.
Evidence exists in studies that have found biochemical and hematological changes such as fluctuating hepatic enzyme levels, increased C-reactive protein, and abnormalities of leukocyte and platelet count (38,43). In the present study, 53.2% of leukocytosis cases were found, followed by neutrophilia (23.4%) and lymphocytosis (13.0%). In their study, Liu et al (26) found 41 (68.3%) thrombocytopenia and 49 (81.7%) leucopenia cases. In the present study, 15.6% and 7.8% thrombocytosis and thrombocytopenia were identified, respectively. Serum creatinine and C-reactive protein were elevated by 13.0% and 40.0%, respectively, in our study samples. Patients with scrub typhus, as an apparent generic outcome of feverish sickness, have been reported to have elevated serum creatinine kinase levels (44). A raised creatinine kinase level was linked to lower serum phosphate, lowered blood urea, tremor, and soreness in the muscles in a group of feverish patients in Israel (45). In Laos, intramuscular injections prior to hospitalization are a widespread practice that may have contributed to an increase in creatinine kinase levels. Muscle discomfort is probably linked to modest muscle injury since patients with rickettsioses who have myalgia have greater serum creatinine kinase concentrations than those who do not (46). Above 83%, 45.2%, and 13.0% of cases demonstrated higher pus cell proteinuria and ketonuria in their urine samples, respectively. When rickettsial infection occurs, renal abnormalities can vary from hematuria or proteinuria to acute kidney injury and, in rare cases, chronic kidney disease (47).
Conclusion
The virulence of the Rickettsia species and host variables, such as immunocompetence, determine the severity of rickettsial infections, which are associated with considerable mortality if the sick person is not identified and treated promptly. In Teknaf, rickettsial infection is a significant but little-known public health issue. Prevention is the key to rickettsial illnesses. Avoiding tick, lice, mites, and flea bites is essential for prevention, especially when living in or visiting endemic areas. We advise medical professionals to consider rickettsial infection as a potential cause of fever while treating patients. More studies including, larger populations and longer time spans, are required to provide a comprehensive picture of Rickettsia prevention and control in Teknaf. This will help us develop programs to stop the disease from spreading.
Acknowledgement
We express our heartfelt gratitude to the governments of Bangladesh and Canada for their invaluable, unrestricted support. In addition, we appreciate to the research participants and all the employees in the Respiratory Disease Hospital, icddr,b Teknaf, Cox’s Bazar, Bangladesh, for providing the patient’s record data and related cooperation.
Authors’ Contribution
Conceptualization: Md. Jahangir Alam, Ziaul Islam, Tareq Mahmud Rakib, Md. Munirul Islam.
Data curation: Md. Jahangir Alam, Al Amin, Md. Abbas Ali.
Formal analysis: Md. Jahangir Alam, Neamul Hasan Tomal, Al Amin, Md. Abbas Ali.
Investigation: Md. Jahangir Alam, Ziaul Islam, Md. Munirul Islam.
Methodology: Md. Jahangir Alam, Ziaul Islam, Tareq Mahmud Rakib, Ireen Sultana Shanta, Md. Munirul Islam.
Project administration: Md. Jahangir Alam, Ziaul Islam, Md. Munirul Islam.
Resources: Md. Jahangir Alam, Ziaul Islam, Md. Munirul Islam.
Software: Md. Jahangir Alam.
Supervision: Md. Jahangir Alam, Ziaul Islam, Md. Munirul Islam.
Validation: Md. Jahangir Alam, Ireen Sultana Shanta.
Visualization: Md. Jahangir Aalm, Neamul Hasan Tomal.
Writing–original draft: Md. Jahanir Alam, Ziaul Islam, Md. Munirul Islam.
Writing–review & editing: Md. Jahangir Alam, Tareq Mahmud Rakib, Ziaul Islam, Md. Munirul Islam, Ireen Sultana Shanta.
Competing Interests
The authors state that they have no financial conflicts or personal connections that could have affected their work in this paper.
Data Availability Statement
Interested readers may ask the senior and corresponding authors for the information used and analyzed in this study. All the data are available to authors at any time.
Ethical Approval
Consent was waived by all participants in this study. Institutional Ethics Committee, International Centre for Diarrhoeal Disease Research, Bangladesh issued approval PR-23064. Ethics were approved by the Institutional Ethics Committee (IEC) and the Institutional Review Board (IRB).
Funding
This study received no specific money from organizations that provide funding, such as the government, businesses, or non-profit groups.
References
- Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 2005; 18(4):719-56. doi: 10.1128/cmr.18.4.719-756.2005 [Crossref] [ Google Scholar]
- Blanton LS. Rickettsial infections in the tropics and in the traveler. Curr Opin Infect Dis 2013; 26(5):435-40. doi: 10.1097/QCO.0b013e328363811b [Crossref] [ Google Scholar]
- Angelakis E, Bechah Y, Raoult D. The history of epidemic typhus. Microbiol Spectr 2016;4(4). 10.1128/microbiolspec.PoH-0010-2015.
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm Rep 2016; 65(2):1-44. doi: 10.15585/mmwr.rr6502a1 [Crossref] [ Google Scholar]
- Walker DH, Occhino C, Tringali GR, Di Rosa S, Mansueto S. Pathogenesis of rickettsial eschars: the tache noire of boutonneuse fever. Hum Pathol 1988; 19(12):1449-54. doi: 10.1016/s0046-8177(88)80238-7 [Crossref] [ Google Scholar]
- Kirkland KB, Marcom PK, Sexton DJ, Dumler JS, Walker DH. Rocky Mountain spotted fever complicated by gangrene: report of six cases and review. Clin Infect Dis 1993; 16(5):629-34. doi: 10.1093/clind/16.5.629 [Crossref] [ Google Scholar]
- Sekeyová Z, Danchenko M, Filipčík P, Fournier PE. Rickettsial infections of the central nervous system. PLoS Negl Trop Dis 2019; 13(8):e0007469. doi: 10.1371/journal.pntd.0007469 [Crossref] [ Google Scholar]
- Helminiak L, Mishra S, Kim HK. Pathogenicity and virulence of Rickettsia. Virulence 2022; 13(1):1752-71. doi: 10.1080/21505594.2022.2132047 [Crossref] [ Google Scholar]
- Parola P, Miller RS, McDaniel P, Telford SR 3rd, Rolain JM, Wongsrichanalai C. Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis 2003; 9(5):592-5. doi: 10.3201/eid0905.020511 [Crossref] [ Google Scholar]
- Adjemian JZ, Krebs J, Mandel E, McQuiston J. Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001-2005. Am J Trop Med Hyg 2009; 80(1):72-7. [ Google Scholar]
- Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, Richards AL. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg 2004; 70(4):351-6. [ Google Scholar]
- De Vito A, Geremia N, Mameli SM, Fiore V, Serra PA, Rocchitta G. Epidemiology, clinical aspects, laboratory diagnosis and treatment of rickettsial diseases in the mediterranean area during COVID-19 pandemic: a review of the literature. Mediterr J Hematol Infect Dis 2020; 12(1):e2020056. doi: 10.4084/mjhid.2020.056 [Crossref] [ Google Scholar]
- Rathi N, Rathi A. Rickettsial infections: Indian perspective. Indian Pediatr 2010; 47(2):157-64. doi: 10.1007/s13312-010-0024-3 [Crossref] [ Google Scholar]
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 2015; 9(8):e0003971. doi: 10.1371/journal.pntd.0003971 [Crossref] [ Google Scholar]
- Abdad MY, Abou Abdallah R, Fournier PE, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol 2018; 56(8):e01728-17. doi: 10.1128/jcm.01728-17 [Crossref] [ Google Scholar]
- Tshokey T, Stenos J, Durrheim DN, Eastwood K, Nguyen C, Graves SR. Seroprevalence of rickettsial infections and Q fever in Bhutan. PLoS Negl Trop Dis 2017; 11(11):e0006107. doi: 10.1371/journal.pntd.0006107 [Crossref] [ Google Scholar]
- Khan SA, Bora T, Chattopadhyay S, Jiang J, Richards AL, Dutta P. Seroepidemiology of rickettsial infections in Northeast India. Trans R Soc Trop Med Hyg 2016; 110(8):487-94. doi: 10.1093/trstmh/trw052 [Crossref] [ Google Scholar]
- Devamani CS, Schmidt WP, Ariyoshi K, Anitha A, Kalaimani S, Prakash JAJ. Risk factors for scrub typhus, murine typhus, and spotted fever seropositivity in urban areas, rural plains, and peri-forest hill villages in South India: a cross-sectional study. Am J Trop Med Hyg 2020; 103(1):238-48. doi: 10.4269/ajtmh.19-0642 [Crossref] [ Google Scholar]
- Paulraj PS, Renu G, Ranganathan K, Leo VJ, Veeramanoharan R. First seroprevalence report of scrub typhus from the tribal belts of the Nilgiris district, Tamil Nadu, India. Indian J Med Res 2021; 153(4):503-7. doi: 10.4103/ijmr.IJMR_1223_19 [Crossref] [ Google Scholar]
- Kularatne SA, Rajapakse RP, Wickramasinghe WM, Nanayakkara DM, Budagoda SS, Weerakoon KG. Rickettsioses in the central hills of Sri Lanka: serological evidence of increasing burden of spotted fever group. Int J Infect Dis 2013; 17(11):e988-92. doi: 10.1016/j.ijid.2013.05.014 [Crossref] [ Google Scholar]
- Kingston HW, Hossain M, Leopold S, Anantatat T, Tanganuchitcharnchai A, Sinha I. Rickettsial illnesses as important causes of febrile illness in Chittagong, Bangladesh. Emerg Infect Dis 2018; 24(4):638-45. doi: 10.3201/eid2404.170190 [Crossref] [ Google Scholar]
- Young EJ. An overview of human brucellosis. Clin Infect Dis 1995; 21(2):283-90. doi: 10.1093/clinids/21.2.283 [Crossref] [ Google Scholar]
- Bartels H, Böhmer M, Heierli C. [Serum creatinine determination without protein precipitation]. Clin Chim Acta 1972; 37:193-7. doi: 10.1016/0009-8981(72)90432-9.[German] [Crossref] [ Google Scholar]
- Hansen AP, Haischer-Rollo GD, Shapiro JB, Aden JK, Abadie JM, Mu TS. The novel use of umbilical cord blood to obtain complete blood counts for critical neonatal assessment. Cureus 2022; 14(8):e28009. doi: 10.7759/cureus.28009 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). GP16-A3: Urinalysis; Approved Guideline. 3rd ed. CLSI; 2009.
- Liu H, Li Q, Zhang Y, Huang D, Yu F. Consistency analysis of the Sysmex UF-5000 and Atellica UAS 800 urine sedimentation analyzers. J Clin Lab Anal 2022; 36(9):e24659. doi: 10.1002/jcla.24659 [Crossref] [ Google Scholar]
- Miah MT, Rahman S, Sarker CN, Khan GK, Barman TK. Study on 40 cases of Rickettsia. Mymensingh Med J 2007; 16(1):85-8. doi: 10.3329/mmj.v16i1.259 [Crossref] [ Google Scholar]
- Yasmin T, Yusuf MA, Mowla MG, Afrin MJ, Akhter S, Sayam MA. Status of triple antigen test among community acquired febrile illness patient attended at tertiary care hospital, Bogura. Bangladesh J Infect Dis 2020; 7(1):18-21. doi: 10.3329/bjid.v7i1.48672 [Crossref] [ Google Scholar]
- Faruque LI, Zaman RU, Gurley ES, Massung RF, Alamgir AS, Galloway RL. Prevalence and clinical presentation of Rickettsia, Coxiella, Leptospira, Bartonella and chikungunya virus infections among hospital-based febrile patients from December 2008 to November 2009 in Bangladesh. BMC Infect Dis 2017; 17(1):141. doi: 10.1186/s12879-017-2239-6 [Crossref] [ Google Scholar]
- D’Cruz S, Perumalla SK, Yuvaraj J, Prakash JAJ. Geography and prevalence of rickettsial infections in Northern Tamil Nadu, India: a cross-sectional study. Sci Rep 2022; 12(1):20798. doi: 10.1038/s41598-022-21191-7 [Crossref] [ Google Scholar]
- Maude RR, Maude RJ, Ghose A, Amin MR, Islam MB, Ali M. Serosurveillance of Orientia tsutsugamushi and Rickettsia typhi in Bangladesh. Am J Trop Med Hyg 2014; 91(3):580-3. doi: 10.4269/ajtmh.13-0570 [Crossref] [ Google Scholar]
- Hopkins RS, Jajosky RA, Hall PA, Adams DA, Connor FJ, Sharp P. Summary of notifiable diseases--United States, 2003. MMWR Morb Mortal Wkly Rep 2005; 52(54):1-85. [ Google Scholar]
- ISCG reports on host community in Teknaf & Ukhia Upazilas, Cox’s Bazar. 2017. Available from: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/reach_bgd_msna_hc_overall_teknaf_ukhia_upazila.pdf. Accessed March 5, 2023.
- Livelihood in Ukiah and Teknaf - Household Economy Analysis. 2017. Available from: https://www.humanitarianresponse.info/en/operations/bangladesh/assessment/livelihood-ukiah-and-teknaf-household-economy-analysis. Accessed March 6, 2023.
- Mansoor T, Fomda BA, Koul AN, Bhat MA, Abdullah N, Bhattacharya S. Rickettsial infections among the undifferentiated febrile patients attending a tertiary care teaching hospital of northern India: a longitudinal study. Infect Chemother 2021; 53(1):96-106. doi: 10.3947/ic.2020.0147 [Crossref] [ Google Scholar]
- Kato CY, Chung IH, Robinson LK, Eremeeva ME, Dasch GA. Genetic typing of isolates of Rickettsia typhi. PLoS Negl Trop Dis 2022; 16(5):e0010354. doi: 10.1371/journal.pntd.0010354 [Crossref] [ Google Scholar]
- Premaratna R, Loftis AD, Chandrasena TG, Dasch GA, de Silva HJ. Rickettsial infections and their clinical presentations in the Western Province of Sri Lanka: a hospital-based study. Int J Infect Dis 2008; 12(2):198-202. doi: 10.1016/j.ijid.2007.06.009 [Crossref] [ Google Scholar]
- Crespo P, Seixas D, Marques N, Oliveira J, da Cunha S, Meliço-Silvestre A. Mediterranean spotted fever: case series of 24 years (1989-2012). Springerplus 2015; 4:272. doi: 10.1186/s40064-015-1042-3 [Crossref] [ Google Scholar]
- Madeddu G, Fiore V, Mancini F, Caddeo A, Ciervo A, Babudieri S. Mediterranean spotted fever-like illness in Sardinia, Italy: a clinical and microbiological study. Infection 2016; 44(6):733-8. doi: 10.1007/s15010-016-0921-z [Crossref] [ Google Scholar]
- Mahajan SK, Kashyap R, Kanga A, Sharma V, Prasher BS, Pal LS. Relevance of Weil-Felix test in diagnosis of scrub typhus in India. J Assoc Physicians India 2006; 54:619-21. [ Google Scholar]
- Mathai E, Lloyd G, Cherian T, Abraham OC, Cherian AM. Serological evidence for the continued presence of human rickettsioses in southern India. Ann Trop Med Parasitol 2001; 95(4):395-8. doi: 10.1080/00034980120065804 [Crossref] [ Google Scholar]
- Central Research Institute. Antigens. 2023. Available from: https://crikasauli.nic.in/Antigens#:~:text=Description%3A,to%20humans%20as%20accidental%20host. Accessed May 24, 2023.
- Brouqui P, Bacellar F, Baranton G, Birtles RJ, Bjoërsdorff A, Blanco JR. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect 2004; 10(12):1108-32. doi: 10.1111/j.1469-0691.2004.01019.x [Crossref] [ Google Scholar]
- Young PC, Hae CC, Lee KH, Hoon CJ. Tsutsugamushi infection-associated acute rhabdomyolysis and acute renal failure. Korean J Intern Med 2003; 18(4):248-50. doi: 10.3904/kjim.2003.18.4.248 [Crossref] [ Google Scholar]
- Cohen O, Leibovici L, Mor F, Wysenbeek AJ. Significance of elevated levels of serum creatine phosphokinase in febrile diseases: a prospective study. Rev Infect Dis 1991; 13(2):237-42. doi: 10.1093/clinids/13.2.237 [Crossref] [ Google Scholar]
- Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis 2006; 12(2):256-62. doi: 10.3201/eid1202.050900 [Crossref] [ Google Scholar]
- Hwang K, Jang HN, Lee TW, Cho HS, Bae E, Chang SH. Incidence, risk factors and clinical outcomes of acute kidney injury associated with scrub typhus: a retrospective study of 510 consecutive patients in South Korea (2001-2013). BMJ Open 2017; 7(3):e013882. doi: 10.1136/bmjopen-2016-013882 [Crossref] [ Google Scholar]