Avicenna Journal of Clinical Microbiology and Infection. 10(4):131-136.
doi: 10.34172/ajcmi.3514
Original Article
The Antimicrobial Activity of Propolis Ethanolic Extract and Silver Nanoparticles Synthesized by Green Method on Gram-Positive and Negative Bacteria
Zahra Karimitabar 1  , Abbas Farmani 2, Masoud Azimzadeh 3, Mohammad Sina Alikhani 3, Masoud Moghadam Shakib 1, Mohammad Yousef Alikhani 4, *
, Abbas Farmani 2, Masoud Azimzadeh 3, Mohammad Sina Alikhani 3, Masoud Moghadam Shakib 1, Mohammad Yousef Alikhani 4, * 
Author information:
1Department of Microbiology, Hamadan University of Medical Sciences, Hamadan, Iran
2Dental Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
3Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
4Infectious Disease Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: The increasing resistance of bacteria to different classes of antibiotics has become an important public health concern. This study was aimed at the effectiveness of the antibacterial effect of silver nanoparticles and propolis (AgNPs@propolis) on bacteria.
Methods: A hydroalcoholic extract of propolis was used for prepare of silver nanoparticles (AgNPs@propolis). The characteristics, anti-bacterial effect and cell toxicity of AgNPs@propolis were examined in vitro.
Results: The size of the synthesized nanoparticles was 32 to 85 nm. The AgNPs@propolis had no toxic effect up to a concentration of 200 μg/mL. Compared to AgNPs and propolis, AgNPs@ propolis showed a greater inhibitory effect on the growth of gram-positive and gram-negative bacteria. propolis as a natural substance has an inhibitory effect on the growth of bacteria.
Conclusion: Green synthesis of AgNPs@propolis has a low toxic effect on the cell and has a high effect in inhibiting the growth of various bacteria.
Keywords: Propolis, Silver, Nanoparticle, Antimicrobial effect
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Karimitabar Z, Farmani A, Azimzadeh M, Alikhani MS, Moghadam Shakib M, Alikhani MY. The antimicrobial activity of propolis ethanolic extract and silver nanoparticles synthesized by green method on gram-positive and negative bacteria. Avicenna J Clin Microbiol Infect. 2023; 10(4):131-136. doi:10.34172/ajcmi.3514
Introduction
Antibiotics are important factors for fighting infections caused by bacteria, fungi and some parasites, which reduce the growth and death of this group of microorganisms (1). One of the worrisome problems related to antibiotics is the resistance of microorganisms to drugs. The resistance of bacteria to antibiotics is often changed by mechanisms such as the production of enzymes that can break down drugs, the lack of penetration of drugs in bacteria, and the surface proteins of bacteria (2,3).
Propolis is a type of resinous substance obtained by bees from the secretions of trees, plants, buds and leaves. On average, propolis contains 50%-55% resin, 30% wax, 10% essential oil, 5% pollen and various other substances. Due to the presence of flavonoids such as quercetin, propolis has anti-microbial and anti-viral properties, and the inhibition mechanism of these compounds is related to inhibition of viral polymerase and binding of nucleic acid and capsid protein of viruses. There are more than 150 different compounds including terpenoids, polyphenols, steroids and amino and organic acids in propolis, the amount of which varies according to the geographical region, type of plant and type of extraction. Propolis and its extracts have antiseptic, anti-inflammatory, antioxidant, antibacterial, anti-microbial, anti-fungal, anti-cancer properties and regulation of the body’s immune system, which has led to its use in the treatment of various diseases (4).
One of the most important common antimicrobial agents is silver nanoparticles (AgNPs). Antimicrobial mechanisms of silver include: lipid peroxidation, reactive oxygen species (ROS) production, inhibition of cytochrome, inhibition of cell wall synthesis, ribosome instability and increased membrane permeability (5,6). Silver as a nanoparticle is one of the important metals that are used for antibacterial purposes and can bind to the cell and break down its structure by destroying proteins and lipids, as well as destroy bacterial biofilms. AgNPs are prepared by two chemical, physical and biological methods. Thermal decomposition and spark discharge are common physical methods. Stabilizers such as borohydride, 2ME and thioglycerol cause chemical regeneration of silver, using these materials and methods to prepare AgNPs is toxic and dangerous (7). One of the important problems of the chemical and physical synthesis methods of AgNPs is the potential for toxicity (8). Therefore, the use of methods according to green preparation of AgNPs can significantly reduce its toxic effects. Plant extracts can be used as a reducing and stabilizing agent in the nanoparticle structure, which not only reduces the toxic effects of AgNPs, but also in some plant extracts such as propolis (which have antimicrobial effects), It also increases its effectiveness (9,10).
Therefore, this study aimed to investigate the antibacterial effect of AgNPs and propolis (AgNPs@propolis) on gram negative bacteria: P. aeruginosa, E. coli, K. pneumoniae, A. baumannii and gram positive bacteria: S. aureus and E. faecalis in laboratory conditions.
Material and Methods
Preparation of the Propolis
To prepare propolis hydroalcoholic extract, 50 mg of propolis was converted into powder and added to 20 mL of 7:3 hydroalcoholic solution (V/V) and kept at room temperature for one week. The hydroalcoholic composition based on absolute ethanol leads to the extraction of its polyphenolic compounds from propolis and its extraction percentage increases. The resulting suspension was filtered using Whatman® cellulose filter papers and the remaining propolis was separated from it. Then, the obtained solution was centrifuged (400 rpm, 5 minutes) and after separating the remaining solids, it was placed at 4 ˚C. For antimicrobial tests, the sample was diluted with dimethyl sulfoxide (11).
Preparation of AgNPs@Propolis
AgNPs@propolis was prepared by a one-step method. Briefly, propolis solution was added dropwise to aqueous AgNO3 solution until the solution turned bright yellow. The change of yellow color to yellowish-brown indicates the success of the preparation process of AgNPs@propolis (12).
Cytotoxicity Assay
Investigation of the cytotoxicity of propolis and AgNPs@propolis was performed on fibroblastic cell line (cell line L 929). Different concentrations of propolis and AgNPs@propolis were used for treated in cultured cells (100, 200, 300, 400 and 500 µg/mL). MTT method was used to test cell viability (13).
Bacteria Strain
Gram positive bacteria: S. aureus (ATCC: 25923) and E. faecalis (ATCC: 29212) and gram-negative bacteria: P. aeruginosa (ATCC: 27853), E. coli (ATCC: 25922), K. pneumoniae (ATCC: 1290), A. baumannii (ATCC: 1855) used in this study.
Antibiotic Sensitivity Testing
Antibiotic sensitivity test by standard Kirby-Bauer disk agar diffusion (DAD) for antibiotics including ciprofloxacin (5 μg), vancomycin (30 μg), imipenem (10 μg), clindamycin (3 μg) gentamicin (10 μg), and ampicillin (10 μg) were performed.
MIC And Well Diffusion
Well diffusion and minimum inhibitory concentration for AgNPs@propolis, AgNPs and propolis were performed according to CLSI guidelines. Concentrations (37.5, 75, 150 and 300 μg/mL) of AgNPs@propolis, AgNPs and propolis were prepared. 0.5 McFarland suspension of bacterial strains was cultured on Mueller-Hinton agar, 6 mm diameter wells were created in the culture medium, and 100 mL of each concentration of AgNPs@propolis, AgNPs and propolis was first sonicated and poured into each well and for a period of time. After 24 hours, the diameter of the inhibition zone was checked. For determining the minimum concentration of AgNPs@propolis, AgNPs and propolis that had the ability to inhibit the growth of bacteria, 96-well plates with flat bottoms were used. The first concentration that was used was the lowest amount of AgNPs@propolis, AgNPs and propolis that was obtained in the well diffusion test. 100 mL of different dilutions were added to each well. Next, 100 mL of Mueller Hinton Broth culture medium was added to each well and finally 5 mL of 0.5 McFarland suspension of bacteria was added to all wells and incubated for 24 hours at 37 ˚C. After this time, it was checked visually to determine the minimum concentration, and it was considered as the minimum concentration that inhibits the growth of bacteria in the wells where the bacteria had not grown (14,15).
Results
AgNPs@Propolis Characteristics
Figure 1 shows the XRD pattern of the AgNPs@propolis. The peaks recorded at 20 of 32.28, 38.18, 44.53, 64.68, and 77.58, are identified as differences in (111), (200), (220), and (311), respectively. Figure 2 shows the UV-vis absorption spectrum of AgNPs@propolis. The main absorption peak at 372 nm is related to surface resonance absorption characteristics.
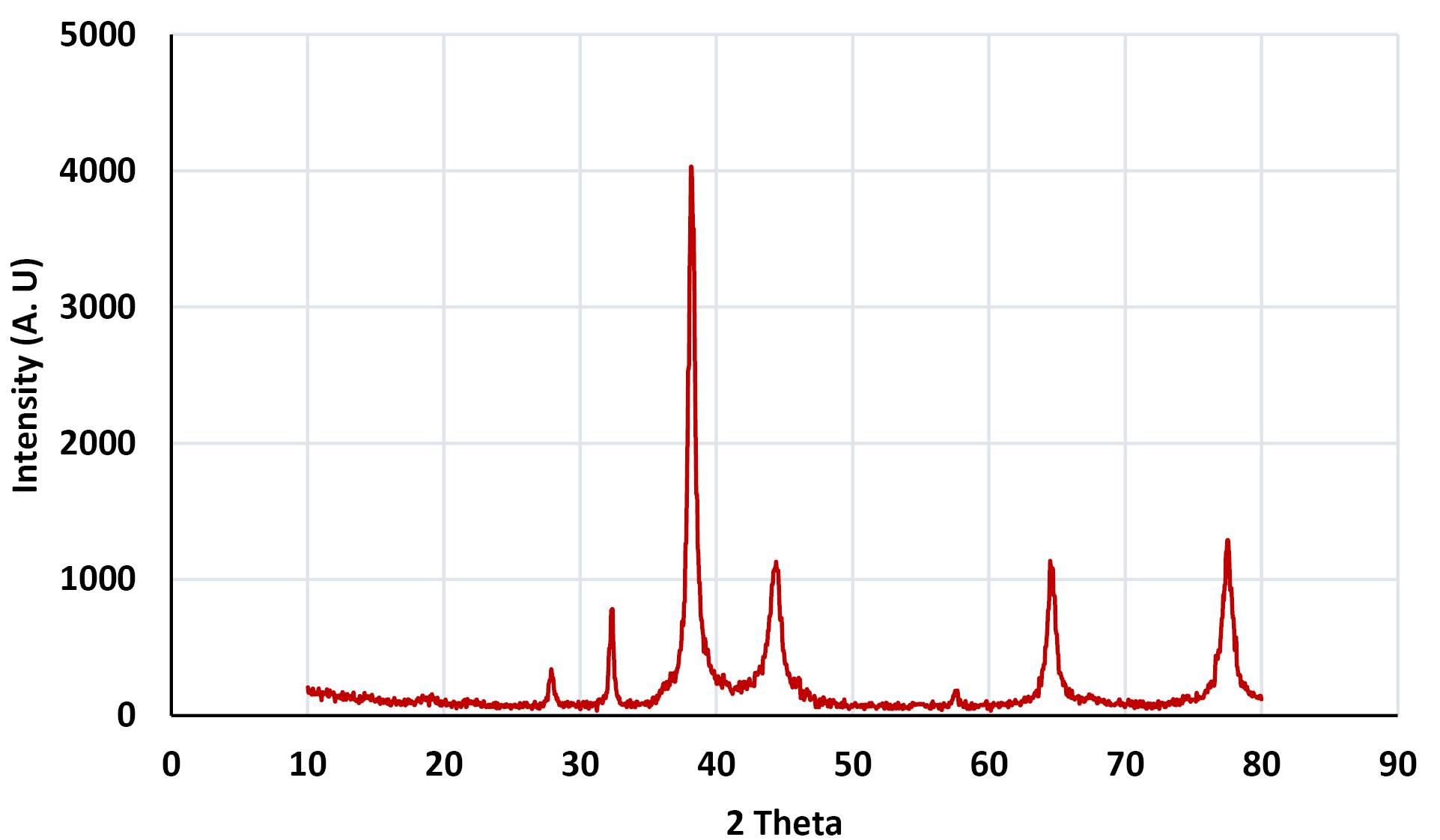
Figure 1.
XRD Pattern of AgNPs@Propolis
.
XRD Pattern of AgNPs@Propolis
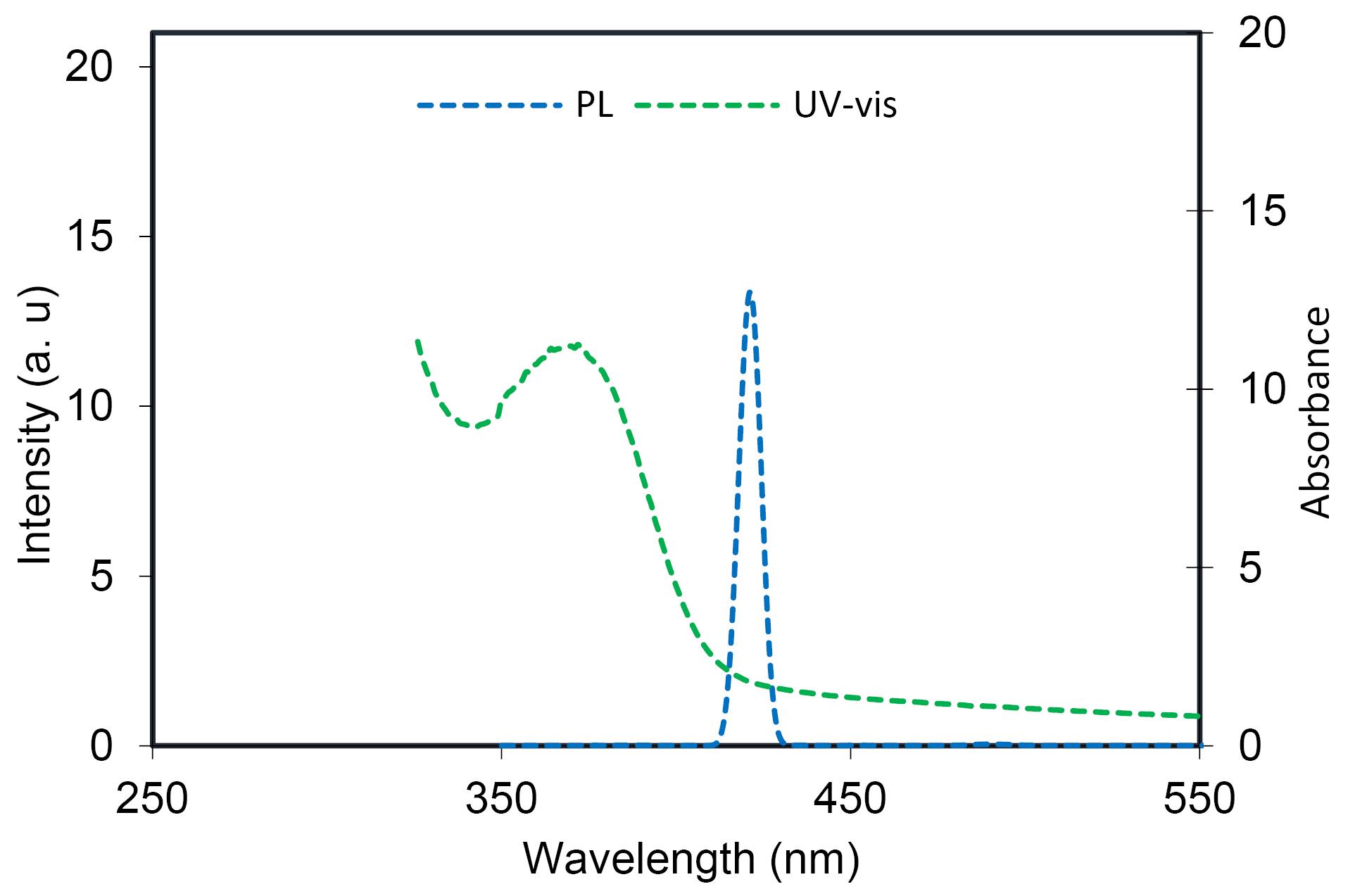
Figure 2.
UV-Vis and PL Spectra of AgNPs@Propolis
.
UV-Vis and PL Spectra of AgNPs@Propolis
The photoluminescence (PL) spectrum of AgNPs@propolis indicated that the AgNPs@propolis has a high and intense emission at 420 nm, in addition, a PL spectrum was recorded for the excitation wavelength of 380 nm (Figure 2).
TEM Image of the AgNPs@Propolis
Figure 3 shows the TEM image of the AgNPs@propolis. As can be seen in the image, the particles are spherical with a size of 32 to 85 nm.
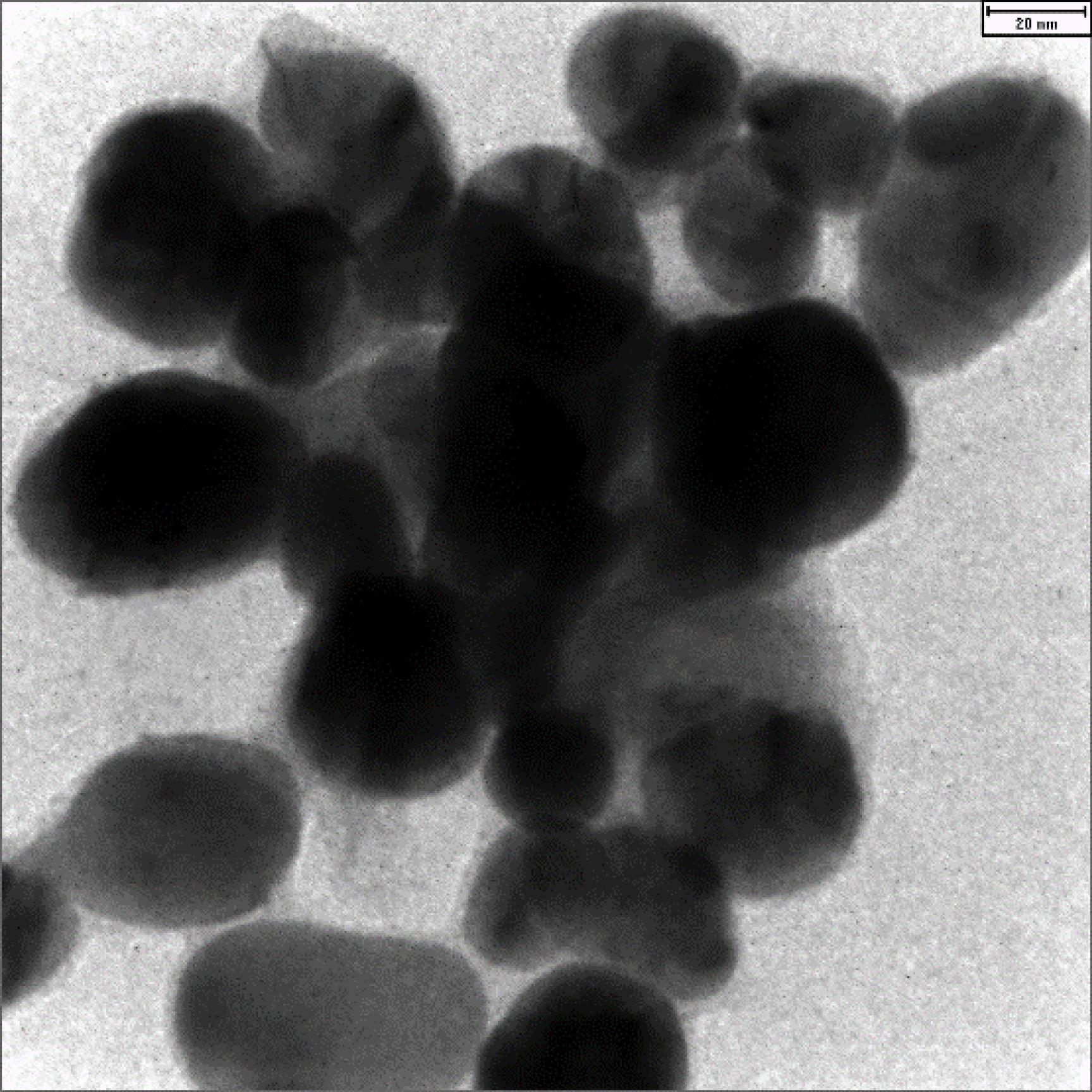
Figure 3.
Transmission Electron Microscopy Image of the AgNPs@Propolis
(Scale bar is 50 nm)
.
Transmission Electron Microscopy Image of the AgNPs@Propolis
(Scale bar is 50 nm)
The Results of Cytotoxicity Test
According to the results obtained from the toxicity test, AgNPs@propolis in concentrations of 100 and 200 μg/mL does not have a toxic or lethal effect on cells. In concentrations of 400 and 500 μg/mL, up to 40% caused cell death. The important and promising point was that when the cells were treated with propolis alone, the toxicity of the particles was significantly reduced (Figure 4).
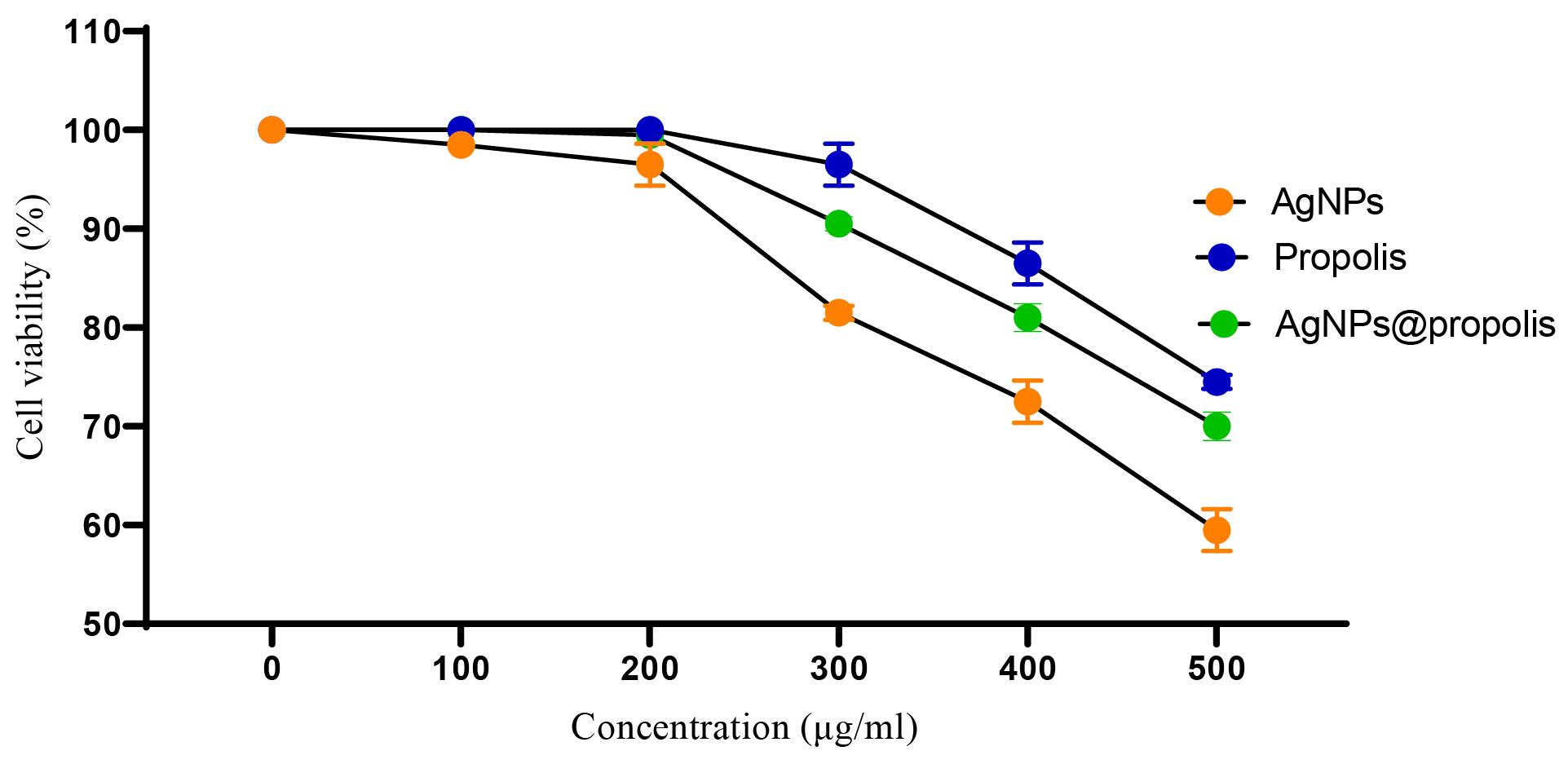
Figure 4.
Toxicity Effect of Formulations on Fibroblastic Cell Line (cell line L 929)
.
Toxicity Effect of Formulations on Fibroblastic Cell Line (cell line L 929)
The Results of Antibiotic Sensitivity Testing
In order to determine the antibiotic resistance profile for the studied bacteria, an antibiogram test was performed. According to the obtained results (Table 1), gram-positive bacteria were resistant to ampicillin and intermediate resistance to ciprofloxacin. Gram-negative bacteria had variable resistance to ampicillin and all were sensitive to other antibiotics.
Table 1.
The Result of Antibiotic Sensitivity Test
|
Bacterial Strain
|
Ciprofloxacin
|
Vancomycin
|
Gentamicin
|
Imipenem
|
Clindamycin
|
Ampicillin
|
|
S. aureus
|
I |
S |
S |
ND |
S |
R |
|
E. faecalis
|
I |
S |
S |
ND |
R |
R |
|
E. coli
|
S |
ND |
S |
S |
ND |
R |
|
P. aeruginosa
|
S |
ND |
S |
S |
ND |
I |
|
K. pneumoniae
|
S |
ND |
S |
S |
ND |
R |
|
A. baumannii
|
S |
ND |
S |
S |
ND |
I |
ND: Not done; S: Sensitive; I: Intermediate; R: Resistant.
The Results of Well Diffusion and MIC of AgNPs@Propolis
The findings of well diffusion (Table 2) showed that propolis alone had no inhibitory effect on bacterial strains. Also, the best performance in inhibiting the growth of bacteria was AgNPs@propolis. This shows the synergistic effect of propolis and silver, the statistical analysis of growth inhibition by different formulations showed that AgNPs@propolis has a significant difference in inhibiting bacteria compared to AgNPs and propolis (P value < 0.05).
Table 2.
The Result of Well Diffusion Test
|
Antibacterial activity
|
|
Bacterial Strain
|
Formulations
|
Zone of Inhibition (mm) in Four Concentration (μg/mL)
|
|
300
|
150
|
75
|
37.5
|
|
S. aureus
|
AgNPs |
10 ± 1 |
6 ± 0.2 |
Resistant |
Resistant |
| Propolis |
6 ± 0.5 |
Resistant |
Resistant |
Resistant |
| AgNPs@propolis |
12 ± 2.5 |
7 ± 0.4 |
Resistant |
Resistant |
|
E. faecalis
|
AgNPs |
9 ± 1.2 |
Resistant |
Resistant |
Resistant |
| Propolis |
Resistant |
Resistant |
Resistant |
Resistant |
| AgNPs@propolis |
10 ± 2.1 |
6 ± 0.9 |
Resistant |
Resistant |
|
E. coli
|
AgNPs |
11 ± 1.3 |
6 ± 0.5 |
Resistant |
Resistant |
| Propolis |
8 ± 0.5 |
Resistant |
Resistant |
Resistant |
| AgNPs@propolis |
15 ± 1.9 |
13 ± 2.0 |
11 ± 0.9 |
7 ± 0.5 |
|
P. aeruginosa
|
AgNPs |
7 ± 0.2 |
Resistant |
Resistant |
Resistant |
| Propolis |
Resistant |
Resistant |
Resistant |
Resistant |
| AgNPs@propolis |
12 ± 1.3 |
11 ± 0.9 |
Resistant |
Resistant |
|
K. pneumoniae
|
AgNPs |
8 ± 0.8 |
Resistant |
Resistant |
Resistant |
| Propolis |
6 ± 0.21 |
Resistant |
Resistant |
Resistant |
| AgNPs@propolis |
13 ± 2.1 |
10 ± 1.1 |
Resistant |
Resistant |
|
A. baumannii
|
AgNPs |
8 ± 1.0 |
Resistant |
Resistant |
Resistant |
| Propolis |
Resistant |
Resistant |
Resistant |
Resistant |
| AgNPs@propolis |
12 ± 1.9 |
9 ± 0.9 |
Resistant |
Resistant |
Considering that propolis and silver have little penetrating power in the agar culture medium, therefore, the MIC test has more realistic results than the effectiveness of different formulations. As seen in Figure 5, AgNPs@propolis have a very good effect on bacterial strains. The important point is that, in general, AgNPs@propolis have a greater inhibitory effect on gram-positive bacteria than gram-positive bacteria.
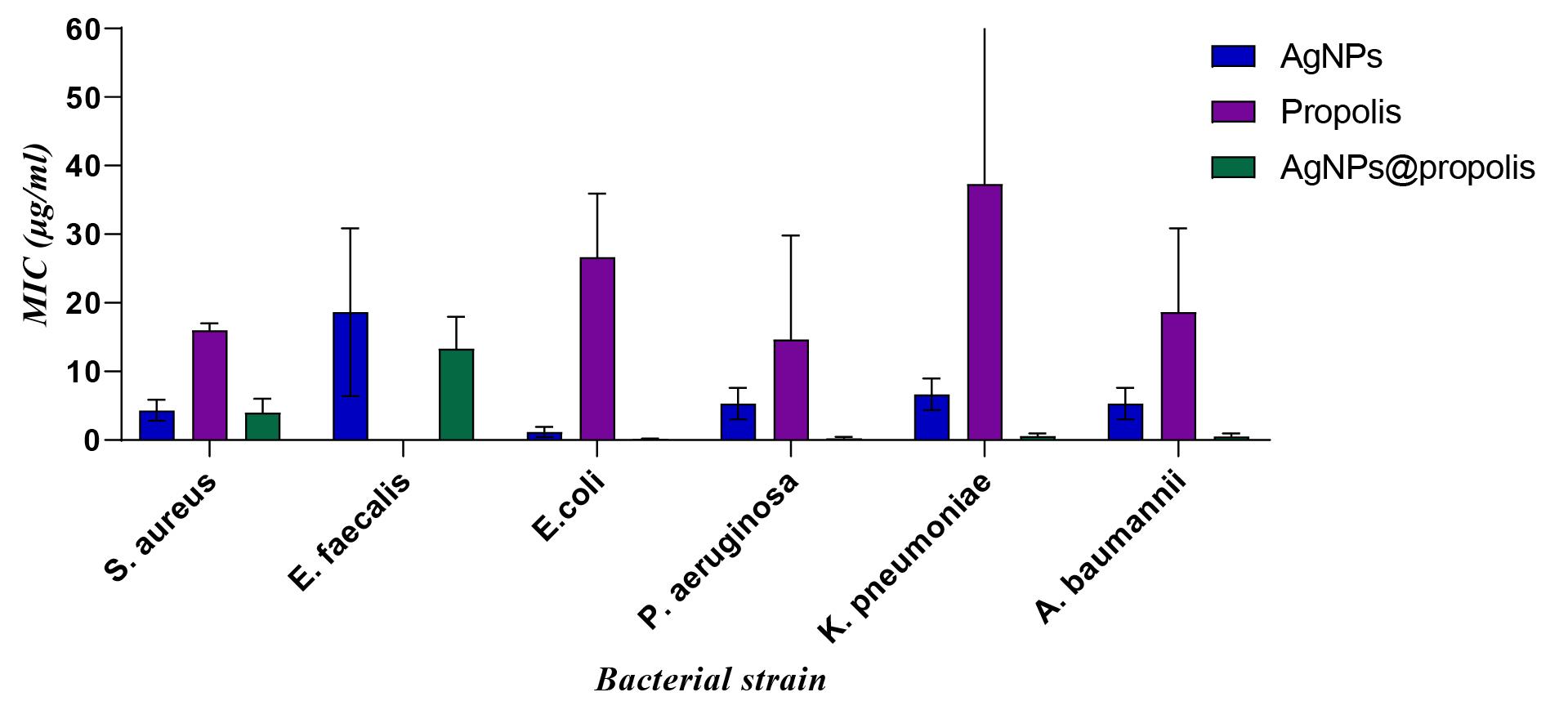
Figure 5.
The Result of MIC Test
.
The Result of MIC Test
Discussion
Colloidal AgNPs have been proven to be one of the most common antimicrobial agents. The antibacterial properties of these nanomaterials were investigated against various organisms, including fungi and bacteria. Many studies have reported that AgNPs can inhibit the growth of bacteria (16). In this study, we have tested the effectiveness of propolis at different concentrations on bacteria separately or in co-treatment with AgNPs@propolis.
The findings of our study showed that propolis alone has no inhibitory effect on bacterial strains. This finding is in contrast with the study of Wieczynska et al (17) and Seibert et al (18), reasons for this discrepancy can be pointed to the type of extract extraction as well as the geographical region. Also, the well diffusion test results showed that the best performance in inhibiting the growth of bacteria was AgNPs@propolis. This indicates the synergistic effect of propolis and silver, the statistical analysis of growth inhibition with different formulations showed that AgNPs@propolis has a significant difference in the inhibition of bacteria compared to AgNPs and propolis. Habibipour et al (11) conducted a study titled green synthesis of AgNPs@PPE and biofilm formation activity of Pseudomonas aeruginosa compared to pomegranate peel extract in 2019. The results of this group’s study showed that AgNPs@PPE has an inhibitory effect against P. aeruginosa at concentrations of 0.1 to 0.5 mg/mL. This finding was consistent with the results of our research.
According to the MIC results, propolis alone at a concentration between 20 and 30 μg/mL had the ability to inhibit bacterial growth. This finding is contrary to the results of well diffusion. The point that should be noted is that propolis does not penetrate the agar medium. Therefore, inhibition zone was not observed in the well diffusion test. But in the MIC method, due to the fact that the bacteria are directly exposed to propolis, its effect on the bacteria is visible. AgNPs also had a good effect on gram-positive and especially gram-negative bacteria in the MIC method, and in concentrations between 2.5 and 5 μg/mL, it had an inhibitory effect on the growth of bacteria. An interesting point to note was the better effect of AgNPs@propolis than AgNPs and propolis on inhibiting the growth of bacteria, which indicates the synergistic effect of AgNPs and propolis.
Dziedzic et al associate the antimicrobial property of propolis with its mechanism on cell division, changing the nature of cytoplasmic and bacterial membranes. Of course, the role of propolis affects the activity of DNA-dependent RNA polymerase and glucose-transferase enzymes of bacteria, which is probably related to the antimicrobial properties of propolis on bacteria (19). The study of Turnia et al (20) showed that the alcoholic extract of propolis can be effective in the treatment of various pathogenic bacteria such as B. cereus, S. aureus, S. enterica and E. coli. Although they stated that the effect of propolis in controlling gram positive bacteria is more than gram negative bacteria. Tosi et al (21) investigated the effects of ethanol, glycerin, propylene glycol and oil extracts (extracted from edible legumes) on bacteria and fungi and found all of them to be effective.
The mode of action of AgNPs@propolis on bacteria is still not fully understood. Defects in the cell membrane, Defects of energy transfer, formation of ROS and release of toxic elements are proposed as possible mechanisms of antibacterial effects of AgNPs@propolis. Negatively charged AgNPs@propolis can be electrostatically removed from negatively charged bacterial membranes (22).
Conclusion
Due to the increasing resistance of bacteria to different classes of antibiotics, the use of nanoparticles, plant extracts and natural materials to deal with this problem has become seriously important. The results of the current research showed that propolis as a natural substance has an inhibitory effect on the growth of bacteria. Green synthesis of AgNPs@propolis has a low toxic effect on the cell and has a high effect in inhibiting the growth of gram positive and negative bacteria.
Authors’ Contribution
Conceptualization: Zahra Karimitabar, Mohammad Yousef Alikhani.
Data curation: Zahra Karimitabar, Abbas Farmani, Masoud Azimzadeh, Mohammad Sina Alikhani.
Formal analysis: Masoud Azimzadeh.
Funding acquisition: Zahra Karimitabar
Investigation: Zahra Karimitabar, Abbas Farmani, Masoud Azimzadeh.
Methodology: Zahra Karimitabar, Mohammad Sina Alikhani, Masoud Moghadam Shakib.
Project administration: Mohammad Yousef Alikhani.
Resources: Mohammad Yousef Alikhani, Zahra karimitabar.
Supervision: Mohammad Yousef Alikhani.
Validation: Abbas Farmani, Masoud Azimzadeh.
Visualization: Mohammad Yousef Alikhani.
Writing–original draft: Masoud Azimzadeh, Zahra Karimitabar.
Writing–review & editing: Mohammad Yousef Alikhani, Masoud Azimzadeh.
Competing Interests
The authors declare that they have no conflict of interests.
Funding
This study was supported by Hamadan University of Medical Sciences [Grant No. 140003182187].
References
- Papaneophytou C, Giannenas I, Dragomir C. Resistance of bacteria, fungi, and parasites to antibiotics or natural substances of botanical origin. In: Florou-Paneri P, Christaki E, Giannenas I, eds. Feed Additives. Academic Press; 2020. p. 339-54. 10.1016/b978-0-12-814700-9.00019-4.
- Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G. Nanotechnology approaches for global infectious diseases. Nat Nanotechnol 2021; 16(4):369-84. doi: 10.1038/s41565-021-00866-8 [Crossref] [ Google Scholar]
- Hosseini SM, Taheri M, Nouri F, Farmani A, Morovati Moez N, Arabestani MR. Nano drug delivery in intracellular bacterial infection treatments. Biomed Pharmacother 2022; 146:112609. doi: 10.1016/j.biopha.2021.112609 [Crossref] [ Google Scholar]
- Kalia A, Morya S, Neumann A. Health from the hive: therapeutic potential of propolis-a review. Journal of Food Bioactives 2022;18.
- Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G. Silver nanoparticles as potential antibacterial agents. Molecules 2015; 20(5):8856-74. doi: 10.3390/molecules20058856 [Crossref] [ Google Scholar]
- Arya V, Komal R, Kaur M, Goyal A. Silver nanoparticles as a potent antimicrobial agent: a review. Pharmacologyonline 2011; 3:118-24. [ Google Scholar]
- Silva LP, Silveira AP, Bonatto CC, Reis IG, Milreu PV. Silver nanoparticles as antimicrobial agents: past, present, and future. In: Ficai A, Grumezescu AM, eds. Nanostructures for Antimicrobial Therapy. Elsevier; 2017. p. 577-96. 10.1016/b978-0-323-46152-8.00026-3.
- dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci 2014; 103(7):1931-44. doi: 10.1002/jps.24001 [Crossref] [ Google Scholar]
- de Souza TA, Rosa Souza LR, Franchi LP. Silver nanoparticles: an integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol Environ Saf 2019; 171:691-700. doi: 10.1016/j.ecoenv.2018.12.095 [Crossref] [ Google Scholar]
- Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics 2020; 10(20):8996-9031. doi: 10.7150/thno.45413 [Crossref] [ Google Scholar]
- Habibipour R, Moradi-Haghgou L, Farmany A. Green synthesis of AgNPs@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int J Nanomedicine 2019; 14:6891-9. doi: 10.2147/ijn.s209912 [Crossref] [ Google Scholar]
- Elhakim HKA, Azab SM, Fekry AM. A novel simple biosensor containing silver nanoparticles/propolis (bee glue) for microRNA let-7a determination. Mater Sci Eng C Mater Biol Appl 2018; 92:489-95. doi: 10.1016/j.msec.2018.06.063 [Crossref] [ Google Scholar]
- Karimitabar Z, Chegini Z, Shokoohizadeh L, Morovati Moez N, Arabestani MR, Hosseini SM. Use of the quantum dot-labeled solid lipid nanoparticles for delivery of streptomycin and hydroxychloroquine: a new therapeutic approach for treatment of intracellular Brucella abortus infection. Biomed Pharmacother 2023; 158:114116. doi: 10.1016/j.biopha.2022.114116 [Crossref] [ Google Scholar]
- Barabadi H, Mojab F, Vahidi H, Marashi B, Talank N, Hosseini O. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg Chem Commun 2021; 129:108647. doi: 10.1016/j.inoche.2021.108647 [Crossref] [ Google Scholar]
- Gheidari D, Mehrdad M, Maleki S, Hosseini S. Synthesis and potent antimicrobial activity of CoFe2O4 nanoparticles under visible light. Heliyon 2020; 6(10):e05058. doi: 10.1016/j.heliyon.2020.e05058 [Crossref] [ Google Scholar]
- Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver Nanoparticles and Their Antibacterial Applications. Int J Mol Sci 2021; 22(13):7202. doi: 10.3390/ijms22137202 [Crossref] [ Google Scholar]
- Wieczynska A, Wezgowiec J, Wieckiewicz W, Czarny A, Kulbacka J, Nowakowska D. antimicrobial Activity, cytotoxicity and total phenolic content of different extracts of propolis from the west Pomeranian region in Poland. Acta Pol Pharm 2017; 74(2):715-22. [ Google Scholar]
- Seibert JB, Bautista-Silva JP, Amparo TR, Petit A, Pervier P, dos Santos Almeida JC. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem 2019; 287:61-7. doi: 10.1016/j.foodchem.2019.02.078 [Crossref] [ Google Scholar]
- Dziedzic A, Kubina R, Wojtyczka RD, Kabała-Dzik A, Tanasiewicz M, Morawiec T. The antibacterial effect of ethanol extract of Polish propolis on mutans streptococci and lactobacilli isolated from saliva. Evid Based Complement Alternat Med 2013; 2013:681891. doi: 10.1155/2013/681891 [Crossref] [ Google Scholar]
- Turnia I, Nongkhlaw F, Joshi S, Prasad S. Antibacterial and antitumor activity of methanolic extract of propolis from Meghalaya. World J Pharm Pharm Sci 2015; 4(11):1809-21. [ Google Scholar]
- Tosi B, Donini A, Romagnoli C, Bruni A. Antimicrobial activity of some commercial extracts of propolis prepared with different solvents. Phytother Res 1996; 10(4):335-6. doi: 10.1002/(sici)1099-1573(199606)10:4<335::aid-ptr828>3.0.co;2-7 [Crossref] [ Google Scholar]
- Ahmad N, Sharma Sharma, Rai R. Rapid green synthesis of silver and gold nanoparticles using peels of Punica granatum. Adv Mater Lett 2012; 3(5):376-80. doi: 10.5185/amlett.2012.5357 [Crossref] [ Google Scholar]